Abstract
Graesiella emersonii was cultivated in an osmotic membrane photobioreactor (OMPBR) for nutrients removal from synthetic wastewater in continuous mode. At 1.5 days of hydraulic retention time and under continuous illumination, the microalgae removed nitrogen (N) completely at influent NH4+-N concentrations of 4–16 mg/L, with removal rates of 3.03–12.1 mg/L-day. Phosphorus (P) removal in the OMPBR was through biological assimilation as well as membrane rejection, but PO43−-P assimilation by microalgae could be improved at higher NH4+-N concentrations. Microalgae biomass composition was affected by N/P ratio in wastewater, and a higher N/P ratio resulted in higher P accumulation in the biomass. The OMPBR accumulated about 0.35 g/L biomass after 12 days of operation under continuous illumination. However, OMPBR operation under 12 h light/12 h dark cycle lowered biomass productivity by 60%, which resulted in 20% decrease in NH4+-N removal and nearly threefold increase in PO43−-P accumulation in the OMPBR. Prolonged dark phase also affected carbohydrate accumulation in biomass, although its effects on lipid and protein accumulation were negligible. The microalgae also exhibited high tendency to aggregate and settle, which could be attributed to reduction in cell surface charge and enrichment of soluble algal products in the OMPBR. Due to a relatively shorter operating period, membrane biofouling and salt accumulation did not influence the permeate flux significantly. These results improve the understanding of the effects of N/P ratio and light/dark cycle on biomass accumulation and nutrients removal in the OMPBR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
.
Introduction
Microalgae application in wastewater treatment, particularly in nitrogen (N) and phosphorus (P) removal, has many advantages. The autotrophic microalgae has the potential to integrate wastewater treatment with carbon capture, which can provide a single-stage solution to greenhouse gas emission as well as eutrophication (Hwang et al. 2016; Judd et al. 2015). Moreover, carbon and nutrients removed from the waste streams can be recovered as biomass, which may be valuable in manufacturing a wide variety of useful products, including biofuels (Jones and Mayfieldt 2012). While microalgae application in waste treatment is an excellent approach towards sustainability, use of wastewater in microalgae cultivation can reduce the cost of biomass production significantly (Delrue et al. 2016; Ventura et al. 2013).
Several microalgae strains, belonging mainly to Chlorella and Scenedesmus genus, have exhibited excellent nutrient removal and biomass accumulation potential during wastewater treatment (Delrue et al. 2016; Wu et al. 2014). Most of these studies have been conducted in batch or fed-batch mode though and very few studies have been conducted on microalgae cultivation under continuous operation. This is mainly due to low growth rate of microalgae, which would require continuous operation either at high hydraulic retention time (HRT) or in large volume photobioreactors (PBR) (Praveen et al. 2018). Nevertheless, the application of microalgae in wastewater treatment can benefit immensely from continuous operation. It would not only provide continuous supply of microalgae biomass, but it would also facilitate seamless integration of microalgae-based tertiary wastewater treatment system with conventional activated sludge process treating municipal wastewater (Viruela et al. 2018).
Recently, several studies have been conducted on continuous cultivation of microalgae in wastewater using membrane photobioreactor (MPBR) (Boonchai and Seo 2015; Najm et al. 2017; Noguchi et al. 2017). The MPBRs are based on integration of membrane filtration to conventional PBRs. Since the effluent is filtered through semi-permeable membranes, the MPBRs operate with high biomass retention without any cell washout concerns. High-retention MPBRs also yield high nutrient removal rates, and these are expected to reduce the costs of biomass harvesting (Gao et al. 2015; Naira et al. 2018).
One of the most crucial parameters for microalgae cultivation is PBR illumination. Both light intensity and duration affect biomass accumulation and nutrients removal performance in PBRs (Wang et al. 2012). It has also been reported that microalgae cultivation in wastewater treatment can be economical only if it is based on the use of solar radiation, as the cost of using artificial light is high (Praveen et al. 2016). However, the availability of sunlight is limited by day/night cycle, and the disruption of photosynthesis at night would be disadvantageous for microalgae growth and wastewater treatment. Since MPBRs operate under high-biomass retention, it would also mean that these are more susceptible to light limitation due to enhanced self-shading effects (Carvalho et al. 2011). Another challenge in MPBR operation can be the fluctuations in wastewater nutrients composition and large variations in N/P ratio (Beuckels et al. 2015). In addition, there are reports of microalgae aggregation in MPBR under prolonged operation. The biomass aggregation may lower productivity and affect PBR performance adversely (Praveen and Loh 2016).
In this research, a PBR was fitted with forward osmosis (FO) filtration module to design a submerged osmotic membrane photobioreactor (OMPBR). The OMPBR was operated with Graesiella emersonii to study the effects of wastewater composition, pH, and light exposure on nutrients removal and biomass accumulation. The changes in microalgae size, surface charge, and biomass composition were estimated and a mechanism for microalgae aggregation was proposed. The use of FO for filtration was motivated by the low pore size of FO membranes and absence of hydraulic pressure in the process, which result in enhanced contaminant rejection, low energy demand, and low membrane fouling (Wang et al. 2016).
