Abstract
In this study, the effect of sludge retention time (SRT) on biomass production and nutrient removal was determined by constant hydraulic retention time (HRT) with mixed microalgae culture. The SRTs of 2, 3, 6, 12, and 24 days with constant 24 h HRT were studied in microalgae membrane photobioreactor (msMpBR) by using hollow fiber (HF) membranes with a pore diameter of 0.45 μm. According to the results, the best removal was achieved within 3 days of SRT. Chlorophyll-a/mixed liquor suspended solid (MLSS) ratios were found to be 0.033. Total nitrogen (TN) and phosphate phosphorus (PO4–P) removal rates were found to be 5.55 mg N/L day−1, and 0.4 mg PO4–P/L day−1, respectively. The volumetric microalgae production was found to be 0.118 g/L day−1. Also, Chaetophora sp. and Navicula sp. cultures were found to be dominant in steady state. The percentage of lipid and protein in dry biomass was obtained to be 8.94% and 30.34%, respectively. It is advisable to use algal membrane photobioreactor, and mixed microalgae cultures instead of specific microalgae cultures, which could be readily affected by seasonal changes and outdoor conditions in wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A substantial interest is growing in the cultivation of microalgae as a source of biofuel production, considering their relatively high lipid content, fast growth rates, use of alternative water sources, and growth on non-arable land. The production of microalgae as a source of chemical energy has received a substantial scholarly attention, primarily due to fast growth rates and relatively high lipid content of microalgae biomass product in comparison with terrestrial crops [1]. Microalgae are considered sustainable renewable producers of some value-added bioactive macro-molecules that have the potential for commercial production of essential oils, proteins, enzymes, and pigments as well as feed concentrate for animals/fish etc. and for third-generation biofuel energy. Use of wastewater effluent as a pond medium to grow microalgae biomass has been shown not only to significantly reduce the need for chemical fertilizers and associated life cycle burdens but also to reduce the use of fresh water during algae cultivation. Utilizing waste nutrients and wastewater resources for algae cultivation alleviate economic constraints on large-scale algae cultivation [2]. Algae present several advantages over other types of biomass. Algae are the fastest growing photosynthetic organisms. Algae can efficiently remove carbon dioxide and synthesize polysaccharides or oil that can be used for biofuel production. Generally, algal carbohydrates can be used for bioethanol fermentation after relatively easy scarification due to the absence of lignin. Oil can be trans-esterified into biodiesel. In addition, algal biomass can be directly used for heat and power generation using anaerobic digestion and pyrolysis [3].
Since the amount of nutrients in treated domestic or industrial wastes is above the extraction capacity of the receiving medium, many problems arise, primarily eutrophication, which disrupts the beneficial use of the receiving medium [4]. Various advanced treatment methods are available to reduce the quality of these wastewaters to the receiving environment. In almost all of these methods, even if the discharge quality can be achieved, tertiary products are formed which require disposal such as dewatered sludge or concentrated waste [5, 6]. However, thanks to new treatment technologies within the scope of sustainable waste management, it is possible to prevent waste formation and carry out advanced treatment. One of the most foremost new treatment technologies is using microalgae. Microalgae advanced treatment also enables biomass to be formed, which can be used as a raw material in chemistry, pharmacological, and food industry. In addition, recovering the biomass-derived oil creates an alternative energy source to fossil fuel [7,8,9].
