Abstract
In present research, a microalgae membrane bioreactor (MMBR) was constructed by combining the optical panel photobioreactor (OPPBR) and membrane bioreactor (MBR). Experiments were conducted in MMBR pilot-plant configuration for 150 days. A biomass productivity of 2.53 g/l/day with light transmittance of 94 % at a 300-mm depth in the OPPBR was achieved. The total reduction of chemical oxygen demand (COD) and biochemical oxygen demand (BOD) in the MMBR were found to be 96.99 and 97.09 %, respectively. Additionally, the removal of total nitrogen (TN), NH4-N, NO3-N, total phosphorus (TP), and PO4-P were 96.38, 99.80, 97.62, 92.75, and 90.84 % in MMBR, respectively. These results indicated that the MMBR process was highly effective for COD, BOD, and nutrient removal when compared to the OPPBR or MBR process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae cultures offer an interesting alternative for wastewater treatment, because they provide a biotreatment coupled with the production of potentially valuable biomass with implications for the reduction of greenhouse gas emission [1]. However, the nutrient removal using microalgae was lower as compared to the other available process. Especially, biochemical oxygen demand (BOD), chemical oxygen demand (COD), total phosphorus (TP), and PO4-P removal was found to be lower than the removal of TN and NH4-N. Many researchers reported that the wastewater treatment utilizing the algal-bacterial system was capable for ∼80 % removal of COD, 68–75 % BOD, ∼70 % TP, ∼70 % PO4-P, ∼95 % NH4-N, and ∼90 % TN [2–7].
Based on the aforementioned reasons, in this study, we developed a new process by combining microalgae photobioreactor with membrane bioreactor (MMBR) process for cultivation of microalgae and to enhance the nutrient removal rate in the wastewater. The MMBR process could reduce energy cost, as it does not require the addition of chemicals and is simplified without numerous modes of operations and internal recycles will be preferred. In present research, a newly developed optical panel photobioreactor (OPPBR) was employed for cultivation and nutrient removal of microalgae. The MMBR was performed in two step processes: in the first step, OPPBR was used for cultivation of microalgae and primary nutrient removal, and in the second step, MBR was used for nutrient removal. A closed photobioreactor (PBR) with OP system was used in an attempt to enhance the light utilization throughout the reactor in the mass cultivation of microalgae and nutrient removal from domestic wastewater. In addition to the above, the aim of present study was to quantitatively evaluate the effect of nutrient removal on the growth of a Chlorella vulgaris (C. vulgaris) biomass with simultaneous nutrient removal in the MMBR process.
Materials and Methods
Microalgae Cultures and Medium
C. vulgaris microalga is chosen for the mass cultivation of biomass and nutrient removal in wastewater. C. vulgaris (FC-16) cells were cultivated in Jaworski’s medium prepared by using deionized water under LED lamps at ambient temperature. Jaworski’s medium comprises 4.0 g of Ca(NO3)2H2O, 2.48 g of KH2PO4, 10.0 g of MgSO4·7H2O, 3.18 g of NaHCO3, 0.45 g of EDTAFeNa, 0.45 g of EDTANa2, 0.496 g of H3BO3, 0.278 g of MnCl2·4H2O, 0.20 g of (NH4)6Mo7O24·4H2O, 0.008 g of cyanocobalamin, 0.008 g of thiamine HCl, 0.008 g of biotin, 16.0 g of NaNO3, and 7.2 g of Na2HPO4·12H2O in 200 ml deionized water. The cultures are incubated at a constant temperature (23 ± 1 °C) for 15 days. The C. vulgaris (FC-16) cells are round in shape and 3–8 μm in diameter. Culturing is performed at a neutral pH (7.2 ± 0.3) and with a dark and light cycles maintained for 8 and 16 h, respectively.
Characterization of Wastewater
The municipal wastewater was obtained from preliminary sedimentation of a sewage plant in Gangneung (∼1852.56 ha), South Korea. The characteristics of the municipal wastewater used in present investigation were shown in Table 1. The wastewater employed in this study was considered to be favorable for possible nutrient removal along with the enhanced growth of microalgae. This wastewater had an excess ratio of COD/N/P (100/20/2), which is recommended for nutrient removal in activated sludge plants. The BOD5-to-PO4 ratio (19.49) is found to be reasonably high. However, the PO4-to-TP (0.88) and BOD5-to-TP ratios (17.28) are found to be at the lower range for municipal wastewater.
