Abstract
A dispersive liquid–liquid microextraction (DLLME) method followed by gas chromatography/mass spectrometry (GC/MS) was applied for the trace determination of organochlorine pesticides in honey samples. The type and volume of organic extraction and disperser solvents, pH, effect of added salt content and centrifuging time and speed were optimized to find the appropriate extraction conditions. In DLLME, 30 µL of 1,2-dibromomethane (serving as extractant) and 1.5 mL of acetonitrile (serving as disperser) were applied. The limit of detection (3 s) and limit of quantification (10 s) for all the analytes of interest (organochlorine pesticides) varied from 0.004 to 0.07 and from 0.02 to 0.3 ng g−1, respectively. The extraction recovery ranged from 91 to 100 %, and the enrichment factors ranged from 171 to 199. The relative standard deviation was <6 % for intraday (n = 6) and <8 % interday (n = 4) measurements. The proposed DLLME–GC/MS method was confirmed to be fast, simple to perform, friendly to environment and suitable for analysis of organochlorine pesticide residues at trace levels in honey samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey is a food product consumed worldwide and has high-quality nutritional properties. It is a sweet food product made by bees [1]. To ensure its safe consumption, honey must be free of all contaminants (especially pesticides) at trace levels [2].
Pesticides are broadly used in agriculture, and the presence of pesticides residue in food products like honey is unacceptable. Organochlorine pesticides (OCPs) are highly poisonous, carcinogenic and extremely resistant to environmental degradation [3]. The manufacture and use of OCPs have been restricted or forbidden in many countries such as German, England, France, Portugal and the USA [4]. However, their residues have remained in the environment from prior agricultural use [5] and transfer in atmosphere as surplus to the honey [6].
Several studies have shown that OCPs bio-accumulate in plants from contaminated soil [7]. This bio-accumulation in plants resources can spread 10–1000 times more pesticide than that which is spread in the air and water [8]. OCPs can also easily contaminate non-fatty foods such as honey as well as fatty-enriched food products [9]. Honey can become contaminated directly from contact with honeybees carrying polluted pollen, nectar, soil and water and from inhalation during flight [10]. Moreover, OCPs occurrence has been widely documented in different studies [11–16].
The contaminants in honey exist at trace levels, and honey has a complex matrix. For this reason, sample preparation and isolation of the analytes are significant analytical stage in honey analysis [17]. The procedure most commonly used for extraction and purification of analytes from samples such as honey matrix is classical liquid–liquid extraction [18, 19], solid-phase extraction [20], supercritical fluid extraction [21], matrix solid-phase dispersion [22], accelerated solvent extraction [23], quick, easy, cheap, effective, rugged and safe extraction [24] and solid-phase microextraction [25].
Most of these pretreatment methods have disadvantages such as being labor intensive, time-consuming, achieving low enrichment factors (EFs) and requiring large amounts of hazardous solvents. In 2006, Rezaee et al. introduced a liquid-phase microextraction technique termed dispersive liquid–liquid microextraction (DLLME). The DLLME method is based on a ternary component solvent system. Briefly, a cloudy solution is formed when a suitable mixture of extractant and disperser is thoroughly injected into an aqueous phase. The target analytes are rapidly transferred to fine microdroplets of extraction phase. After that, phase separation is performed by: centrifugation [26], in-line filtration [27], column adsorption [28], demulsificaton [29], floatation [30] or passive sedimentation [31] of extraction phase. The extracted analyte is then collected for determination step. With regard to the fine mixing of both extraction and aqueous phase, several recent papers utilized kinetic energy instead of dispersive solvent using: magnetic stirring [32], vortexing, ultrasound irradiation [33], multiple aeration [34], repeated up and down agitation [35] or in-single-step-performed automatic vigorous solvent injection [36].
The advantages of DLLME are: rapidity, low-cost operation, simplicity and achievement of high recovery and ERs. Furthermore, many research groups and projects have been attracted to using DLLME method up to date [26, 37–43].
In this work, DLLME-GC/MS method was developed and validated in order to make it simple, rapid and environmentally friendly. This method was successfully applied for the determination of OCPs at trace levels in honey samples using select ion monitoring mode [44]. The effect of different experimental factors was studied to optimize extraction conditions. In contrast to previous analysis of OCPs in honey using DLLMEs, better sensitivity, shorter extraction time and comparable reproducibility represented by intraday and interday precision and accuracy were achieved.