In biomass production experiments, it is known that feeding waters differ in terms of nutrients and trace elements such as BG11, ZM, and F/2 [10,11,12]. In addition, synthetic and real wastewater was also worked, and removal efficiency was examined. In most of these studies, optimization studies were carried out by examining the basic parameters such as pH, temperature, light intensity, light/dark ratio, CO2 feeding status, and nutrient quality using specific microalgae culture. As a result of the findings, potential utility rates such as oil content and protein ratio of the biomass formed and nutrient removal efficiencies were determined at the same time [13, 14]. In these studies, conventional microalgae production methods have been used, but microalgae were not found to have good sedimentation potential as activated sludge microorganisms. High mixed liquor suspended solid (MLSS) concentrations could not be reached because of the microalgae escapes in classical reactors and the effluent water quality was not reached at the desired level [15, 16]. Solid-liquid separation membranes were used both to prevent MLSS escape and to increase the quality of the effluent. In these studies, the reactors are usually operated by batch and rarely continuously. Most of these studies were carried out in cultivation studies and no sludge was wasted [17,18,19]. In addition to these findings, several researchers into sludge retention time (SRT) studies reported that they achieved good volumetric microalgae production and nutrient removal efficiencies. However, specific microalgae culture was used in these studies [17, 20, 21].
Currently, the open raceway design is the common choice for low-cost algal cultivation. Driven by paddlewheels to maintain the cultures in suspension, these raceways are often sparged with CEA (CO2-enriched air) to provide the carbon needs to the cultures. To avoid light drop-off in such raceways, the culture depth has to be shallow (< 0.4 m) and the cell density has to be low (< 0.8 g /L); both these confines have negative impacts on the overall process. Shallow depths translate to larger footprint and surface areas resulting in prohibitive water loss by evaporation. Shallow depths also limit the bubble detention time of the sparged CEA, resulting in poor transfer of CO2 to the culture and, consequently, low biomass productivity and energy yield. In addition, low biomass densities translate to inefficient harvesting in downstream processing, and higher overall costs. Further, open raceways are susceptible to contamination and predation by invaders. The current pathway for algal biomass to biofuel is limited also by the energy extraction processes that involve drying of the harvested wet biomass, cell disruption, and extraction of its lipid content for further processing and refining to yield biofuel [22]. As a result, the need to maximize energy generation efficiency has remained a challenge under three main areas: (1) microalgae growth, (2) harvesting, and (3) energy generation when microalgae are used for the generation of energy [23].
For the maximizing of microalgae growth, membrane photobioreactor (MPBR) may be a useful technology for concentrated microalgae cultivation, particularly, with diluted wastewater such as secondary effluent. In this study, optimum SRT was experimented in synthetic wastewater of secondary wastewater effluent, by which mixed microalgae cultures are fed. When stable conditions were reached in the system where HF membranes were used, dominant microalgae were determined in mixed culture. Microalgae production efficiencies and nutrient removal efficiencies were determined. Also, the amount of lipid and protein of obtained biomass was examined.
Material and Methods
Laboratory Scale Membrane Photobioreactor
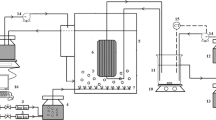
In this study, msMpBR was used with an active volume of 5.5 L (Plexiglas material, transparent, 7.1-cm radius, and 49 cm high). In the reactor, temperature, pH, oxidation reduction potential (ORP), and dissolved oxygen (DO) parameters were measured and recorded by a programmable logic controller (PLC). Schematic diagram of msMpBR is shown in Fig.1.
The reactor was set up in 10 m2 fully closed daylight and illuminated with white fluorescent lamps providing 6000-lx lights. The automatic timer was used for the light/dark time to be 12/12 h. The reactor was aerated with constant compressed air for membrane fouling control and mixing of MLSS. The solid/liquid separation process at the reactor was done with hollow fiber membranes made from polyvinylidene fluoride (PVDF) material with a nominal pore size of 0.45 μm and a maximum membrane flux of 20 L/m2 h−1 supplied by National Membrane Technology Research Center (MEM-TEK) in Istanbul, Turkey.
Microalgae Inoculation and Feeding Water Characterization
Mixed microalgae culture inoculated with the reactor were collected from the effluent of Kayseri Organized Industrial Zone Wastewater Treatment Plant, Turkey. Then, they were drained and removed from their rough particles. Samples were taken from the reactor every week to determine the dominant species and examined by a microscope.