Experimental Design
Optical Panel Photobioreactor Construction and Operation Conditions
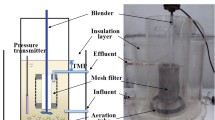
A schematic diagram of the OPPBR is shown in Fig. 1a. The OPPBR was operated at 40 l total volume. A batch culture was prepared using an initial cell concentration of 1.12 ± 0.05 g/l. The experiments were conducted at neutral pH (7.2 ± 0.3) and with dark and light cycles of 8 and 16 h, respectively. The experiment was conducted at 25 ± 2 °C using LEDs for a period of 3 days. The OPPBRs were aerated continuously at a rate of 0.5 l/min. An equivalent CO2 aeration rate of 0.02 vvm was used for cultivation. The OPPBR was designed in such a way that the LED light source (22 LEDs, bar type panel) was placed in the middle of the OPPBR (Fig. 1c). An optical panel, v-grooved, was inserted underneath of this photoreactor. The incident light was uniformly distributed across both sides of the OP in the reactor, providing greater functionality. The LED light source was employed because it was efficient and provided light of the required wavelength to be selective for microalgal growth, i.e., 430 to 670 nm. Moreover, the light intensity, which represents the amount of light used for photosynthesis, was found to be between 270–310 μE/m2/s.
A high biomass concentration in the PBR caused pronounced shading effects, which significantly reduced the intensity of light with depth in the photobioreactor and limited microalgal growth during cultivation [8, 9]. Therefore, an effective utilization of light energy was restricted in these operations. An OP-based photobioreactor with a uniform light distribution within the reactor might enhance the efficiency of the reactor in the cultivation of microalgae. The characteristics of the OP are listed in Table 2. The OP dimensions were 210 mm (L) × 290 mm (H) × 6 mm (W) and were constructed from a transparent panel of pure poly(methyl methacrylate) (PMMA), as shown in Fig. 1b. This material has good transparency, while its absorption of light in the visible region is almost negligible.
A v-cut OP was designed in this study (Fig. 1b). This was employed to quantitatively evaluate and assess the effects of the illumination area and the OP arrangement on cell growth and biomass productivity. In v-cut technology, the light was guided to the v grooves that have x-, y-, and z directional grooves, i.e., enlarged horizontal and vertical grooves. The vertical v grooves are widely spaced when they are near to the light source and narrow with distance away from the light source. The enlarged horizontal v grooves were arranged in straight lines along the x direction from the end edge of the OP and were maximally enlarged portions located on the other edge of the OP. In addition, the v cut was varied to give a uniform distribution of light in the PBR [3].
Membrane Bioreactor
The membrane bioreactor (MBR) was operated using commercial polymeric membrane of pore size 0.2 μm (PES, Millipore). The operating conditions of the MBR system are summarized in Table 3. A hydrophilic commercial membrane of pore size 0.2 μm (PES, Millipore) was submerged in the tank from wastewater and was withdrawn at a constant flux of 8 l/m2/h. Air was fed to the MMBR reactor at a constant rate of 4 l/min. The membranes with an effective area of approximately 0.2 m2 were used in flat sheet mode of operation. The total volume of MBR reactor was 40 l.
Microalgae Membrane Bioreactor
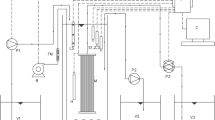
The MMBR is combined OPPBR and MBR. By the MMBR system, OPPBR was installed in front of MBR. When the MBR installed in front of OPPBR, microalgae could not grow well, because of the lack of nutrient in the wastewater. The schematic diagram of MMBR is depicted in Fig. 2. The HRT of the MMBR was set for 3.4 days (72 h for OPPBR and 9 h for MBR) throughout. This combined MMBR system was operated discontinuously for 150 days. The microalgae concentration in the OPPBR was monitored daily. All experiments were conducted at room temperature (25 ± 2 °C).