Materials and methods
Microorganisms, culture conditions, and chemicals
All the chemicals used in this research were of analytical grade and purchased either from Sigma-Aldrich (St. Louis, USA) or Merck (Darmstadt, Germany). G. emersonii ATCC 13482 was used throughout this study. The microalgae were cultivated in Bold’s basal medium (BBM) supplemented with 5% CO2 enriched air at a rate of 0.2 gas volumes per reactor volume per minute (VVM). The microalgae culture was illuminated using fluorescent light at 7000 lx intensity. All media, pipette tips, and Erlenmeyer flasks fitted with cotton plugs were autoclaved before use.
OMPBR
Experimental setup
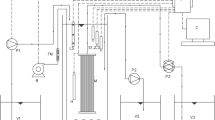
Figure 1 shows the laboratory scale OMPBR setup. A detailed description of the setup was provided elsewhere (Praveen and Loh 2016). Briefly, a plate-and-frame membrane module was prepared using commercial cellulose triacetate FO membranes (HTI, USA) with an effective filtration area of 0.036 m2. The membrane module was immersed in a 5.5 L tank, such that the active layer of the membranes faced the wastewater, whereas the support layer faced the draw solution (DS). The OMPBR was illuminated from all four sides and the top using fluorescent lights of 7000–8000 lx intensity. Humidified 3% CO2-enriched air was sparged in the OMPBR at the rate of 0.4 VVM. A 2 L beaker designed with an overflow outlet at 1.5 L was used as the DS reservoir. DS concentration in the reservoir was kept constant using a feedback control system (eChem, Singapore). Excess water overflowing from the DS reservoir was collected as OMPBR effluent. The wastewater feed tank was placed above a weighing balance (Sartorius, Germany) and connected to a PC for periodic mass monitoring, which was used to compute the permeate flux.
Schematic diagram of the OMPBR setup: (1) compressed air; (2) compressed CO2; (3) flow meter; (4) humidification tank; (5) MBR tank; (6) membrane module; (7) air diffuser; (8) feed tank; (9) weighing scale; (10) stirring plate; (11) DS; (12) concentrated DS stock; (13) effluent; (14) peristaltic pump; (15) conductivity meter; and, (16) data logger
Operation
Synthetic wastewater was used throughout the OMPBR operation, with NH4+-N concentration varying between 4 and 16 mg/L and a fixed PO43−-P concentration of 2.4 mg/L. Other micronutrients were added to the synthetic wastewater, based on BBM composition, to prevent limitations on microalgae growth. NH4+-N was chosen as the nitrogen source in this study, as it is more readily assimilated by G. emersonii, as compared to NO3−-N (Praveen et al. 2018). A 5 M stock solution of sodium chloride was used to prepare the DS, such that the HRT was maintained at 1.5 days. The OMPBR was operated with complete biomass retention, and only a small amount of liquid was removed every day to monitor biomass and nutrients concentration.
A total of four runs were conducted in the OMPBR. (1) NH4+-N = 4 mg/L, continuous illumination; (2) NH4+-N = 8 mg/L, continuous illumination; (3) NH4+-N = 16 mg/L, continuous illumination, and; (4) NH4+-N = 16 mg/L, light/dark cycle. For light/dark cycle, a digital plug-in timer was used to illuminate the OMPBR for 12 h from 8 am, and to switch off the lights from 8 pm. All the runs were started with fixed biomass concentration and the OMPBR was operated for 12–18 days under each condition.
Analytical methods
Microalgae biomass density was determined by measuring the optical density (OD) of the aqueous medium at 540 nm using an ultraviolet-visible spectrophotometer (Shimadzu, Japan). The OD was used to compute the biomass concentration by the formula: dry cell weight (mg/L) = 542 × OD540 (Praveen and Loh 2016). The concentrations of NH4+-N, NO3−-N, and PO43−-P were measured using standard methods (Rice et al. 2012). The overall removal efficiency and the removal rates were calculated based on the difference between the feed and the effluent concentrations.
Microalgae biomass was harvested from the OMPBR through centrifugation (5810R, Eppendorf, Germany) at 13600 g for 5 min. Surface charge and particle size of microalgae were measured using a Zetasizer (Malvern, UK) with disposable folded capillary cells. Total algal lipid content was measured gravimetrically after extraction using methanol and chloroform. Carbohydrates from biomass were extracted using 80% sulfuric acid solution, and quantified using phenol/sulfuric acid method. Proteins were extracted from the biomass using 0.1 M sodium hydroxide, and quantified using Lowry’s method. Chlorophyll content of the microalgae was measured spectrophotometrically after extraction using 90% methanol at 4 °C for 48 h. Detailed procedures for these assays are described elsewhere (Praveen and Loh 2016).