As feeding water to the reactor, the simulated sewage-treated effluent was prepared with glucose (C6H12O6 H2O), potassium hydrogen phthalate (KH2PO4), and ammonium chloride (NH4Cl) dissolved in tap water. The average concentrations of the simulated wastewater are as follows: COD, 32.89 mg/L (± 2.3); TN, 18.35 mg/L (± 0.30) NH4–N, 18.01 mg/L (± 0.38); PO4–P, 8.81 mg/L (± 0.17).
Analytical Methods
In the samples taken from the reactor, daily MLSS analysis was performed according to SM 2540-D and mixed liquor volatile suspended solid (MLVSS) analyses were performed according to SM 2540-E [24]. Chlorophyll-a was analyzed with 10 ml of the sample which was centrifuged at 3000 rpm for 3 min and the supernatant was discarded. 5 ml of 90% methanol was added to the remaining precipitate and stirred for 5 min in a water bath of 65 °C. It was centrifuged again and the supernatant water was read at 665 and 650 nm versus the pure methanol solution and the result is calculated according to the equation which is (16.5 × A665) − (8.3 × A650) to Becker [25]. Lipid analysis in dry biomass was carried out according to the modified Bligh and Dyer method [26]. The content of protein in the dried biomass sample was obtained by measuring the nitrogen content using an element analyzer (Thermo Flash 2000) and multiplying by the conversion factor of 6.25 [10]. In addition, chemical oxygen demand (COD) was determined according to SM 5220-D and phosphate phosphorus analysis according to SM 4500-P.D. [24]. Total nitrogen analysis was performed with the LCK138 (Hach-Lange) kit and NH4 analysis with the DR2800 spectrophotometer with a test kit Merck, Germany.
Operational Conditions
We worked at SRTs of 2, 3, 6, 12, and 24 days respectively with HRT being set constant at 24 h in the continuous feed system. The reactors were operated continuously for 12, 37, 33, 72, and 99 days at the indicated SRTs, respectively. In order to achieve these SRTs, 2750 mL, 1833 mL, 915 mL, 458 mL, and 229 mL of excess sludge per day were wasted from the reactor, respectively. Re-inoculation was performed at the beginning of each study to determine which type of microalgae became dominant. A time-controlled peristaltic pump was used for homogenous sludge wasting over 24 h. Also, NaOH was added to prevent pH drop.
The peristaltic pump connected to the membrane module to provide effluent from the reactor was operated at intervals of 2–6-min vacuum, 2-min rest, 1–3-min backwash, and 2-min rest to get the desired outlet at the specified SRTs. The temperature of the reactor was maintained at 23 ± 2 °C by means of a heater. In addition, the pH of the reactor was around 6.4–8.2. Dissolved oxygen concentration in the reactor was at 5–6 mg/L and ORP value ranged from 110 to 140 mV.
Results and Discussion
Microalgae Growth
The increase or decrease of biomass in the reactor was observed with MLSS concentration, but the presence of microalgae was determined by chlorophyll-a assay. The concentrations of MLSS and chlorophyll-a for each SRT are shown in Fig. 2. In addition, when stable conditions are reached, MLSS, MLVSS, chlorophyll-a, and predominant microalgae in the reactor are given in Table 1. According to this, 800 mg/L MLSS concentration at an SRT of 2 days has been started and day-to-day decline has been observed. As the amount of sludge recovered in the reactor is less than that in the slurry, a decrease in the concentrations of MLSS and chlorophyll-a is observed. In the literature, it was reported that Chlorella sp. culture and 4 days of HRT and 4 days of SRT study were performed and the MLSS concentration was found to be 163 mg/L. The same researcher group reported that they had reached 809 mg/L (MLSS) in the study with 1 day of HRT and 10 days of SRT using the membrane [27]. On the other hand, a different researcher group conducted a treatment study in the classical system with mixed culture and it was reported that the MLSS concentration of 485 mg/L was reached by using algal-bacterial mixed culture [28]. At SRTs of 3, 6, 12, and 24 days, when stable conditions are reached, the concentrations of MLSS have been measured to be 354, 352, 610, and 1025 mg/L, respectively. The ratio of MLVSS/MLSS has been calculated as 0.798, 0.823, 0.801, and 0.860 respectively.