Experimental
Chemicals and solutions
All OCPs used: chlorthalonil, chlorpyrifos, dicofol, O,P′-DDE, P,P′-DDE, O,P′-DDT and P,P′-DDD were of >98 % purity and purchased from Dr. Ehrenstorfer (Germany, Merck). The extraction solvents were 1,1,1-trichloroethane (1,1,1-TCE), chloroform, 1,2-dibromoethane (1,2-DBE) and 1,2 dichloromethane (1,2-DCE) obtained from Merck (Germany) and 1,1,2,2-tetrachloroethane (1,1,2,2-TCE) obtained from Janssen Chimica (Belgium).
Solvents such as acetonitrile (ACN) of HPLC grade, acetone and methanol and ethyl acetate were obtained from Merck (Germany). The deionized water was prepared by Milipore-Direct-Q3 system and used for the preparation of aqueous solutions. Sodium hydroxide, sodium chloride and hydrochloric acid were of analytical grade and also purchased from Merk (Germany).
Standard stock solutions of pesticides were supplied by dissolving an appropriate amount of each analyte in ethyl acetate (at 1000 mg L−1). Working solutions were prepared in ethyl acetate from the stock solutions and stored at −18 °C.
Apparatus
The pesticides were analyzed using a Agilent 7890A gas chromatograph equipped with a 5975C mass-selective detector (Agilent Technologies; USA) and a splitless injector operated at 250 °C in splitless/split mode (sampling time: 1 min). Chromatographic separation was carried out in an HP-5 MS capillary column of 30 m × 0.25 mm ID with a film thickness of 0.25 µm (Agilent J and W; USA). The initial oven temperature of 75 °C was held for 3 min, ramped up at 25 °C min−1 to 180 °C, then finally ramped up at 5 °C min−1 to a final temperature of 300 °C and held for 25 min. Helium was used as carrier gas at a flow rate of 1.0 mL min−1. The target analytes were quantified in SIM mode, and one qualifier ion was selected for each analyte (Table 1). A Hettich centrifuge (Universal 320 R; Germany) was used to accelerate phase separation.
Sample collection
Four honey samples of different floral origins were purchased from local markets in the city of Tabriz in Iran during the winter of 2014. To optimize and validate the proposed method, one further pesticide-free honey sample was obtained from beehives in a virgin mountainous region of Kurdistan, a western province of Iran.
All the samples were stored in their original containers at ambient temperature until analysis. A homogenous solution (aqueous sample), 25 g of honey sample was dissolved in deionized water at first and the then refilled with water up to 50 mL. The solution was left to equilibrate for at least 15 min prior to extraction. It was then strictly exposed to the developed technique without pretreatment.
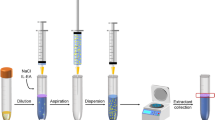
DLLME protocol
5 mL of honey prepared solution was spiked with 20 ng g−1 of each pesticide and transferred to a glass test tube. Then, the mixture of dispersive and extraction solvent consisting of 1.5 mL ACN (disperser) and 30 μL 1,2-DBE (extractant) was quickly injected in one stage to sample using a 5 mL glass syringe. At this step, a cloudy state was formed by dispersion of fine microdroplets into aqueous solution. At the same time, OCPs were transferred immediately to the extractant. To separate the organic phase from the aqueous phase, the mixture was centrifuged at 4000 rpm for 5 min so that the dispersed droplets of 1, 2-DBE can settle at the bottom of the conical test tube (25 ± 1 μL). Finally, 1 μL of the extractant (sediment phase) was injected into a GC/MS for analysis.
Results and discussion
In the proposed study, DLLME–GC/MS method was used to determine OCPs in honey samples. Experimental factors such as the type and volume of extractant and disperser, salt addition, pH adjustment, centrifugation time and speed were investigated.
Effect of extraction solvent
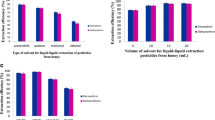
The type of extraction solvent is a key parameter affecting the efficiency of DLLME [26] and should possess the following features: immiscibility with water, density higher than water, good chromatographic behavior (in case, chromatography is used as detection technique) and high extraction capacity for target compounds. For this goal, five organic solvents having these qualifications were studied: chloroform, 1,2-DBE, 1,1,1-TCE, 1,2-DCE and 1,1,2,2-TCE. Investigation was accomplished using 1.5 mL of ACN and different volumes of extractant solvent to achieve a comparable volume for the sediment organic phase (10 ± 1 µL). The tested volumes were 35 µL 1,2-DBE, 40 µL chloroform, 45 µL 1,2-DCE, 40 µL 1,1,2,2-TCE and 38 µL 1,1,1-TCE.