Analytical Methods
A direct microscopic count was performed on the microalgal sample suspension using a Brightline Hemacytometer (BOECO, Hamburg, Germany) and a Nikon Eclipse TS100 inverted metallurgical microscope (Nikon Corporation, Tokyo, Japan). Biomass growth was calculated from the microalgal dry weight produced per liter (g/l). The dry cell weight of the microalgal biomass was determined using the following procedure: 50-ml samples were removed once every day and were then centrifuged at 648 g for 15 min. The centrifuged sample was washed twice with distilled water and was dried at 105 °C for 16 h. The biomass productivity, C B, is defined by using Eq. (1).
where C b and C b0 were the biomass concentrations at time t and at starting time t 0, respectively.
The specific growth rate (μ/h) was calculated using Eq. (2).
where C 1 and C 2 were the biomasses in gram per liter at time t 1 and time t 2, respectively. In this study, we used biomass (g/l) to quantify C. vulgaris (FC-16) in culture.
Uniformity was calculated using Eq. (3)
where L min was the minimum light intensity and L max was the maximum light intensity. All samplings and measurements were conducted at the same time every day.
The PO4-P and NH3-N were analyzed by using an ion chromatograph (Dionex ISP 2000, Dinox, Sunnyvale, California), according to DIN standards (German Institute for Standardization). The total phosphorus (TP), total nitrogen (TN), biological oxygen demand (BOD), and chemical oxygen demand (COD) were obtained photometrically by using a spectrophotometer (UV-vis 1240, Tecator Co., Germany).
Results and Discussion
Diffuse Light Rate in the OPPBR
In general, light is one of the most vital factors that influences the growth and productivity of microalgae [9, 10]. The uniformity was defined in Eq. 3; hence, the luminance uniformity (%) was obtained as a function of the varied depth level for OP. It was found that the light transmittance was 94 % at 300-mm depth in the OPBR. The OP radiates light with adequate uniformity, prevailing from the top to the bottom of the PBR. The results clearly demonstrated that an enhanced light intensity was observed even deep inside the PBR using an OP. Moreover, the performance of PBR containing OP was better compared to the PBR without OP. The OP of PBR increases the illumination surface-to-volume ratio as well; providing efficient utilization of light radiation for biomass production. Moreover, the use of OP provides an equal amount of light energy passed through the reactor and the irradiance profile is redistributed due to diffuse light from the OP [3, 11]. The redistributed irradiance profile results in higher photosynthetic efficiency of microalgae and leads to effective light utilization. Consequently, the photobioreactor with OP not only increases the illumination area for cultivation, but also provides conditions for effective utilization of light energy to increase the growth rate of microalgae when compared to OP unused PBR.
Biomass Growth Rate in the OPPBR
The biomass productivity observed in this study was 2.53 g/l/day. Various researchers reported good biomass productivity. In an 11 l undular row turbular photobioreactor for Arthrospira platensis, the productivity was reported about 2.7 g/l/day [12]. However, many reports still show low biomass productivity. Hsieh and Wu [13] reported the biomass productivity 0.340 g/l/day in a transparent rectangular chamber PBR. Furthermore, about 0.27 g/l/day was obtained in 440-l outdoor flat-plate photobioreactor, which was used for cultivation of Nannochloropsis [14]. Garcia-Malea Lopez et al. [15] reported a 55-l bubble column photobioreactor (for the outdoor cultivation of Haematococcus pluvialis); the biomass productivity was obtained 0.06 g/l/day. It should be noted that aside from volumetric productivity (productivity per unit of reactor volume per unit of time) algal biomass productivity can be evaluated in photobioreactors based on areal productivity (productivity per unit of occupied-land area per unit of time), photosynthetic efficiency or biomass yield (g-biomass per unit of solar radiation).
The specific biomass growth rate known as the increase in cell mass per unit time was calculated by using Eq. (2), and this was considered to be an indicator of the photosynthetic efficiency of the microalgae. The specific growth rate from the initial cell concentration was found to be significant as 2.20 ± 0.015 l/day. This may be due to the fact that the OP affected the high transparent surfaces, high illumination surfaces, and high mass transfer rates to high biomass and specific growth rate in the OPPBR. Hsieh and Wu [13] reported 1.745 l/day of specific growth rate using Chlorella sp. in transparent rectangular chambers PBR. Ong et al. [16] obtains 0.238 l/day specific growth rate using Chlorella sp. during 8-day cultivation in outdoor photobioreactor.