The chlorophyll-a concentrations, indicative of the life status of photosynthetic organisms, are 11.54 mg/L, 10.70 mg/L, 8.66 mg/L, and 8.42 mg/L at SRTs of 3, 6, 12, and 24 days, respectively. According to the ratio of chlorophyll-a concentration to the concentration of MLSS in the reactor, this ratio is the highest within 3 days of work. According to these results, as the SRT increases, the MLSS concentrations increase in the reactors; however, an increase in chlorophyll-a concentration has not been observed.
When the volumetric microalgae production efficiencies are examined, it has been specified that the best production is 0.118 g/L day−1 at an SRT of 3 days. A comparison of the obtained results to the literature is given in Table 2. For systems using solid-liquid separation membranes according to the literature, the volumetric microalgae production rate varies between 0.043 and 2.53 g/L day−1 [19, 30, 32]. When looking at the systems that do not use membranes, it is in the range of 0.010–0.029 g/L day−1 [21, 34]. Accordingly, the use of membranes for solid-liquid separation in the reactor provides a significant contribution to the production of volumetric microalgae.
Microalgae Presence in Mixed Culture
No investigation was done at the SRT of 2 days because there was no reproduction of microbial community in the reactor. At the SRT of 3 days, it was observed that over 90% of Amphora sp. predominated in the first inoculation. After 16 days, Amphora sp. was reduced to the levels of 10%, but Chaetophora sp. was found to be dominant. About 50% of Chaetophora sp. and about 50% of Navicula sp. were detected on day 26. In the last days when stable water quality was maintained, the same microalgae observed on day 26 were detected.
At the SRTs of 6 days, it was observed that over 99% of Amphora sp. predominated in the first inoculation. On the 15th day of operation, it was again observed that Amphora sp. maintained its dominance, but in the meanwhile, Chaetophora sp. began to appear. On the 20th day, Amphora sp. still retained its dominance while Chaetophora sp. reached about 10–15%. When stable water quality was maintained, about 60–70% Amphora sp. and about 30–40% Chaetophora sp. were observed.
At SRTs of 12 days, it was observed that more than 99% of the Amphora sp. dominated the inoculation. In the following days, the dominance of Amphora continued while the presence of Chaetophora sp. increased day by day. The microalgae community was predominantly around 90% of Chaetophora sp. and about 5% of Amphora sp.
At SRTs of 24 days, it was observed that more than 99% of the Amphora sp. dominated the inoculation. In the following days, the dominance of Amphora sp. continued while the presence of Chaetophora sp. increased day by day. The stable conditions of approximately 90% of Chaetophora sp. and about 5% of Amphora sp. were observed.
Previous studies found that Desmodesmus sp. was dominant in the mixed culture [35]. It was also reported that Botryococcus braunii predominated in the mixed culture [20]. According to our current study, Chaetophora sp. becomes dominant as the SRT is increased. This finding indicates that mixed culture is favorable over specific cultures in microalgae studies for being free of energy, isolation, and purification costs.
In addition, in the experiments done in real scale in the external environment, it could be not easy to keep the isolated species over the year due to seasonal changes in temperature, which makes the mixed culture to have wider applications in real scale.
Nutrient Removal Efficiencies
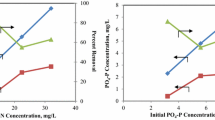
TN removal efficiencies at 3, 6, 12, and 24 days of SRTs are 5.5 mg/L day−1, 4.02 mg/L day−1, 5.05 mg/L day−1, and 4.88 mg/L day−1 (Table 2). In the literature, for systems without solid-liquid separation membranes, synthetic wastewater was treated with Chlorella vulgaris (specifies culture) and TN removal efficiency was found to be 6.19 and SRT was 2 days; also, no sludge was removed [18]. Also in another study, TN removal rate was found to be 4.13 with HBS for 1 day and 10 days for treatment with C. vulgaris culture while treating real treated wastewater [21]. On the other hand, synthetic wastewater was treated with the same culture (C. vulgaris) and 6.1 values were obtained [33]. However, in the treatment with conventional cultivation without the membrane, the anaerobic digester output was purified with Desmodesmus sp. culture and TN removal rate was found to be 4.54 [29]. However, highlighted in our study, the best yield was obtained at the 3 days of SRT, but this value was obtained with mixed culture instead of uniform culture.