The effect of extraction solvent type on extraction efficiency of OCPs is shown in Fig. 1. The results showed that 1,1,2,2 TCE and 1,2-DBE provide better extraction efficiency for the rest of the solvents. Due to comparable lower utilization of solvent volume required for 1,2-DBE—35 µL over 1,1,2,2-TCE—40 µL and higher peak areas for majority of the determined OCPs, 1,2-DBE was selected as optimal extraction solvent for further experiment.
Effect of dispersive solvent
To achieve high preconcentration of target analytes, type of the disperser solvent is very important. The appropriate disperser solvent must meet the following requirements: fine miscibility with both aqueous and organic extraction phase, easy achievement of cloudy state to enlarge contact area between sample and extraction phase, which serves for effective transfer of target analyte. Three solvents: acetone, methanol and ACN, were tested using 1.5 mL volume. The results showed that ACN achieved better results after addition to the honey sample than acetone and methanol due to slightly higher response in peak area (Fig. 2).
Effect of extraction solvent volume
Extraction efficiency and repeatability of results could be affected by the final volume of the extraction phase. Concentrations of analytes in organic phase decrease as its volume is larger due to the effect of dilution. To evaluate the effect of extraction solvent volume, different volumes: 30, 40, 50 and 60 µL of 1,2-DBE, were dissolved in 1.5 mL ACN and used in the proposed DLLME protocol. As shown in Fig. 3, the average peak areas of the extracted OCPs decreased at 30–60 µL volume range of 1,2-DBE. The maximum peak was recorded using 30 µL of extraction solvent. By using less than 30 µL of extraction solvent, DLLME procedure suffered from insufficient volume for sedimentation and thus recollection for GC/MS analysis. Therefore, 30 µL was selected as the optimal volume of extraction solvent.
Effect of disperser solvent volume
To evaluate the influence of ACN volume on extraction efficiency, 0.5–2 mL using 0.5 increments was studied. The amount of organic phase recovered and the average peak areas of most pesticides increased as the ACN volume increased up to 1.5 mL. The results indicated that at 2 mL ACN, the quantity of the upper phase was increased, but the extraction efficiency decreased; this could be the result of the higher solubility of OCPs in the aqueous phase. Figure 4 shows that the highest analytical responses were found for all the extracted OCPs as the 1.5 mL of ACN was used; thus, this volume was selected as optimal for the next investigation.
Salting-out effect
The addition of salt usually reduces the ionic strength of analytes as their solubility in aqueous sample decreases; this is known as the ‘salting-out effect.’ To investigate the effect of salt addition on DLLME, NaCl was tested up to 6 % (w/v). The results indicated that the peak areas for most OCPs were approximately constant and showed a slight decrease with an increase in salt content for most analytes. This finding suggests that it was not necessary to add salt during testing, and it was omitted from further experimentation.
Effect of pH adjustment
The effect of pH adjustment on extraction efficiency was tested for pH of 2–12. The results show that within this range, peak areas of most extracted OCPs remained stable; thus, pH of natural matrix solution was used.
Effect of centrifugation time and speed
Another important parameter that affects DLLME is the choice of proper technique for droplets recollection. For this purpose, centrifugation (time and speed) as the most efficient recollection technique was chosen. The centrifugation time and speed were tested from 2 to 6 min at 2000–6000 rpm, respectively. The results showed that highest extraction of OCPs was performed using 5 min centrifugation at 4000 rpm, which were taken as optimal for further analysis.
Analytical performance
Several analytical parameters such as correlation coefficient (R), repeatability, linear range, extraction recovery (ER) and EF were also evaluated under optimal conditions. The limit of detection (LOD) and limit of quantification (LOQ) were calculated on the basis of the signal-to-noise ratio (S/N) using 3 and 10 s criterion. The results are summarized in Table 1.