Results
Cell growth and nutrients removal
In the beginning, the OMPBR was operated using feed wastewater with NH4+-N and PO43−-P concentrations of 4 mg/L and 2.4 mg/L, respectively. There was no pH control in the OMPBR during the first 10 days of operation. The pH was subsequently adjusted and maintained at 7.5.
Figure 2 shows temporal profiles for biomass accumulation and nutrients removal in the OMPBR. It can be seen that two different concentration profiles developed on either side of the FO membranes, and most of the nutrients were retained in the OMPBR due to low pore size and enhanced rejection by the FO membranes. In the absence of any pH adjustment, NH4+-N accumulation in the OMPBR reached a maximum of 29 mg/L on day 2 (Fig. 2a). However, as biomass concentration in the OMPBR increased gradually, NH4+-N was metabolized and its concentration stabilized at about 2.5 mg/L after 5 days of operation. In contrast, NH4+-N concentration in the effluent was unaffected by low biomass concentration or high NH4+-N accumulation in the OMPBR, and it remained stable at 2–3 mg/L during this period. On the other hand, PO43−-P concentration in the effluent was nearly negligible, owing to excellent rejection by the FO membranes (Fig. 2b). Consequently, there was a gradual increase in PO43−-P concentration in the OMPBR in the beginning, and the concentration stabilized at 21 mg/L after 6 days of operation.
The pH of the OMPBR was adjusted to 7.5 on day 11, when the measured pH was 4.5. This resulted in quick improvements in cell growth and nutrients assimilation, as shown in Fig. 2. While NH4+-N concentration in the OMPBR and in the effluent dropped nearly to zero, PO43−-P level in the OMPBR decreased by 35% to 13.8 mg/L. The removal efficiencies for PO43−-P and NH4+-N were calculated as 100% and 99.8%, respectively, whereas the removal rates were 1.82 mg/L-day and 3.03 mg/L-day, respectively. The effects of pH were also evident in microalgae growth profiles (Fig. 3). Although microalgae concentration increased gradually from 17 mg/L in the beginning to 353 mg/L on day 18, there was a significant difference between microalgae growth rate before and after day 11. It was observed that the doubling time for the microalgae had increased to over 3 days under acidic pH conditions. The pH in the OMPBR was maintained constant for all the subsequent experiments.
Effects of NH4 +-N concentration
In order to investigate the effects of NH4+-N concentrations on OMPBR performance, NH4+-N concentration was varied from 4 to 16 mg/L. At NH4+-N concentrations of 8 mg/L and 16 mg/L, biomass growth trends were nearly identical (Fig. 4a). The microalgae exhibited relatively higher growth rates, and biomass accumulation increased to 335–382 mg/L after 12 days of operation. It was also observed that suspended biomass concentration in the OMPBR did not stabilize during the operation, even when NH4+-N had been completely exhausted.
Figure 4b shows NH4+-N removal in the OMPBR at different initial NH4+-N concentrations. It can be seen that under each experimental condition, NH4+-N accumulated briefly in the OMPBR due to partial membrane rejection, before it was removed completely through microalgae assimilation. NH4+-N accumulation was expected to increase with increasing NH4+-N concentration in the wastewater. However, the maximum accumulation was observed at initial NH4+-N concentration of 4 mg/L under low pH conditions. NH4+-N removal at initial concentrations of 8–16 mg/L was nearly complete with removal efficiencies >99.8%. Average NH4+-N removal rates increased proportionately with NH4+-N concentration, and these were observed to be 3.03, 6.07, and 12.1 mg/L-day, at influent NH4+-N concentrations of 4, 8, and 16 mg/L, respectively. Apart from NH4+-N, NO3−-N concentration was also measured during the OMPBR operation (data not shown). However, no NO3−-N was detected in the OMPBR throughout the operation.
Unlike NH4+-N, which was partially rejected by the FO membranes and completely metabolized by microalgae, PO43−-P rejection by the FO membranes was nearly complete. Since PO43−-P concentration in the influent remained unchanged throughout the operation, the removal efficiency and removal rate for PO43−-P was constant at about 100% and 1.82 mg/L-day, respectively. It was also observed that PO43−-P accumulation in the MPBR decreased from 13.8 to 4.7 mg/L, when NH4+-N concentration in the wastewater was increased from 4 to 16 mg/L (Fig. 4c). Clearly, NH4+-N concentration in wastewater influenced PO43−-P uptake by the microalgae.
Effects of light/dark cycle
In order to understand the effects of light/dark cycle on wastewater treatment by microalgae in the OMPBR, experiments were conducted under alternating light and dark cycle of 12 h each, at NH4+-N concentration of 16 mg/L, and an HRT of 1.5 days.