The removal efficiency of PO4–P was found to be 0.4 mg/L day−1, 0.29 mg/L day−1, 1.36 mg/L day−1, and 1.61 mg/L day−1 at SRTs of 3, 6, 12, and 24 days, respectively. These results are higher than the values of 0.08 and 0.29 obtained in the literature studies in which membranes are not used in the liquid-liquid separation [21, 29]. The value of 0.35–1.72 mg/L day−1 is reported to be obtained in the study where membranes are used [18, 30]. Moreover, when the reactor pH is taken into consideration, it is clear that the phosphorus removal is due to the assimilation of the microalgae cell as well as the pH change [18].
When the basic components of the microalgae cell are examined, the theoretical component of the C. vulgaris cell is calculated as CO0.48H1.83N0.11P0.01 by Chisti [36]. According to this formula, the cell has 6.59% N and 1.33% P and the N/P ratio is 4.97. In the same manner, they reported the formula of the C. vulgaris cell as CO1.176H0.459N0.148S0.06P0.01 in the study and that it has 13.16% N and 1.97% P, and the N/P ratio was 6.68 [37]. As for the values obtained from the removal efficiencies, they reported the ratios of Gao et al. [21] and Ji et al. [29] to be N/P 7.78 and 15.66 respectively. For systems using membranes, Xu et al. [30] and Marbelia et al. [31] reported the values N/P 5.28 and between 15.5 and 22.8 respectively. In our SRTs of 3, 6, 12, and 24 day studies, the N/P ratios are 13.88, 13.86, 3.71, and 3.03, respectively, which are acceptable values.
Lipid and Protein Ratio of Dry Biomass
Lipid and protein ratios in dry biomass obtained at different SRTs are measured and compared to the literature in Table 3. When stable conditions were reached, the rate of lipid in the dry biomass obtained from SRT of 3 days was measured to be 8.90%. This value is lower than the value of 27.5–36.6 obtained in the study using pure culture of Scenedesmus dimorphus in BG-11 medium [10]. However, it is quite close to the ratio of 10–11% obtained by Ruiz-Marin et al. [40] using S. obliquus culture and synthetic artificial wastewater. On the other hand, in the study using S. platensis culture in the Zarrouk environment, 7.8% of fat was obtained and the same researchers succeeded in obtaining oil twice as much by changing the feed water [11].
Based on the protein ratios, 34.20% of the protein was obtained from dry biomass. It is reported that 45.31% of protein was achieved in the studies where f/2 in vitro and Isochrysis aff. galbana microalgae culture was used [41]. But in their study, Praveen and Loh [38] stated that pure C. vulgaris contained 8.95% protein in synthetic tertiary treatment water. When the obtained and literature values are examined together, it is obvious that the ratio of lipid and protein varies according to microalgae, quality of feed medium, and ambient conditions.
Conclusion
This study investigated the effect of SRT on algal growth and nutrient removal from the simulated secondary wastewater effluent by using an algal photo membrane reactor. The different SRTs have been tested to investigate the nutrient removal, biomass production rate, and algal concentration. The results demonstrate that control of SRT is important in maximizing algal productivity and nutrient removal from wastewaters. Under experiment conditions, MLSS in effluent is prevented by membranes, and higher MLSS concentrations have been achieved in the reactor compared to conventional systems. In addition, the volumetric microalgae production and nutrient removal rates obtained by mixed microalgae culture are very close to the specific culture. This study demonstrates that using mixed microalgae culture and membrane treatment can offer a new approach to algae production for the renewable energy, and nutrient removal for the advanced wastewater treatment instead of the conventional processes with specific culture which poses difficulties in real-scale application. Furthermore, msMpBR with the membrane is also a useful reactor providing high microalgae concentrations in the reactor.
References
Shahnazari M, Bahri PA, Parlevliet D, Minakshi M, Moheimani NR (2017) Sustainable conversion of light to algal biomass and electricity: a net energy return analysis. Energy 131:218–229. https://doi.org/10.1016/j.energy.2017.04.162
Khanzada ZT, Övez S (2017) Microalgae as a sustainable biological system for improving leachate quality. Energy 140:757–765
Lee OK, Lee EY (2016) Sustainable production of bioethanol from renewable brown algae biomass. Biomass Bioenergy 92:70–75
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30(3):673–690
De-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101(6):1611–1627
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev 19:360–369
Rawat I, Kumar RR, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88(10):3411–3424
Chen G, Zhao L, Qi Y (2015) Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: a critical review. Appl Energy 137:282–291
Molinuevo-Salces B, Mahdy A, Ballesteros M, González-Fernández C (2016) From piggery wastewater nutrients to biogas: microalgae biomass revalorization through anaerobic digestion. Renew Energy 96:1103–1110
Wang L, Li Y, Sommerfeld M, Hu Q (2013) A flexible culture process for production of the green microalga Scenedesmus dimorphus rich in protein, carbohydrate or lipid. Bioresour Technol 129:289–295
Chang Y, Wu Z, Bian L, Feng D, Leung DY (2013) Cultivation of Spirulina platensis for biomass production and nutrient removal from synthetic human urine. Appl Energy 102:427–431
Ra CH, Kang C-H, Kim NK, Lee C-G, Kim S-K (2015) Cultivation of four microalgae for biomass and oil production using a two-stage culture strategy with salt stress. Renew Energy 80:117–122
Jacob-Lopes E, Scoparo CHG, Lacerda LMCF, Franco TT (2009) Effect of light cycles (night/day) on CO 2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process Process Intensif 48(1):306–310
Koller M, Salerno A, Tuffner P, Koinigg M, Böchzelt H, Schober S, Pieber S, Schnitzer H, Mittelbach M, Braunegg G (2012) Characteristics and potential of micro algal cultivation strategies: a review. J Clean Prod 37:377–388
Feng Y, Li C, Zhang D (2011) Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour Technol 102(1):101–105
Ruiz-Martínez A, Serralta J, Romero I, Seco A, Ferrer J (2015) Effect of intracellular P content on phosphate removal in Scenedesmus sp. experimental study and kinetic expression. Bioresour Technol 175:325–332
Singh G, Thomas PB (2012) Nutrient removal from membrane bioreactor permeate using microalgae and in a microalgae membrane photoreactor. Bioresour Technol 117:80–85
Gao F, Yang Z-H, Li C, Zeng G-M, Ma D-H, Zhou L (2015) A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour Technol 179:8–12
Gao F, Li C, Yang Z-H, Zeng G-M, Feng L-J, J-z L, Liu M, H-w C (2016) Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol Eng 92:55–61
Honda R, Boonnorat J, Chiemchaisri C, Chiemchaisri W, Yamamoto K (2012) Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour Technol 125:59–64
Gao F, Yang Z-H, Li C, Wang Y-j, W-h J, Deng Y-b (2014) Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour Technol 167:441–446
Selvaratnam T, Henkanatte-Gedera S, Muppaneni T, Nirmalakhandan N, Deng S, Lammers P (2016) Maximizing recovery of energy and nutrients from urban wastewaters. Energy 104:16–23
Naraharisetti PK, Das P, Sharratt PN (2017) Critical factors in energy generation from microalgae. Energy 120:138–152
APHA/AWWA/WEF (2012) Standard methods for the examination of water and wastewater. ISBN 9780875532356
Becker EW (1994) Microalgae: biotechnology and microbiology, vol 10. Cambridge University Press, Cambridge
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Tang T, Hu Z (2016) A comparison of algal productivity and nutrient removal capacity between algal CSTR and algal MBR at the same light level under practical and optimal conditions. Ecol Eng 93:66–72
Kang D, Kim K, Jang Y, Moon H, Ju D, Jahng D (2018) Nutrient removal and community structure of wastewater-borne algal-bacterial consortia grown in raw wastewater with various wavelengths of light. Int Biodeterior Biodegrad 126:10–20
Ji F, Liu Y, Hao R, Li G, Zhou Y, Dong R (2014) Biomass production and nutrients removal by a new microalgae strain Desmodesmus sp. in anaerobic digestion wastewater. Bioresour Technol 161:200–207
Xu M, Li P, Tang T, Hu Z (2015) Roles of SRT and HRT of an algal membrane bioreactor system with a tanks-in-series configuration for secondary wastewater effluent polishing. Ecol Eng 85:257–264
Marbelia L, Bilad MR, Passaris I, Discart V, Vandamme D, Beuckels A, Muylaert K, Vankelecom IF (2014) Membrane photobioreactors for integrated microalgae cultivation and nutrient remediation of membrane bioreactors effluent. Bioresour Technol 163:228–235
Choi H (2015) Intensified production of microalgae and removal of nutrient using a microalgae membrane bioreactor (MMBR). Appl Biochem Biotechnol 175(4):2195–2205
Gao F, Peng Y-Y, Li C, Cui W, Yang Z-H, Zeng G-M (2018) Coupled nutrient removal from secondary effluent and algal biomass production in membrane photobioreactor (MPBR): effect of HRT and long-term operation. Chem Eng J 335:169–175
Ji Y, Hu W, Li X, Ma G, Song M, Pei H (2014) Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with diluted monosodium glutamate wastewater. Bioresour Technol 152:471–476
Komolafe O, Orta SBV, Monje-Ramirez I, Noguez IY, Harvey AP, Ledesma MTO (2014) Biodiesel production from indigenous microalgae grown in wastewater. Bioresour Technol 154:297–304
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Hadj-Romdhane F, Jaouen P, Pruvost J, Grizeau D, Van Vooren G, Bourseau P (2012) Development and validation of a minimal growth medium for recycling Chlorella vulgaris culture. Bioresour Technol 123:366–374
Praveen P, Loh K-C (2016) Nitrogen and phosphorus removal from tertiary wastewater in an osmotic membrane photobioreactor. Bioresour Technol 206:180–187
Olsson J, Feng XM, Ascue J, Gentili FG, Shabiimam M, Nehrenheim E, Thorin E (2014) Co-digestion of cultivated microalgae and sewage sludge from municipal waste water treatment. Bioresour Technol 171:203–210
Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T (2010) Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol 101(1):58–64
Valenzuela-Espinoza E, Millán-Núñez R, Núñez-Cebrero F (2002) Protein, carbohydrate, lipid and chlorophyll a content in Isochrysis aff. galbana (clone T-Iso) cultured with a low cost alternative to the f/2 medium. Aquac Eng 25(4):207–216
Lam MK, Yusoff MI, Uemura Y, Lim JW, Khoo CG, Lee KT, Ong HC (2017) Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: growth condition and kinetic studies. Renew Energy 103:197–207
Acknowledgements
I would like to express my special thanks of gratitude to Aksaray University (BAP Project No: 2015-043), Kayseri Organized Industrial Zone Management, and Dr. Murat KAYA of Aksaray University, Biotechnology and Molecular Biology Department because of their contributions and supports in defining the species in mixed cultures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solmaz, A., Işik, M. Effect of Sludge Retention Time on Biomass Production and Nutrient Removal at an Algal Membrane Photobioreactor. Bioenerg. Res. 12, 197–204 (2019). https://doi.org/10.1007/s12155-019-9961-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-9961-4