The calibration curves were constructed by analyzing the honey sample spiked with 0.02–200 ng g−1 chlorthalonil, 0.02–200 ng g−1 chlorpyrifos, 0.04–200 ng g−1 dicofol, 0.06–300 ng g−1 2,4-DDE, 0.09–300 ng g−1 4,4-DDE, 0.2–300 ng g−1 2,4-DDT and 0.3–300 ng g−1 4,4-DDT. Good linearity was achieved for all the pesticides at R > 0.991. The LODs were 0.004–0.07 ng g−1, and the LOQs were 0.02–0.3 ng g−1, which are considerably low values. The EFs and ERs for the analytes ranged from 171–199 and 91–100 %, respectively. The precision of the DLLME method was calculated in terms of RSD of 3–5 % for intraday (n = 6) and 5–8 % for interday (n = 4) and indicated that the technique was satisfactorily repeatable.
Analysis of honey samples
The proposed method was applied for the analysis of commercially available honey samples from diverse floral origins in East Azerbaijan province in Iran. Figure 5 shows a typical GC/MS blank honey sample, honey samples spiked with 20 ng g−1 of each pesticide and standard solution (100 ng g−1) of each analyte in 1,2-DBE. All the honey samples were analyzed by the proposed DLLME technique. The results showed that they were free of the target analytes.
To evaluate the applicability and matrix effect of the method, the samples were spiked with the target analytes at 10, 30 and 50 ng g−1. The data indicated recovery of pesticides in comparison with the deionized water samples spiked at the three fortification levels, as shown in Table 2. The results confirmed that recoveries range from 90 to 98 %, and the matrices of the real samples had no significant effect on the efficiency of the method. This indicates that the proposed method is satisfactory and appropriate for quantitative analysis of OCPs in real samples and can be used for routine analysis.
Comparison of the recommended method and other procedures
The analytical features of the proposed DLLME method combined with GCMS for the determination of OCPs in honey were compared with results from other methods reported in the literature for organic solvent volume, extraction time (min), LOD, LOQ, RSD and separation technique. The results are summarized in Table 3. The volume of organic solvent required for this method was lower than that of the other approaches. It was found that the extraction time of the proposed method was much lower than that of the other techniques and the proposed method recorded lower LOD, LOQ and RSD values. Also, the linearity range of method was better. The DLLME method offered advantages such as low cost and no need for specialized instruments. The DLLME method is a rapid, simple, efficient and repeatable method that can be used for the extraction and preconcentration of organochlorine pesticides from honey samples.
Conclusion
In this study, the DLLME-GC/MS method was developed and applied for the determination of OCPs in honey samples. The experimental results indicate that the proposed method shows qualities such as low LOD and LOQ, low cost, low consumption of organic solvent, simplicity, shorter extraction time, better repeatability and high ERs and EFs. In conclusion, the proposed DLLME-GC/MS serves as appropriate tool for analysis of the selected OCPs at trace levels in honey samples.
References
Y. Zhang, H.K. Lee, Determination of ultraviolet filters in water samples by vortex-assisted dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. J. Chromatogr. 1249, 25–31 (2012)
D. Tsipi, M. Triantafyllou, A. Hiskia, Determination of organochlorine pesticide residues in honey, applying solid phase extraction with RP-C18 material†. Analyst 124(4), 473–475 (1999)
M. Fontcuberta et al., Chlorinated organic pesticides in marketed food: Barcelona, 2001–06. Sci. Total Environ. 389(1), 52–57 (2008)
D.Q. Hung, W. Thiemann, Contamination by selected chlorinated pesticides in surface waters in Hanoi, Vietnam. Chemosphere 47(4), 357–367 (2002)
L. Guzzella, F. Pozzoni, G. Giuliano, Herbicide contamination of surficial groundwater in Northern Italy. Environ. Pollut. 142(2), 344–353 (2006)
S. Meijer et al., Air-soil exchange of organochlorine pesticides in agricultural soils. 1. Field measurements using a novel in situ sampling device. Environ. Sci. Technol. 37(7), 1292–1299 (2003)
M. Gonzalez et al., Occurrence and distribution of organochlorine pesticides (OCPs) in tomato (Lycopersicon esculentum) crops from organic production. J. Agric. Food Chem. 51(5), 1353–1359 (2003)