Figure 5a shows the effects of light/dark cycle on biomass growth and accumulation in the OMPBR. Under light/dark cycle, microalgae growth rate decreased significantly, and biomass concentration increased from 18 to 143 mg/L after 12 days of operation. Thus, both biomass accumulation and nutrients removal decreased substantially under light/dark cycle. NH4+-N removal was slow and its accumulation in the OMPBR increased by 40%, from 21.1 mg/L under continuous illumination to 34.7 under light/dark cycle (Fig. 5b). NH4+-N removal was incomplete after 12 days of operation, and its level in the OMPBR and the effluent were 4.5 mg/L and 3 mg/L, respectively. The corresponding removal efficiency and removal rate were computed as 81% and 8.64 mg/L-day, respectively.
Similarly, PO43−-P assimilation by microalgae decreased significantly under light/dark cycle, even though PO43−-P was completely removed from the effluent due to its rejection by the FO membranes. Low PO43−-P uptake by microalgae was evident in PO43−-P accumulation in the OMPBR, which increased nearly threefold from 4.66 mg/L under continuous illumination to 15.2 mg/L under light/dark cycle. It was also observed that PO43−-P assimilation (based on accumulation in OMPBR) by microalgae under light/dark cycle was lower than that observed during continuous illumination at a lower NH4+-N concentration of 8 mg/L.
Flux and fouling
In the OMPBR, the permeate flux and salinity were monitored regularly. There were changes in salinity, which increased gradually from 2.66 to 12.5 mS, after 3 weeks of operation. On an average, conductivity increased by about 3 mS every week, which indicated that nearly 30 mM NaCl diffused into the OMPBR tank from the DS through the FO membranes. Although salt buildup inside the OMPBR reduced the nett osmotic pressure gradient across the membranes, these changes were small, and could be compensated easily by increasing DS concentration (data not shown).
Compared to the effects of salt accumulation on permeate flux in the OMPBR, the contribution of biofouling on flux were nearly negligible. Although microalgae deposits and biofilms were observed on the FO membranes (results not shown), biofilm developed within 2–3 weeks of operation were weak, and it could be easily washed away at the end of each run. Since microalgae also exhibited biomass aggregation in the OMPBR, their effective size increased over time, further diminishing their ability to block the membrane pores.
Biomass aggregation analysis
During OMPBR operation, microalgae readily aggregated and settled at the bottom of the bioreactor tank. While aggregation resulted in low suspended biomass concentration, there was also risk of biomass deposited at the bottom receiving poor light exposure. In order to avoid the deposition, the bottom of the bioreactor tank was agitated with compressed air every day to prevent thick microalgae deposits. However, mechanical agitation did not prevent gradual aggregation of biomass in the OMPBR. In order to investigate the factors affecting biomass aggregation, microalgae surface charge and cell size were monitored during the experiments.
Table 1 shows changes in surface charge and size of the microalgae during different experiments. In the beginning of the OMPBR operation, zeta potential of the microalgae was about −35 mV. However, the surface charge decreased nearly to one-third of the value within a week of operation, and varied between −10.6 mV and − 13.1 mV. Although a higher surface charge of −18.7 mV was measured during the third week during OMPBR operation at influent NH4+-N concentration of 4 mg/L, it was half of the potential seen during the control experiments. Simultaneously, microalgae size increased from 8 to 10 μm at the beginning of operation to nearly 60 μm after 1–2 weeks of operation. A higher value of 75 μm was also recorded in third week of OMPBR operation at influent NH4+-N concentration of 4 mg/L. It was suspected that biomass aggregation was a result of bacterial contamination, but investigations with optical microscopy did not show presence of any invading microorganisms in the OMPBR.
Biomass composition analysis
In order to investigate the effects of OMPBR operating conditions on nutrients accumulation in microalgae, microalgae biomass was collected at the end of each experiment, and carbohydrate, lipid, protein, and chlorophyll composition were measured (Table 2). Under continuous illumination, changes in wastewater N/P ratio did not influence lipid and polysaccharides content of microalgae, which remained constant at 18–21% and 43–46%, respectively. On the other hand, protein content of the microalgae increased slightly from 2.5 to 6.9%, when NH4+-N concentration was raise from 4 to 16 mg/L. Under continuous illumination, pigment accumulation increased with increasing NH4+-N concentration as well. The concentrations of chlorophyll A at 4 mg/L, 8 mg/L, and 16 mg/L NH4+-N concentration were 2.0 mg/g, 17. 2 mg/g, and 23.0 mg/g, respectively. Under light/dark cycle, biochemical composition of the microalgae changed significantly as there was nearly 30% decrease in protein and lipid content of the biomass, whereas carbohydrate content increased by more than 10%. Although pigments accumulation decreased under cyclic illumination conditions, chlorophyll content in biomass was higher than those observed under continuous illumination at feed NH4+-N concentration of 4 mg/L.
Apart from biochemical composition, elemental analysis of the biomass was also conducted under each operating condition to investigate the changes in biomass composition (results not shown). Under continuous illumination, C, N, and H composition of the biomass remained stable at 48.2 ± 1.6%, 7.62 ± 0.40%, and 8.49 ± 0.05%, respectively, although P content of the biomass increased gradually from 3.46 to 3.81% with increasing N/P ratio in influent wastewater. On the other hand, both N and P accumulation in the biomass decreased to 7.45% and 2.53%, when the OMPBR was operated under 12 h light/12 h dark cycle.
Discussion
Application of microalgae in polishing secondary wastewater requires integration and operation of a microalgae cultivation system, in tandem with an activated sludge process treating secondary wastewater. The integration of these two processes is challenging due to the disparity in growth rates of microalgae and bacteria. Microalgae are highly susceptible to washout, when cultivated under continuous operation at HRTs optimized for bacterial processes (Praveen and Loh 2016). Although these concerns can be alleviated through operation at high HRTs, it would result in dramatic increase in the footprint of the algal cultivation system.
The integration of membrane filtration with conventional PBRs has the potential to alleviate the challenges in continuous microalgae cultivation. Although the OMPBR was operated at a relatively low HRT of 1.5 days with G. emersonii cells of 18 h doubling time, the OMPBR retained a high amount of biomass and achieved excellent NH4+-N and PO43−-P removal. This efficacy of OMPBR was not solely due to nutrients rejection by the FO membranes, and biological uptake of the nutrients was key to achieve complete removal of NH4+-N and stabilization of PO43−-P levels in the OMPBR and the effluent. Several other studies on microfiltration-based MPBR have reported continuous microalgae cultivation at HRTs ranging from 1 to 4 days (Luo et al. 2017). MPBR studies conducted at relatively high light intensities have also reported operation at HRTs as low as 0.25 days, which is comparable to the HRTs typically used in activated sludge process (Xu et al. 2015). Since microalgae in the OMPBR were able to remove NH4+-N completely under continuous illumination, it can be inferred that it would be possible to operate the OMPBR at lower HRTs, as well as, at higher NH4+-N loadings under identical conditions, without any significant loss in performance.
The concentration of nutrients in tertiary wastewater varies widely, depending upon the operating conditions used in the preceding activated sludge process. Typically, total N in tertiary wastewater is below 20 mg/L, whereas P content can vary between 1 and 5 mg/L (Cai et al. 2013). In the OMPBR, the assimilation of NH4+-N was nearly complete at different feed concentrations under continuous illumination. The absence of NO3−-N in the OMPBR indicated low or no presence of heterotrophic microorganisms, including nitrifying bacteria, in the OMPBR. Thus, it could be concluded that the removal of NH4+-N in the OMPBR was through direct assimilation by microalgae. On the other hand, the assimilation of PO43−-P by microalgae was lower due to a relatively low N/P ratio in wastewater. However, PO43−-P removal by microalgae improved significantly with increasing NH4+-N concentration in the wastewater. While the improvement in PO43−-P uptake by microalgae can be attributed to an increase in the limiting nutrient NH4+-N, there was a slight increase in P accumulation in microalgae biomass when N/P ratio was increased. The biomass P content were 3.46%, 3.68%, and 3.81%, when influent NH4+-N concentrations were 4 mg/L, 8 mg/L, and 16 mg/L, respectively. These results indicated that the improvement in PO43−-P assimilation was not simply to support assimilation of a higher concentration of NH4+-N, but it was also a result of a higher P accumulation in microalgae biomass. It should also be noted that higher P accumulation can result from luxury uptake or from P precipitation on cell surface. Since P was not the limiting nutrient in the OMPBR, the possibility for luxury P uptake was low (Solovchenko et al. 2016). On the other hand, a relatively high biomass P concentration of 3.5–4% suggested that P could have been precipitated on cell surface as well as in flocs matrix in the presence of chelating ions (Xu et al. 2014).
These results are consistent with previous studies reporting improvements in P intake by microalgae at higher N concentration due to enhanced P accumulation in microalgae biomass (Beuckels et al. 2015). Apart from influencing the rate of removal and biomass composition, wastewater N/P ratio also influenced the effluent quality. Typically, the N/P ratio (weight basis) in tertiary wastewater varies from 2 to 4 at the lower end to over 100 at the higher end (Kesaano and Sims 2014). In the OMPBR, the N/P ratio was rather low and varied from 1.67–6.67. Since NH4+-N was the limiting nutrient under these conditions, it could be inferred that the optimal N/P ratio for microalgae in the OMPBR was above 6.67.
Due to a relatively low N/P ratio, low PO43−-P removal efficiency was expected in the OMPBR. On the contrary, the results obtained in Figs. 4 and 5 showed nearly complete PO43−-P removal from the effluent, irrespective of the variations in wastewater composition or duration of illumination. This discrepancy can be attributed to excellent rejection properties of the FO membranes in the OMPBR (Wang et al. 2016). Due to small pore size, the FO membranes exhibited high rejection properties for the nutrients. While NH4+-N was rejected partially, PO43−-P rejection was nearly 100% due to larger size of these molecules. Consequently, two different nutrients concentration profiles developed on either side of the FO membranes, depicting nutrients accumulation in the OMPBR, as well as nutrients concentration in the effluent. For NH4+-N, the differences between the two profiles were visible only under the transient state (in the first few days), when NH4+-N briefly accumulated in the OMPBR. In contrast, FO membranes played a dominant role in PO43−-P removal, and facilitated nearly complete removal of PO43−-P from the effluent. The rejected PO43−-P accumulated in the OMPBR, and the level of accumulation decreased with increasing NH4+-N availability in influent wastewater. Such high PO43−-P removal in wastewater, characterized by low N/P ratio, has not been reported in microalgae-based wastewater treatment processes (Cai et al. 2013). These results also highlighted the advantages of OMPBR over microfiltration-based MPBRs, as membrane filtration in the OMPBR not only improved biomass retention, but it also enhanced effluent quality.
The trapping of PO43−-P in the OMPBR and other FO-based wastewater treatment processes has the potential to lower the risk of eutrophication from the filtered effluent, when it is discharged into the environment. On the other hand, the resulting enrichment of excess PO43−-P in the OMPBR can be useful in precipitation, and subsequent recovery of phosphate salts from wastewater (Guzzon et al. 2008; Praveen and Loh 2016). This approach has been demonstrated successfully in several recent studies utilizing FO-based separation processes and bioreactors for PO43−-P recovery (Ansari et al. 2016; Xue et al. 2015). Thus, selective rejection and enrichment of PO43−-P through FO can not only help in fulfilling the stringent regulations regarding PO43−-P discharge in environment, but it would also help in mitigating the concerns over rapidly falling reserves of phosphate rocks, which is key to sustain the agriculture industry (Solovchenko et al. 2016; Xue et al. 2015). Therefore, the accumulation of large amounts of PO43−-P in the OMPBR is highly desirable, as there is potential for its recovery and recycle.
The high-throughput photosynthetic assimilation of NH4+-N and PO43−-P by microalgae in the OMPBR (Fig. 4) was driven by incessant illumination of the microalgae using fluorescent lights. In contrast, under 12 h light/12 h dark cycle, OMPBR performance deteriorated significantly, resulting in 20% decrease in NH4+-N removal efficiency. Although PO43−-P removal was still complete owing to FO-based separation, there was threefold increase in PO43−-P accumulation in the OMPBR, which indicated poor PO43−-P assimilation by the microalgae. These results are consistent with literature highlighting the crucial role of illumination on microalgae growth and biomass production (Jacob-Lopes et al. 2009). However, there are also reports of enhancement in PBR productivity, when it was operated under light/dark cycle (Carvalho et al. 2011). Some studies have also indicated that light intensity as well as wavelength may offset the adverse effects of light/dark cycle on microalgae (Wang et al. 2014). Thus, the influence of light/dark cycle on PBRs may depend on several factors, including microalgae strain, light source, and intensity. However, considering the fact that artificial light incurs high operating costs in PBRs (Praveen et al. 2016), it is likely that the application of microalgae in wastewater treatment would be supported through solar radiation with light/dark cycle of approximately 12 h each, and it would also deteriorate nutrients assimilation by microalgae. An approach to alleviate this limitation is by using an algal-bacterial consortium, or by using a mixture of microalgae comprising of both autotrophic and heterotrophic microalgae strains (Acien et al. 2016).
Since the OMPBR was operated under complete biomass retention, it resulted in large biomass accumulation. Biomass concentration in the OMPBR was about 0.2 g/L after a week of operation, and it doubled to 0.4 g/L after 2 weeks of operation. However, biomass growth was adversely affected by the acidic pH and the light/dark period. Under acidic condition, biomass growth rate decreased by about 50%, and only 0.12 g/L biomass was accumulated after a week of operation. On the other hand, biomass growth was about 60% slower under 12 h light/12 h dark cycle, and only 0.092 g/L biomass was accumulated after a week of operation, whereas biomass concentration after 12 days was 0.142 g/L. Thus, poor nutrients removal under these conditions can be attributed to the loss in biomass productivity and low biomass accumulation in the OMPBR.
Microalgae in the OMPBR also exhibited high tendency to aggregate, and the larger aggregates readily settled at the bottom of the tank. This phenomena of cell attachment and aggregation has previously been reported in OMPBR, wherein the loss of planktonic biomass resulted in underestimation of biomass concentration (Praveen et al. 2016; Praveen and Loh 2014). Biomass aggregation in the OMPBR started in the first few days, and microalgae size increased from 10-12 μm in the beginning to 60–70 μm after 2–3 weeks of operation. An analysis of cell surface charge and cell size indicated gradual lowering of zeta potential of the microalgae from −35 mV in the beginning, to −12 mV after 2 weeks of operation. This change in cell surface charge was accompanied by a gradual increase in the cell size, from 10–12 μm to 60–70 μm in the same period. It was evident from these results that the reduction in surface charge aided biomass aggregation. These changes in microbial cell surface properties can be attributed to the production of soluble algal products (SAPs). SAPs comprise of extracellular and intracellular organic matter produced by living and dead algal cells, respectively (Zhuang et al. 2016). The SAPs may comprise of large proteins and polysaccharides, some of which may act as coagulants. Since microalgae aggregation was not observed under batch operation (results not shown), it is possible that FO led to enrichment of these SAPs in the OMPBR, which accelerated changes in microalgae surface properties and facilitated aggregation and subsequent flocculation of the biomass (Praveen and Loh 2016).
The biomass harvested at the end of operation did not show any changes in the lipid, protein, or carbohydrate content, when the OMPBR was operated under continuous illumination. However, under light dark cycle, the polysaccharide content of the biomass decreased from 45 to 26%, indicating poor photosynthetic efficiency (Araujo and Garcia 2005). The accumulation of chlorophyll in the microalgae exhibited significant differences in the range of 2–23%. Chlorophyll a content of biomass increased with increasing NH4+-N availability, indicating a higher growth activity in microalgae at enhanced N levels. Under light/dark cycle, chlorophyll a accumulation was relatively low at 6.5 mg/g, but it was higher than the lowest chlorophyll a content seen under NH4+-N concentration of 4 mg/L. The minor increase could have been a result of high pigment production to negate the effects of poor illumination of the microalgae under light/dark conditions (Rossignol et al. 2000). Typically, N limitation in microalgae cultivation leads to enhanced lipid accumulation in the biomass (Gomez-Serrano et al. 2015). However, continuous supply of NH4+-N in the OMPBR could have prevented high lipid enrichment in the biomass.
Two factors affected FO permeate flux during OMPBR operation—salt accumulation in the OMPBR and membrane biofouling. However, the effects of salt accumulation on flux were observed immediately, and there was nearly sixfold increase in salt concentration within 2 weeks of operation. Thus, salt accumulation can be considered the primary challenge to maintain high flux during OMPBR operation (Praveen and Loh 2016; Wang et al. 2016). In contrast, 2 weeks were relatively short time period to observe strong algae biofilms on the membranes, especially in synthetic medium inoculated with pure culture. Besides, microalgae generally do not form very strong biofilms due to their low growth rate and high polysaccharide content of their extracellular polymeric substances. Nevertheless, during long term operation and in association with compatible heterotrophic bacteria, microalgae may be able to exhibit high biofouling tendency (Schnurr and Allen 2015).
Based on these results, it can be concluded that the OMPBR, integrating excellent rejection properties of FO membranes with high nutrients assimilation capacity of microalgae, can be an excellent platform for recovery of nutrients from wastewater. Both N/P ratio in wastewater and light/dark cycle were found to be important parameters, which could affect biomass accumulation, nutrient removal, and biomass quality in the OMPBR. Interesting results were obtained highlighting the correlation between biomass aggregation and cell surface charge, which may be explored further to design efficient and economical processes to simplify biomass harvesting from photobioreactors.
References
Acien FG, Gomez-Serrano C, Morales-Amaral MM, Fernandez-Sevilla JM, Molina-Grima E (2016) Wastewater treatment using microalgae: how realistic a contribution might it be to significant urban wastewater treatment? Appl Microbiol Biotechnol 100(21):9013–9022. https://doi.org/10.1007/s00253-016-7835-7
Ansari AJ, Hai FI, Guo WS, Ngo HH, Price WE, Nghiem LD (2016) Factors governing the pre-concentration of wastewater using forward osmosis for subsequent resource recovery. Sci Total Environ 566:559–566. https://doi.org/10.1016/j.scitotenv.2016.05.139
Araujo SD, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246(1–4):405–412. https://doi.org/10.1016/j.aquaculture.2005.02.051
Beuckels A, Smolders E, Muylaert K (2015) Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res 77:98–106. https://doi.org/10.1016/j.watres.2015.03.018
Boonchai R, Seo G (2015) Microalgae membrane photobioreactor for further removal of nitrogen and phosphorus from secondary sewage effluent. Korean J Chem Eng 32(10):2047–2052. https://doi.org/10.1007/s11814-015-0043-9
Cai T, Park SY, Li YB (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev 19:360–369. https://doi.org/10.1016/j.rser.2012.11.030
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89(5):1275–1288. https://doi.org/10.1007/s00253-010-3047-8
Delrue F, Alvarez-Diaz PD, Fon-Sing S, Fleury G, Sassi JF (2016) The environmental biorefinery: using microalgae to remediate wastewater, a win-win paradigm. Energies 9(3). https://doi.org/10.3390/en9030132
Gao F, Yang ZH, Li C, Zeng GM, Ma DH, Zhou L (2015) A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour Technol 179:8–12. https://doi.org/10.1016/j.biortech.2014.11.108
Gomez-Serrano C, Morales-Amaral MM, Acien FG, Escudero R, Fernandez-Sevilla JM, Molina-Grima E (2015) Utilization of secondary-treated wastewater for the production of freshwater microalgae. Appl Microbiol Biotechnol 99(16):6931–6944. https://doi.org/10.1007/s00253-015-6694-y
Guzzon A, Bohn A, Diociaiuti M, Albertano P (2008) Cultured phototrophic biofilms for phosphorus removal in wastewater treatment. Water Res 42(16):4357–4367. https://doi.org/10.1016/j.watres.2008.07.029
Hwang J-H, Church J, Lee S-J, Park J, Lee WH (2016) Use of microalgae for advanced wastewater treatment and sustainable bioenergy generation. Environ Eng Sci 33(11):882–897. https://doi.org/10.1089/ees.2016.0132
Jacob-Lopes E, Scoparo CHG, Lacerda L, Franco TT (2009) Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process 48(1):306–310. https://doi.org/10.1016/j.cep.2008.04.007
Jones CS, Mayfieldt SP (2012) Algae biofuels: versatility for the future of bioenergy. Curr Opin Biotechnol 23(3):346–351. https://doi.org/10.1016/j.copbio.2011.10.013
Judd S, van den Broeke LJP, Shurair M, Kuti Y, Znad H (2015) Algal remediation of CO2 and nutrient discharges: a review. Water Res 87:356–366. https://doi.org/10.1016/j.watres.2015.08.021
Kesaano M, Sims RC (2014) Algal biofilm based technology for wastewater treatment. Algal Res 5:231–240. https://doi.org/10.1016/j.algal.2014.02.003
Luo Y, Le-Clech P, Henderson RK (2017) Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: a review. Algal Res 24:425–437. https://doi.org/10.1016/j.algal.2016.10.026
Naira VR, Das D, Maiti SK (2018) Designing a CO2 supply strategy for microalgal biodiesel production under diurnal light in a cylindrical-membrane photobioreactor. Bioresour Technol 250:936–941. https://doi.org/10.1016/j.biortech.2017.11.087
Najm Y, Jeong S, Leiknes T (2017) Nutrient utilization and oxygen production by Chlorella vulgaris in a hybrid membrane bioreactor and algal membrane photobioreactor system. Bioresour Technol 237:64–71. https://doi.org/10.1016/j.biortech.2017.02.057
Noguchi M, Hashimoto C, Honda R, Teraoka Y, Yang S, Ninomiya K, Takahashi K (2017) Utilization of anaerobic digestion supernatant as a nutrient source in microalgal biomass production with a membrane photobioreactor. J Water Environ Technol 15(6):199–206. https://doi.org/10.2965/jwet.17-006
Praveen P, Loh K-C (2014) Kinetics modeling of two phase biodegradation in a hollow fiber membrane bioreactor. Sep Purif Technol 122(0):350–358. https://doi.org/10.1016/j.seppur.2013.11.033
Praveen P, Loh K-C (2016) Nitrogen and phosphorus removal from tertiary wastewater in an osmotic membrane photobioreactor. Bioresour Technol 206:180–187. https://doi.org/10.1016/j.biortech.2016.01.102
Praveen P, Heng JYP, Loh K-C (2016) Tertiary wastewater treatment in membrane photobioreactor using microalgae: comparison of forward osmosis & microfiltration. Bioresour Technol 222:448–457. https://doi.org/10.1016/j.biortech.2016.09.124
Praveen P, Guo Y, Kang H, Lefebvre C, Loh K-C (2018) Enhancing microalgae cultivation in anaerobic digestate through nitrification. Chem Eng J 354:905–912. https://doi.org/10.1016/j.cej.2018.08.099
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater, 22 edn. American Public Health Association, American Water Works Association & Water Environment Federation, Washington, DC
Rossignol N, Lebeau T, Jaouen P, Robert JM (2000) Comparison of two membrane - photobioreactors, with free or immobilized cells, for the production of pigments by a marine diatom. Bioprocess Eng 23(5):495–501. https://doi.org/10.1007/s004499900186
Schnurr PJ, Allen DG (2015) Factors affecting algae biofilm growth and lipid production: a review. Renew Sust Energ Rev 52:418–429. https://doi.org/10.1016/j.rser.2015.07.090
Solovchenko A, Verschoor AM, Jablonowski ND, Nedbal L (2016) Phosphorus from wastewater to crops: an alternative path involving microalgae. Biotechnol Adv 34(5):550–564. https://doi.org/10.1016/j.biotechadv.2016.01.002
Ventura JRS, Yang BQ, Lee YW, Lee K, Jahng D (2013) Life cycle analyses of CO2, energy, and cost for four different routes of microalgal bioenergy conversion. Bioresour Technol 137:302–310. https://doi.org/10.1016/j.biortech.2013.02.104
Viruela A, Robles Á, Durán F, Ruano MV, Barat R, Ferrer J, Seco A (2018) Performance of an outdoor membrane photobioreactor for resource recovery from anaerobically treated sewage. J Clean Prod 178:665–674. https://doi.org/10.1016/j.jclepro.2017.12.223
Wang B, Lan CQ, Horsman M (2012) Closed photobioreactors for production of microalgal biomasses. Biotechnol Adv 30(4):904–912. https://doi.org/10.1016/j.biotechadv.2012.01.019
Wang SK, