Abstract
Metabolites of synthetic pyrethroids such as cis-3-(2,2-dibromovinyl)-2,2-di-methylcyclo-propane-1-carboxylic acid, cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid), 3-phenoxybenzoic acid (3-PBA), and 4-fluoro-3-PBA are biomarkers for exposure to phenothrin, tetramethrin, cyfluthrin, cypermethrin, deltamethrin, and permethrin. In this study, the pyrethroid metabolites in workers’ urine samples were monitored for the first time with a novel sample pretreatment process combining hollow fiber liquid phase microextraction (HF-LPME) and in-syringe derivatization (ISD) followed by gas chromatography–electron capture detector (GC-ECD) analysis. A micro-syringe pre-filled with derivatizing agents and syringe needle connected to an extracting solvent impregnated hollow fiber segment was used as the LPME probe. Pyrethroid metabolites were extracted and enriched simultaneously from urine samples by HF-LPME sampling and acid hydrolysis at 70 °C for 10 min. After sampling, the ISD was performed by mixing the extracting solution and derivatizing agents through plunger movements, followed by GC-ECD analysis. Parameters influencing the HF-LPME efficiency and ISD were investigated and optimized. Under optimum conditions, the method provided enrichment factors of 69.8–154.6, repeatability from 5.0 to 12% (n = 5), and good linearity (R 2 = 0.9980–0.9998) for interested analytes spiked in urine samples. The method detection limits ranged from 1.6 to 17 ng/mL. A comparison was performed between the proposed method and conventional methods. The proposed method was applied to analyze pyrethroid metabolites in the urine samples collected from workers of pesticide formulation plants. The results suggested that the proposed HF-LPME coupled ISD method was a rapid, simple, efficient, and eco-friendly technique in the biomonitoring of metabolites of pyrethroids in workers’ urine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrethroids and pyrethrins are neurotoxic insecticides which include permethrin, cypermethrin, cyfluthrin, deltamethrin, and fenvalerate. These are commercially available and are widely applied to control agricultural and domestic insects [1]. The use of synthetic pyrethroids in agricultural production and protection of human health (used in hospitals and in homes) has increased widely because of their low mammalian toxicity and high insecticidal activity, and hence have replaced organophosphate and carbamate insecticides as insect control agents [2]. Although the application of synthetic pyrethroids has less impact on humans, occupational studies suggest that excessive exposure to these insecticides can cause serious health effects such as paraesthesia, headache, dizziness, nausea, and skin irritation [3].
Moreover, indoor application of pyrethroids, which is often carried out without adequate expert knowledge, may lead to high pesticide residues, often combined with health hazards for the people concerned. In humans, pyrethroids are rapidly metabolized by esterases through hydrolytic cleavage to form their corresponding acidic metabolites, alcohol moiety metabolites, and some are partially conjugated and finally eliminated in the urine [4, 5]. cis- and trans-3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis- and trans-Cl2CA) are the specific metabolites of permethrin, cypermethrin, and cyfluthrin, whereas deltamethrin is transformed to cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis-Br2CA). The alcohol moiety of most pyrethroids is metabolized to form 3-phenoxybenzoic acid (3-PBA), but some fluoro-substituted pyrethroids such as cyfluthrin is transformed to 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA). Since pyrethroids metabolize rapidly, it is essential to monitor pyrethroid metabolites in the human urine of exposed individuals rather than monitor the actual pyrethroid concentration in blood [6–9].
Many analytical methods accompanied with appropriate pretreatment processes including pre-concentration and derivatization have been applied to monitor the content of pyrethroid metabolites in the urine of exposed individuals [10–15]. In the past two decades, solid phase extraction (SPE) has significantly replaced the conventional liquid–liquid extraction (LLE) based on eco-friendly consideration to enrich analytes from sample matrix [10, 14, 16–18] and has also been used for extraction of pyrethroid metabolites from urine matrix [17]. Moreover, immunoassay methods are on the one hand relatively cheap and simple to perform but on the other hand do not accomplish the sensitivity and specificity of analytical methods for the determination of pyrethroid metabolites in urine samples. Solid phase microextraction was seldom applied to collect the urinary pyrethroid metabolites directly due to the complicated matrix in urine, although it was developed to overcome the disadvantages of LLE and SPE with the advantages of being solvent-free, fast, and simple to perform [18].
Recently, a variety of liquid phase microextraction (LPME) techniques have been developed with the advantages of being simple, fast, convenient, and inexpensive [19–27]. However, using LPME techniques for the collection of pyrethroid metabolites in urine samples has not been reported so far. Since LPME is not for total extraction, several derivatization approaches accompanying LPME have been reported to improve the detection sensitivity [24–26]. With LPME to collect the metabolites of synthetic pyrethroids, an efficient derivatization is also required to enhance detection sensitivity.
In an effort to develop the LPME method to determine pyrethroid metabolites from urine matrix and in order to overcome the loss of extraction solvent while mixing with derivatizing agents, viz., N,N′-diisopropylcarbodiimide (DIC) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), in the derivatization process. For this purpose, we have designed a protocol by using hollow fiber (HF) protected LPME and in-syringe derivatization (ISD) to be a new sample extraction and pretreatment approach. Both derivatizing agents were pre-filled in the micro-syringe barrel, and a hollow fiber segment impregnated with extracting solvent was inserted in the syringe needle to be used as the LPME probe. After extraction, the LPME probe was taken out from the sample solution and the in-syringe derivatization was achieved inside the syringe barrel by mixing the extracting solution and derivatizing agents through the movements of plunger.
In this study, the applicability of the proposed method (HF-LPME-ISD) coupled to GC-ECD was investigated to be an alternative to conventional methods for biomonitoring the metabolites of synthetic pyrethroids in human urine samples.
Experimental
Instrumental and apparatus
Hewlett-Packard 6890plus (Agilent Technologies, Palo Alto, CA, USA) GC system equipped with a split/splitless injector (set at 20:1) and a micro-electron capture detector (μECD, 63Ni) was used to analyze the derivatives of pyrethroid metabolites. Separations were achieved using a fused silica DB-608 capillary column (30-m × 0.25-mm ID, 0.25-μm film thickness; Agilent Technologies). Nitrogen was used as the carrier gas and the makeup gas at flow rates of 1 and 30 mL/min, respectively. Temperatures of injector and detector were held constant at 250 and 300 °C, respectively. The oven was temperature-programmed as follows: 70 °C initially, increased to 160 °C at 6 °C/min (hold for 1.0 min), then increased to 250 °C at 30 °C/min with a final hold for 3 min. Agilent chemstation software was used to control the operation of GC, obtain the chromatogram, and perform data calculations.
Water bath with magnetic stirring (MR2002, Heidolph, Germany) was used to control the sampling temperature and stirring rate. A rotary vaporizer (VV2000, Heidolph) with a vacuum controller (V-880, Buchi, Switzerland) and a cooling system (BL-720, YIH DER, Taiwan) were used to concentrate the extracting solvent in conventional methods. A centrifugator (J2-MC, Beckman, USA) was used to remove suspended particles in urine sample.
Chemicals, reagents, and materials
cis- and trans-Cl2CA (99.8%), cis-Br2CA (98.8%), and 4-F-3-PBA (94%) were purchased from Bayer CropScience GmbH (Leverkusen, Germany); 2-phenoxybenzoic acid (2-PBA) and 3-phenoxybenzoic acid (3-PBA, 99.2%) were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). 2-PBA has served as internal standard. Structures of the pyrethroid metabolites measured in this study were demonstrated in Fig. 1a. Their standard stock solutions (1.0 mg/mL) were prepared by dissolving 100 mg in 100 mL hexane (Merck, Darmstadt, Germany), stored in silanized brown glass bottles with Teflon-lined cap, and kept at 4 °C. Fresh daily working solutions were prepared by appropriate dilutions of the stock solutions with hexane. Derivatizing agents were HFIP (99%, Sigma-Aldrich, St. Louis, MO, USA) and DIC (98%, Merck). Hydrochloric acid (HCl, 37%), sulfuric acid (H2SO4, 95–97%), potassium carbonate (K2CO3), sodium chloride (NaOH), anhydrous sodium sulfate, 1-octanol, 2-octanol, isooctane, toluene, hexane, ethyl acetate, and methanol were obtained from Merck. Dichloromethane was obtained from Mallinckrodt (Paris, Kentucky, USA). Deionized water used for all aqueous solutions was produced in the laboratory using a Barnstead Nanopure water purification system (Bedford, MA, USA). Standard urine sample (KOVA-TROL urine controls, normal range with HCG, HYCOR Biomedical Inc., USA) was used as the matrix to prepare standard metabolite urine samples.
Accurel Q3/2 polypropylene hollow fiber membrane (600-μm ID, 800-μm OD, pore size 0.2 μm) was purchased from Membrana (Wuppertal, Germany) and used for HF-LPME. The hollow fiber membrane was cut into segments of 15, 20, 25, and 30 mm in length, soaked in acetone and sonicated (in order to remove any contaminants on the hollow fiber membrane surfaces and also to activate the pores on the membrane surfaces), and dried by purging with air before use. The solid phase cartridges including C-18 (1,000 mg/6 mL, J&W Scientific, Folsom, CA, USA), EN (200 mg/3 mL, Merck) and EVVI-Carb (500 mg/3 mL, Supelco, Bellefonte, PA, USA), and the Extrelut NT3 liquid phase cartridge (for 1–3 mL aqueous sample, Merck) were used for sample pretreatments. Urine samples were collected from workers of pesticide formulation plants located at central and southern Taiwan.
Urine sample collection
Urine samples were collected from 22 workers in pesticide formulation plants prepared for emulsifiable concentrates of 20% fenvalerate, 10% cypermethrin, and 10% permethrin in the period July 2–September 20, 2004. Urine samples of occupationally exposed individuals were collected for 24 h after working and stored in sterilized containers. Samples were frozen immediately and kept at −30 °C until being analyzed.
HF-LPME sampling and in-syringe derivatization
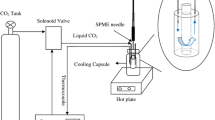
LPME sampling probe was prepared by taking the derivatizing agents (2 μL of DIC and 1 μL of HFIP) into a 10-μL syringe barrel (model 901/26s, Hamilton, USA), followed by inserting a hollow fiber segment onto the syringe needle and impregnating the extracting solvent by immersing the hollow fiber membrane into the extracting solvent and withdrawing 8 μL of the solvent into syringe for HF-LPME sampling. The apparatus for HF-LPME-ISD sampling is demonstrated in Fig. 2.
One milliliter aliquot of urine was placed in a 10-mL sample vial and 1 μg 2-PBA (internal standard) and 0.1 mL conc. HCl were added, and then the sample was diluted with deionized water to 5 mL and heated at 70 °C in a water bath for 10 min with stirring to achieve the hydrolysis of the conjugates of metabolites and adjust the acidity of the sample solution to ensure that metabolites are in their neutral forms. HF-LPME sampling was performed simultaneously by immersing the sampling probe into a sample vial under controlled temperature in a water bath.
After sampling, the LPME probe was shifted out from the urine solution to achieve the derivatization through mixing the extraction solvent and derivatizing agents thoroughly by withdrawing and depressing movements of the syringe plunger ten times. After derivatization and removing the hollow fiber from the syringe needle, the derivatized solution (1 μL each time) was injected into the GC system for analysis.
Conventional liquid–liquid extraction and solid phase extraction methods
LLE method
The sample solution (5 mL) was filled in a 10-mL sample vial and was added 0.5 mL of conc. hydrochloric acid. After mixing thoroughly, the sample solution was heated in s water bath (90 °C) for 1 h. After the solution was cooled to room temperature, the metabolites were extracted by adding 2.5 mL of n-hexane into the sample solution and shaking for 10 min. After centrifuging under 1,500×g for 5 min, the organic layer (n-hexane) was collected. Purification of metabolites was achieved by adding 0.1 mol/L NaOH (1 mL) into the collected n-hexane, shaking, and centrifuging to separate two layers with the same conditions. The aqueous layer was collected, adding 0.1 mL of conc. HCl and 1 mL of n-hexane. After shaking and centrifuging the solution, the n-hexane layer was collected and added 10 μL of HFIP and 15 μL of DIC for derivatization by violently shaking for 3 min. The excess derivatizing agents were removed by adding 5% aqueous K2CO3 (1 mL). After shaking and centrifuging, the n-hexane was collected, purged with nitrogen, and the volume fixed to 1.0 mL with hexane. The n-hexane (1 μL each time) was injected into the GC system for analysis.