C. Blasco et al., Assessment of pesticide residues in honey samples from Portugal and Spain. J. Agric. Food Chem. 51(27), 8132–8138 (2003)
Ö. Erdoğrul, Levels of selected pesticides in honey samples from Kahramanmaraş, Turkey. Food Control 18(7), 866–871 (2007)
S. Bogdanov, Contaminants of bee products. Apidologie 37, 1–18 (2006)
M. Choi, I.-S. Lee, R.-H. Jung, Rapid determination of organochlorine pesticides in fish using selective pressurized liquid extraction and gas chromatography–mass spectrometry. Food Chem. 205, 1–8 (2016)
Z. Cheng et al., Atmospheric pressure gas chromatography quadrupole-time-of-flight mass spectrometry for simultaneous determination of fifteen organochlorine pesticides in soil and water. J. Chromatogr. A 1435, 115–124 (2016)
J.A. Cai et al., Hollow fiber based liquid phase microextraction for the determination of organochlorine pesticides in ecological textiles by gas chromatography–mass spectrometry. Talanta 146, 375–380 (2016)
F.M. Malhat et al., Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 120, 457–461 (2015)
G. Zheng et al., Multiresidue analysis of 30 organochlorine pesticides in milk and milk powder by gel permeation chromatography-solid phase extraction–gas chromatography–tandem mass spectrometry. J. Dairy Sci. 97(10), 6016–6026 (2014)
X. Shi et al., Simultaneous analysis of polychlorinated biphenyls and organochlorine pesticides in seawater samples by membrane-assisted solvent extraction combined with gas chromatography–electron capture detector and gas chromatography–tandem mass spectrometry. J. Chromatogr. B 972, 58–64 (2014)
M. Kahle et al., Azole fungicides: occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 42(19), 7193–7200 (2008)
M.B. Melwanki, M.-R. Fuh, Partitioned dispersive liquid–liquid microextraction: an approach for polar organic compounds extraction from aqueous samples. J. Chromatogr. 1207(1), 24–28 (2008)
K.B. Borges et al., Simultaneous determination of multibenzodiazepines by HPLC/UV: investigation of liquid–liquid and solid-phase extractions in human plasma. Talanta 78(1), 233–241 (2009)
A.L. Saber, On-line solid phase extraction coupled to capillary LC-ESI-MS for determination of fluoxetine in human blood plasma. Talanta 78(1), 295–299 (2009)
S.R. Rissato et al., Supercritical fluid extraction for pesticide multiresidue analysis in honey: determination by gas chromatography with electron-capture and mass spectrometry detection. J. Chromatogr. 1048(2), 153–159 (2004)
C. Sanchez-Brunete et al., Determination of insecticides in honey by matrix solid-phase dispersion and gas chromatography with nitrogen–phosphorus detection and mass spectrometric confirmation. J. AOAC Int. 85(1), 128–133 (2002)
Y. Wang et al., Determination of triazines in honey by dispersive liquid–liquid microextraction high-performance liquid chromatography. J. Chromatogr. 1217(26), 4241–4246 (2010)
A.A. Barakat et al., Simple and rapid method of analysis for determination of pesticide residues in honey using dispersive solid phase extraction and GC determination. J. Food Agric. Environ. 5(2), 97 (2007)
N. Campillo et al., Solid-phase microextraction and gas chromatography with atomic emission detection for multiresidue determination of pesticides in honey. Anal. Chim. Acta 562(1), 9–15 (2006)
M. Rezaee et al., Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 1116(1), 1–9 (2006)
B. Ebrahimpour, Y. Yamini, M. Rezazadeh, A sensitive emulsification liquid phase microextraction coupled with on-line phase separation followed by HPLC for trace determination of sulfonamides in water samples. Environ. Monit. Assess. 187(1), 1–13 (2015)
A.N. Anthemidis, K.-I.G. Ioannou, On-line sequential injection dispersive liquid–liquid microextraction system for flame atomic absorption spectrometric determination of copper and lead in water samples. Talanta 79(1), 86–91 (2009)
H. Chen, R. Chen, S. Li, Low-density extraction solvent-based solvent terminated dispersive liquid–liquid microextraction combined with gas chromatography-tandem mass spectrometry for the determination of carbamate pesticides in water samples. J. Chromatogr. 1217(8), 1244–1248 (2010)
W. Li et al., Development of on-line spectroscopic determination approach of dispersive liquid–liquid microextraction based on an effective device. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 124, 159–164 (2014)