Substrate Consumption in the MMBR
COD and BOD Consumption
The effect of COD and BOD reduction in presence of C. vulgaris in wastewater is graphically represented in Fig. 3.
The average percent of COD and BOD reduction calculated with the present experimental setup is found to be 58.77 and 75.14 % for OPPBR, respectively, from initial COD and BOD level of 209.90 and 159.63 mg/l, respectively. The BOD consumption was ∼16.37 % higher than that of COD consumption in OPPBR. The organic carbon sources can be assimilated either chemo- or photoheterotrophically [5]. In the first case, the organic substrate is used both as the source of energy (through respiration) and as carbon source, while in the second case, light is the energy source. In several algal species, the mode of carbon nutrition can be shifted from autotrophy to heterotrophy when the carbon source is changed; this is the case with, e.g., the green algae Chlorella [10, 17].
The COD and BOD consumption using OPPBR was faster and occurred in 2 days. However, a prolonged operation shows a constant COD and BOD consumption process as mentioned in Fig. 3. Hammouda et al. [18] reported 79 % of COD removal using C. vulgaris and Scenedesmus sp. in a batch system. Govindan [19] reported a 72 % COD reduction in a system with dairy wastewater admixture with sewage. Biological treatment of domestic wastewater using algae indicated a 68.4 % BOD and 67.2 % COD removal [2]. Rana [20] reported 78 % COD reduction using C. vulgaris at 30 °C during 48 h of contact. The wastewater treatment utilizing the algal-bacterial system was capable of removing approximately 70–80 % of the COD [17].
The average percent of COD and BOD reduction was 38.22 and 21.95 % for MBR, from 209.90 mg/l COD concentration and 159.63 mg/l BOD concentration. The total consumption of COD and BOD in the MMBR are found to be 96.99 and 97.09 %, respectively. These results indicated that the OPPBR and MBR combined with MMBR process is more effective and efficient for COD and BOD consumption comparing to the separate OPPBR or MBR process.
TN, NO3-N, and NH4-N Removal
Nitrogen usually exists in wastewater in the form of ammonia, organic nitrogen, and nitrate. Various nitrogen removal techniques were demonstrated in aquaculture systems that can either remove or convert one form of nitrogen to another [21]. The removal of TN, NO3-N, and NH4-N by MMBR was shown in Fig. 4. This clearly indicates that most of the TN, NO3-N, and NH4-N were removed in the MMBR.
The initial concentration of TN, NO3-N, and NH4-N was found to be 40.02, 13.04, and 25.38 mg/l, respectively. From the results, it was observed that TN removal was 70.49 % for OPPBR and 96.38 % for MMBR at initial concentration of 40.02 mg/l TN. These results have exhibited a higher percentage of TN removal as compared with previous reported studies. Valderramna et al. [22] obtained 71.6 % TN removal by C. vulgaris in the treatment of industrial wastewater. Choi et al. [21] found 76.8 % TN removal under fixed bed MBR operated for domestic wastewater.
The maximum NH4-N percent removal in the OPPBR is obtained after 3 days is 79.16 %. Furthermore, it is observed that the total NH4-N percent removal is found to be 99.80 % from the initial level of 25.38 ml NH4-N in the MMBR. The reported NH4-N removal efficiency varies depending on the medium composition and environmental conditions, such as the initial nutrient concentration, light intensity, light/dark cycle, and algae species [23–25]. In the aerobic MMBR, under excess oxygen, NH4-N is converted by nitrifying bacteria to nitrites and nitrates. The MMBR serves as a polishing step to reduce the NH4-N concentrations. The NH4-N removal percentage achieved in this study is higher than several other studies; an average of 72 % nitrogen removal is reported for C. vulgaris from 3–8 mg of NH4-N per liter containing diluted ethanol and citric acid effluent [26]. Martinez et al. [6] reported over 94 % nitrogen removal by Scenedesmus obliquus at an initial concentration of 27.4 mg of N per liter. De-Bashan et al. [4] observed 93 % removal efficiency for NH4-N by C. vulgaris in the treatment of synthetic wastewater after 8 days of operation. Therefore, the NH4-N removal efficiency achieved in this study is found to be slightly higher comparing to other previous reports.
C. vulgaris uses nitrates as a source of food. The microalgal cells are able to consume high concentrations of nitrate ions and, therefore, may help in the purification of industrial and domestic wastewater. NO3-N removal is measured with 66.87 % for OPPBR and total NO3-N removal is 97.62 % in MMBR from an initial concentration of 13.04 mg/l NO3-N. Singh and Thomas [27] reported 35 % of NO3-N removal from MBR with microalgae photoreactor. Similarly TN, NH4-N, and NO3-N removal in MMBR is higher comparing to that of previous studies. The substrate consumption in the MMBR is represented in Table 4.
TP and PO4-P Removal
Phosphorus is another macronutrient essential for growth, which is taken up by algae as inorganic orthophosphate (PO4 3−). The uptake of orthophosphate is an active process that requires energy. Organic phosphates can be converted to orthophosphates by phosphatases at the cell surface, and this occurs especially when inorganic phosphate is in short supply. Microalgae are able to assimilate phosphorus in excess, which is stored within the cells in the form of polyphosphate (volutin) granules. These reserves can be sufficient for prolonged growth in absence of available phosphorus. The growth rate of an alga may therefore not respond at once to changes in the external concentration of phosphorus, in opposite to the immediate responses to temperature and light [6, 28]. The average removal percent of TP and PO4-P was 52.81 and 52.63 % for OPPBR, respectively, from 9.24 mg/l in TP concentration and 8.19 mg/l PO4-P concentrations. Whereas a lower removal was observed for PO4-P and TP compared with TN and NH4-N removal. The plausible reason for this phenomenon is that phosphorus uptake by algae was not always stoichiometric; this can also be affected by algal physiology as well as phosphorus concentration and its chemical forms, light intensity, pH, temperature etc. Phosphorus uptake is inversely related to internal phosphorus concentrations of the cell. It was observed that algae starting with low internal phosphorus concentrations exhibited maximum uptake rate than algae starting with high internal phosphorus concentrations. Therefore, the intracellular phosphorus concentrations could also be an important factor that controls the phosphorus uptake kinetics. Hernandez et al. [29] observed that starvation enhances the algae phosphorus removal from wastewater, because cells which are starved of phosphorus tend to overshoot the necessary phosphorus uptake for the cell growth.
The variation of the TP and PO4-P removal process in MBBR is depicted in Fig. 5. A higher phosphorus removal efficiency compared with previous reported studies was obtained in this study. For instance, an average of 28 % phosphorus removal by C. vulgaris from 1.5–3.5 mg of PO4 per liter containing diluted ethanol and citric acid effluent was obtained [11]. Lardotter [28] observed 55 % phosphorus uptake from agro industrial wastewater using C. vulgaris and Scenedesmus dimorphus. Singh and Thomas [27] reported 60 % PO4-P removal from MBR with microalgae photoreactor. In this study, the result obtained 90.84 % for PO4-P removal and the TP was removed to 92.75 % MMBR. These results indicate that the OPPBR and MBR combined MMBR process was more effective for COD and BOD consumption comparing to individual usage of OPPBR or MBR process.
Conclusions
In present research, a closed photobioreactor (PBR) with optical panel (OP) system was employed to enhance the light utilization throughout the reactor for the mass cultivation of microalgae and nutrient removal from domestic wastewater. In addition to the above, the effect of nutrient removal on the growth of a C. vulgaris algae biomass with simultaneous nutrient removal was evaluated quantitatively by using MMBR. A biomass productivity of 2.53 g/l/day and significant specific growth rate as 2.20 ± 0.015 l/day with light transmittance of 94 % at a 300-mm depth in the OPBR were achieved. It was observed that the specific growth rate was affected by high transparent surfaces, high illumination surfaces and high mass transfer rates to high biomass in the OPPBR. The total consumption of COD and BOD in the MMBR were found to be 96.99 and 97.09 %, respectively. Additionally, the removal of TN, NH4-N, and NO3-N were 96.38, 99.80, and 97.62 % in MMBR, respectively. It was observed that TP (92.75 %) and PO4-P (90.84 %) removal was lower compared to TN and NH4-N removal. These results clearly demonstrate that the MMBR process by combining OPPBR and MBR was highly effective for COD and BOD consumption and nutrient removal, compared to the individual application of OPPBR or MBR process. The MMBR process was more stable than OPPBR for the nutrient removal in the wastewater and can be applied to the field.
References
Brennan, L., & Owende, P. (2010). Renewable and Sustainable Energy Reviews, 14, 557–577.
Aslan, S., & Kapdan, I. K. (2006). Ecological Engineering, 28(1), 64–70.
Choi, H. J., Lee, J. M., & Lee, S. M. (2013). Water Science and Technology, 67(11), 2543–2548.
De-Bashan, L. E., Hernandez, J. P., Morey, T., & Bashan, Y. (2004). Water Research, 38, 466–474.
Griffiths, E. W. (2010). All graduate theses and dissertations. Logan: Utah State University.
Martinez, M. E., Sanchez, S., Jimenez, J. M., Yousfi, F. E., & Munoz, L. (2007). Bioresource Technology, 73(3), 263–272.
Wang, C., Yu, X., Lv, H., & Yang, J. (2013). Journal of Environmental Biology, 34, 421–425.
Posten, C. (2009). Engineering in Life Sciences, 9, 165–177.
Masojidek, J., Torzillo, G., Sven, E. J., & Brian, F. (2008). Ecological Engineering (pp. 2226–2235). Oxford: Academic press.
Muñoz, R., & Guieysse, B. (2006). Water Research, 40(15), 2799–2815.
Choi, H. J., & Lee, S. M. (2014). Bioprocess and Biosystems Engineering, 37(4), 697–705.
Carlozzi, P. (2003). Biotechnology and Bioengineering, 81(3), 305–315.
Hsieh, C. H., & Wu, W. T. (2009). Biochemical Engineering Journal, 46, 300–305.
Chen, C. Y., Yeh, K., Aisyah, R., Lee, D. J., & Chang, J. S. (2011). Bioresource Technology, 102(1), 71–81.
García-Malea López, M. C., Del Río Sánchez, E., Casas López, J. L., Acién Fernández, F. G., Rivas, J., Guerrero, M. G., & Molina Grima, E. (2006). Journal of Biotechnology, 123(3), 329–342.
Ong, S. C., Kao, C. Y., Chiu, S. Y., Tsai, M. T., & Lin, C. S. (2010). Bioresource Technology, 101, 2880–2883.
Luz, E. B., & Yoav, B. (2010). Bioresource Technology, 101(6), 1611–1627.
Hammouda, O., Gaber, A., & Abdel-Raouf, N. (1995). Ecotoxicology and Environmental Safety, 31(3), 205–210.
Govindan, S. (1984). Indian Journal of Environmental Health, 26, 261–263.
Rana, A. A. (2010). Journal of Engineering Technology, 28(4), 785–791.
Choi, H. J., Lee, A. H., & Lee, S. M. (2012). Water Science and Technology, 65(10), 1834–1838.
Valderramna, L. T., Del Campo, C. M., Godriguez, C. M., De-Bashan, L. E., & Bashan, Y. (2002). Water Research, 36(17), 4185–4192.
Kim, J., Lingaraju, B. P., Rheaume, R., Lee, J. Y., & Siddiqui, K. F. (2010). Tsinghua Science and Technology, 15(4), 391–396.
Ogbonna, J. C., & Tanaka, H. (2000). Journal of Applied Phycology, 12, 207–218.
Ugwu, C. U., Aoyagi, H., & Uchiyama, H. (2008). Bioresource Technology, 99, 4021–4028.
Zhang, E., Wand, B., Zhang, S., & Zhao, B. (2008). Bioresource Technology, 99(9), 3787–3793.
Singh, G., & Thomas, P. B. (2012). Bioresource Technology, 117, 80–85.
Larsdotter, K. (2006). Vatten, 62, 31–38.
Hernandez, J. P., de-Bashan, L. E., & Bashan, Y. (2006). Enzyme and Microbial Technology, 38, 190–198.
Acknowledgments
This work is supported by the basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013006899).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, H. Intensified Production of Microalgae and Removal of Nutrient Using a Microalgae Membrane Bioreactor (MMBR). Appl Biochem Biotechnol 175, 2195–2205 (2015). https://doi.org/10.1007/s12010-014-1365-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1365-5