SPE method
Three SPE cartridges (C18, EN, and EVVI-Carb) were applied in the study. The cartridges were conditioned with methanol and water prior to extraction. The sample solution (5 mL) was poured and passed through the SPE cartridge to trap metabolites. After being dried by vacuum pump, 20 mL of the solvent (ethyl acetate/methanol in 1:1) was used to elute out the metabolites. The collected solvent was dried by adding anhydrous sodium sulfate. After removing the solvent by purging with nitrogen, the sample was dissolved in 1 mL of n-hexane for derivatization and analyzed as described above.
Liquid extraction method
The Extrelut NT3 cartridge was used to achieve the liquid phase extraction. Sample solution (3 mL) was poured into the Extrelut NT3 cartridge. After 15 min, dichloromethane (25 mL) was used to extract and elute out the metabolites. The collected dichloromethane was dried by adding anhydrous sodium sulfate. After removing the solvent by purging with nitrogen, the sample was dissolved in 1 mL of n-hexane for derivatization and analyzed as described above.
Results and discussion
Selection of derivatizing agent and in-syringe derivatization
For GC analysis, extractants should be derivatized by various derivatizing agents. For the purpose of the proposed protocol, derivatization should be achieved within a short time. Derivatization by methylation with methanol under conc. H2SO4 [5, 28] and derivatizing with N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide [15] did not seem to meet the demand of the proposed protocol due to their time-consuming, cumbersome, and tedious process. HFIP derivatives which are produced by rapid coupling of the metabolite acids with HFIP in the presence of DIC are highly sensitive and do not produce interfering substances. It has the potential to achieve the ISD. Although the derivatization process could be complicated in the sample extraction and cleanup process [29], the HFIP derivatization can be improved using hollow fiber supported LPME to simplify these tedious procedures. The chemical reaction of derivatization is demonstrated in Fig. 1b.
The derivatizing agent is always added in large excess to ensure the complete derivatization of all the target analytes since the volume available for extracting the solvent and derivatizing agents was only 11 μL in the proposed trace extraction and ISD approach. Moreover, in order to optimize the volume of extraction solvent and derivatizing agents, various lengths of hollow fibers (1.5, 2.0, 2.5, and 3.0 cm with the capacity of 6, 8, 9.5, and 11 μL, respectively) with various amounts of extraction solvent (6, 8, 9.5, and 11 μL) and derivatizing agents (1 μL of DIC + 1 μL HFIP, 2 μL DIC + 2 μL HFIP, 2 μL DIC + 1 μL HFIP, and 1 μL DIC + 2 μL HFIP) were examined. The results show that the extracted quantities of the metabolites increased slightly or insignificantly with the increase of solvent quantity since a dilution effect occurred. Since only 5 mL of the sample solution was used for analysis in a 10-cm test tube, longer hollow fiber was twisted and torn by stirring. Therefore, 2.0 cm of the hollow fiber segment with the capacity of 8 μL was used to prepare the sampling probe. Moreover, derivatizing agents (2 μL DIC + 1 μL HFIP) showed the best derivatization efficiency with 8 μL of 1-octanol. Hence, 2 μL of DIC and 1 μL of HFIP were withdrawn successively into the syringe barrel prior to insertion of the hollow fiber and then the extracting solvent (8 μL of 1-octanol) was withdrawn for further analysis.
After LPME sampling, the extracting solvent was withdrawn into the syringe barrel and derivatization occurred while mixing derivatizing agents and extracting solvent by moving the plunger front and back. The movement was examined, with the results shown in Fig. 3a. It can be seen that the peak areas of derivatives increased with the mixing times, as expected, and plunger movement for 20 times showed maximum extraction efficiency. However, the variation of analytical data increased with the mixing times due to the volatile loss of both derivatizing agents and showed poor RSD. To compromise the detection sensitivity and repeatability, plunger movement for ten times was chosen.
a Influence of mixing time on the peak areas of derivatives. Concentration of metabolites: 5-mL sample containing 5 μg of cis-Cl2CA, trans-Cl2CA, cis-Br2CA, and 4-F-3-PBA and 1 μg of 3-PBA and 2-PBA. HF-LPME sampling: at 70 °C for 10 min. b Hydrolysis efficiency under various concentrations of hydrochloric acid. Conditions as in a. c Influence of extraction temperature on detection peak area. Conditions as in a, except the sampling time (10 min)
Optimization of acid hydrolysis and HF-LPME sampling
In the present approach, LPME was applied to simplify the tedious procedures in conventional LLE and SPE methods to extract pyrethroid metabolites from urine samples. Since acid hydrolysis of metabolite conjugates and HF-LPME sampling were carried out simultaneously, the extracting solvent should be of low volatility since high temperature speeds up the acid hydrolysis. 1-Octanol was chosen as the extracting solvent from series tests of five solvents (ethyl acetate, n-hexane, isooctane, toluene, and 1-octanol). Moreover, the results indicated that the cleanup efficiency and recovery yield of the 1-octanol derivatization was higher than the other tested solvents. Although a worse baseline of chromatogram arose due to the high viscous and low volatile characters of 1-octanol, it can be improved using the splitting injection.
Conc. HCl or H2SO4 was added into urine aliquot to hydrolyze the metabolite conjugates [30]. Both the acids were used for the hydrolysis, and LPME efficiency was examined from 0.1 to 1.0 mL acid additions. It can be seen that 0.1 mL of both acids individually was enough to obtain the acid hydrolysis and LPME efficiency for 1 mL urine sample at 70 °C for 10 min. Addition of 0.1 mL of HCl shows a slight excess extraction quantities and best hydrolysis than sulfuric acid. Therefore, 0.1 mL of HCl was chosen for acid hydrolysis and was used for subsequent analysis. Figure 3b shows the hydrolysis efficiency under various concentrations of conc. HCl addition.
Since high temperature speeds up the acid hydrolysis and also accelerates the diffusion of analytes in sample matrix and in extraction solvent, temperatures from 40 to 90 °C for 5–30 min were examined to find the optimal temperature and time for the HF-LPME. Figure 3c shows the extraction efficiency on the acid hydrolysis with HF-LPME temperature. The results indicated that the highest extraction quantity of the metabolite was apparently obtained at 70 °C for 10 min for the simultaneous acid hydrolysis and LPME and was thus used for subsequent analysis.
Validation of the proposed method
In order to investigate the applicability of the proposed method for quantitative determination of pyrethroid metabolites in urine samples, standard urine matrix sample solutions were used for the calibration after they were subjected to overall treatment procedure, i.e., hollow fiber supported LPME, in-syringe derivatization and GC-ECD analysis. Table 1 illustrated the calibration parameters of metabolites with the proposed method and GC-ECD. The peak area of signal response had good linear relationship with the quantity of individual metabolite in the concentration range. All the linear correlation coefficients of metabolites were between 0.9980 and 0.9998. The method detection limits were calculated based on three times the average background noise (S/N = 3) divided by the detection sensitivity (slope of calibration plot), which varied from 1.6 to 17 ng/mL. The in-house repeatability of detection peak areas was examined with coefficient of variances in 5–12% (n = 5).
The extraction percentage and the enrichment factors of pyrethroid metabolites from urine samples were examined by spiking 0.1 mL of the standard solution containing 0.1 μg/mL of cis-Cl2CA, trans-Cl2CA, and 4-F-3-PBA and 0.02 μg/mL of cis-Br2CA, 3-PBA, and 2-PBA to 5 mL sample solution and analyzed by the proposed method. The extraction percentage (R) and the enrichment factor (E) are evaluated by the equations R = C org V org/C i V aq and E = C org/C i, respectively, where C org and C i are the extracted concentration of analyte in extractant and the initial concentration of analyte in aqueous sample solution, respectively; V org and V aq are the volumes of the extractant and sample solution, respectively. As listed in Table 2, the extraction percentage and the enrichment factors of pyrethroid metabolites were 20.8% and 94.7 for cis-Cl2CA, 22.0% and 99.9 for trans-Cl2CA, 27.2% and 123.6 for cis-Br2CA, 34.1% and 154.6 for 4-F-3-PBA, 23.4% and 106.6 for 3-PBA, and 15.4% and 69.8 for 2-PBA, respectively.
Comparison with LLE and SPE methods
The proposed approach was compared with the conventional LLE and SPE methods in the determination of pyrethroid metabolites in urine samples. The chromatogram of pyrethroid metabolites in urine sample via LLE is shown in Fig. 4a. The complicated noise signal in Fig. 4a depicts that the LLE did not remove some of the matrix interference compared with that via the proposed approach in Fig. 4b and via the SPE in Fig. 4c and thus have comparable or higher detection limit (LOD). Table 2 lists the performance of the proposed method and LLE method in the analysis of pyrethroid metabolites in urine samples. Because the LPME is an extraction based on the equilibrium of analyte between the extractant phase and the aqueous sample phase, only 15.4–34.1% of pyrethroid metabolites were collected in 8 μL of solvent, although the enrichment factors were in 69.8–154.6 when 1 mL of urine sample was spiked with 0.1 μg/mL of cis-Cl2CA, trans-Cl2CA, and 4-F-3-PBA and 0.02 μg/mL of cis-Br2CA, 3-PBA, and 2-PBA were extracted. Therefore, about 1.9–4.3% of pyrethroid metabolites in urine sample were injected into the GC system for 1-μL injection. Although the LLE method is used to extract the total content of analyte, the pyrethroid metabolites spiked with the concentration for the proposed LPME method were not well detected by the LLE method. Therefore, 5 mL of urine sample spiked with1 μg/mL of cis-Cl2CA, trans-Cl2CA, and 4-F-3-PBA and 0.2 μg/mL of cis-Br2CA, 3-PBA, and 2-PBA were used for the LLE method, and about 76.4–88.8% of pyrethroid metabolites, except trans-Cl2CA (with serious interference), were extracted, as listed in Table 2. Because 1 μL of the final 1.0 mL of hexane was injected into GC for analysis, about 0.38–0.44% of pyrethroid metabolites in urine sample were injected into GC system. The higher LOD of LLE than HF-LPME in Table 2 depicts that some matrix interference still existed when LLE was used, which is reflected in the complicated noise signal in Fig. 4a. Moreover, the detection limits of the proposed method were also compared with the previously reported paper, i.e., 10–100 ng/mL, which was obtained by the LLE method [12]. From these results, the proposed HF-LPME method has been identified with a potential to extract lower concentration of pyrethroid metabolites in urine than the LLE method.
Chromatogram of pyrethroid metabolites in urine sample via LLE method (a), via the proposed method (b), and via the SPE method (c). Concentration of metabolites: a same as in Fig. 3a; b one tenth of a; c one fifth of a. SPE method with EN cartridge and elute with 25 mL of methylene chloride. Peaks 1–6 in chromatograms were referred to cis-Cl2CA, trans-Cl2CA, cis-Br2CA, 4-F-3-PBA, 3-PBA, and 2-PBA, respectively
In addition, the proposed method used only 8 μL of 1-octanol as the extracting solvent and one tenth of the derivatizing agent used in LLE and SPE methods within 15 min to achieve the extraction and derivatization compared with 2–4 h in the LLE and SPE methods.
Analysis of pyrethroid metabolites in worker urine samples
The proposed method was examined to analyze pyrethroid metabolites in individual urine samples collected from workers of pesticide formulation plants located in central and southern Taiwan. Also, to estimate the potential matrix effects on real urine samples under the proposed method, urine samples covering a wide range of creatinine contents (0.5–2.0 g/L) were tested. The creatinine content did not show any considerable influence on these parameters. As a result, matrix influences could not be found for our proposed method since the creatinine-adjusted concentration approach allows us to avoid confounding factors such as variations in urine dilution due to variations in liquid intake or variations in creatinine levels associated with factors such as ethnic origin or age or protein intake by the individuals [4, 15, 31]. Therefore, the average creatinine-adjusted concentration (micrograms per milligram) of urine samples was used for the real sample analysis. After the proposed simultaneous acid hydrolysis of real urine samples and HF-LPME sampling followed by the ISD, derivatives of pyrethroid metabolites were analyzed by GC-ECD. The chromatogram is shown in Fig. 5. Interesting pyrethroid metabolites, viz., cis-Cl2CA and trans-Cl2CA, which appeared in the chromatogram were also confirmed by GC-MS. Table 3 lists the concentrations of pyrethroid metabolites that existed in the urine of several workers. The duties of these workers were in the formulation operations. It can be seen that the formulation area in the plant is at high exposure risk to synthetic pyrethroids; hence, the work environment should be improved.
Conclusion
In this work, acid hydrolysis with simultaneous HF-LPME sampling of urine sample as a one-step sample enrichment tool followed by in-syringe derivatization approach was proposed as an alternative pretreatment and biomonitoring method for pyrethroid metabolites in human urine prior to GC-ECD analysis; the optimal conditions have been established. From the results of the applicability test, it has been proved that the presented method is rapid, simple, convenient, sensitive, inexpensive, and consumes much lower organic solvent and derivatization reagent (in the microliter range), making it an environment-friendly procedure to analyze pyrethroid metabolites in human urine. In addition, compared with traditional extraction methods such as LLE and SPE, the current method provides better sensitivity and extraction recovery.
References
Feo ML, Eljarrat E, Barcelo D (2010) Trends Anal Chem 29:692–706
Heudorf U, Butte W, Schulzc C, Angerer J (2006) Int J Hyg Environ Health 209:293–299
You J, Brennan A, Lydy MJ (2009) Chemosphere 75:1477–1482
Fortin MC, Bouchard M, Carrier G, Dumas P (2008) Environ Res 107:343–350
Heudorf U, Angerer J (2001) Environ Health Perspect 109:213–217
Leng G, Lewalter J (1999) Occup Environ Med 56:449–453
Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD, Magsumbol MS, Williams BL, Needham LL (2010) Environ Health Perspect 118:742–748
Naeher LP, Tulve NS, Egeghy PP, Barr DB, Adetona O, Fortmann RC, Needham LL, Bozeman E, Hilliard A, Sheldon LS (2010) Sci Total Environ 408:1145–1153
Arcury TA, Grzywacz JG, Isom S, Whalley LE, Vallejos QM, Chen H, Galván L, Barr DB, Quandt SA (2009) Int J Occup Environ Health 15:339–350
Aprea C, Stridori S, Sciarra G (1997) J Chromatogr B 695:227–236
Barr DB, Leng G, Prei EB, Hoppe HW, Angerer J (2007) Anal Bioanal Chem 389:811–818
Leng G, Gries W (2005) J Chromatogr B 814:285–294
Loper BL, Anderson KA (2003) J AOAC Int 86:1236–1240
Leon-Gonzalez MW, Plaza-Arroyo M, Perez-Arribas LV (2005) Anal Bioanal Chem 382:527–531
Schettgen T, Koch HM, Drexler H, Angerer J (2002) J Chromatogr B 778:121–130
Colume A, Cardenas S, Gallego M, Valcarcel M (2001) Rapid Commun Mass Spectrom 15:2007–2013
Angerer J, Ritter A (1997) J Chromatogr B 695:217–226
Pawliszyn J (1997) Solid phase microextraction theory and practice. Wiley-VCH, New York
Basheer C, Balasubramanian R, Lee HK (2003) J Chromatogr A 1016:1–20
Nerín C, Salafranca J, Aznar M, Batlle R (2009) Anal Bioanal Chem 393:809–835
Hyötyläinen T, Tuutijärvi T, Kuosmanen K, Riekkola ML (2002) Anal Bioanal Chem 372:732–736
Jong JD, Lammertink RGH, Wessling M (2006) Lab Chip 6:1125–1139
Kawaguchi M, Ito R, Endo N, Okanouchi N, Sakui N, Saito K, Nakazawa H (2006) J Chromatogr A 1110:1–5
Pezo D, Salafranca J, Nerín C (2007) J Chromatogr A 1174:85–94
Rodríguez A, Pedersen-Bjergaard S, Rasmussen KE, Nerín C (2008) J Chromatogr A 1198–1199:38–440
Zhang J, Lee HK (2006) J Chromatogr A 1117:31–37
Yan H, Liu B, Du J, Yang G, Row KH (2010) J Chromatogr A 1217:5152–5160
Leng G, Kühn KH, Idel H (1997) Sci Total Environ 199:173–181
Arrebola FJ, Martínez-Vidal JL, Fernández-Gutiérrez A, Akhtar MH (1997) Anal Chim Acta 401:45–54
Payán MR, López MAB, Fernández-Torres R, Bernal JLP, Mochón MC (2009) Anal Chim Acta 653:184–190
Jen JF, Hsiao SL, Liu KH (2002) Talanta 58:711–717
Acknowledgment
The authors thank the National Science Council of Taiwan (grant no. NSC 94-2113-M-005-002, NSC-98-2113-M-005-016-MY3) and the Council of Agriculture (COA) in Taiwan (grant no. 90AS-1.2.2-PI-P2(3)) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, CH., Yan, CT., Kumar, P.V. et al. Determination of pyrethroid metabolites in human urine using liquid phase microextraction coupled in-syringe derivatization followed by gas chromatography/electron capture detection. Anal Bioanal Chem 401, 927–937 (2011). https://doi.org/10.1007/s00216-011-5122-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5122-0