B. Horstkotte et al., In-syringe magnetic stirring assisted dispersive liquid–liquid micro-extraction with solvent washing for fully automated determination of cationic surfactants. Anal. Methods 6(24), 9601–9609 (2014)
E. Ranjbari, M.R. Hadjmohammadi, Magnetic stirring-assisted dispersive liquid–liquid microextraction followed by high performance liquid chromatography for determination of phthalate esters in drinking and environmental water samples. Talanta 100, 447–453 (2012)
J. Zhou et al., Multiresidue determination of tetracycline antibiotics in propolis by using HPLC-UV detection with ultrasonic-assisted extraction and two-step solid phase extraction. Food Chem. 115(3), 1074–1080 (2009)
M. Alexovič et al., A single-valve sequential injection manifold (SV-SIA) for automation of air-assisted liquid-phase microextraction: stopped flow spectrophotometric determination of chromium (VI). Anal Methods 5(10), 2497–2502 (2013)
P.-S. Chen, W.-Y. Haung, S.-D. Huang, Analysis of triazine herbicides using an up-and-down-shaker-assisted dispersive liquid–liquid microextraction coupled with gas chromatography–mass spectrometry. J. Chromatogr. B 955, 116–123 (2014)
M. Alexovič et al., An automatic, vigorous-injection assisted dispersive liquid–liquid microextraction technique for stopped-flow spectrophotometric detection of boron. Talanta 133, 127–133 (2015)
L. Rusnáková et al., A dispersive liquid–liquid microextraction procedure for determination of boron in water after ultrasound-assisted conversion to tetrafluoroborate. Talanta 85(1), 541–545 (2011)
V.P. Jofré et al., Optimization of ultrasound assisted-emulsification-dispersive liquid–liquid microextraction by experimental design methodologies for the determination of sulfur compounds in wines by gas chromatography–mass spectrometry. Anal. Chim. Acta 683(1), 126–135 (2010)
A. Bidari et al., Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction. Food Chem. 126(4), 1840–1844 (2011)
F. Javanmardi et al., Benzoic and sorbic acid in soft drink, milk, ketchup sauce and bread by dispersive liquid–liquid microextraction coupled with HPLC. Food Addit. Contam. Part B 8(1), 32–39 (2014)
S. Zhang et al., Dispersive liquid–liquid microextraction combined with sweeping micellar electrokinetic chromatography for the determination of some neonicotinoid insecticides in cucumber samples. Food Chem. 133(2), 544–550 (2012)
M.A. Farajzadeh et al., Determination of some synthetic phenolic antioxidants and bisphenol A in honey using dispersive liquid–liquid microextraction followed by gas chromatography–flame ionization detection. Food Anal. Methods 8(8), 2035–2043 (2015)
M.A. Farajzadeh et al., Development and validation of a rapid and sensitive gas chromatographic method for the analysis of some phenolic compounds in vegetable oils. Anal. Methods 6(14), 5314–5321 (2014)
P. Reboredo-Rodríguez et al., Ultrasound-assisted emulsification–microextraction for the determination of phenolic compounds in olive oils. Food Chem. 150, 128–136 (2014)
S. Panseri et al., Occurrence of pesticide residues in Italian honey from different areas in relation to its potential contamination sources. Food Control 38, 150–156 (2014)
Ż. Bargańska, M. Ślebioda, J. Namieśnik, Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control 31(1), 196–201 (2013)
G.P. de Pinho et al., Optimization of the liquid–liquid extraction method and low temperature purification (LLE–LTP) for pesticide residue analysis in honey samples by gas chromatography. Food Control 21(10), 1307–1311 (2010)
C.K. Zacharis et al., Dispersive liquid–liquid microextraction for the determination of organochlorine pesticides residues in honey by gas chromatography–electron capture and ion trap mass spectrometric detection. Food Chem. 134(3), 1665–1672 (2012)
Acknowledgments
The authors would like to thank the ‘Drug Applied Research Center of Tabriz University of Medical Science’ for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavi, MM., Nemati, M., Alizadeh Nabili, A.A. et al. Application of dispersive liquid–liquid microextraction followed by gas chromatography/mass spectrometry as effective tool for trace analysis of organochlorine pesticide residues in honey samples. J IRAN CHEM SOC 13, 2211–2218 (2016). https://doi.org/10.1007/s13738-016-0939-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0939-2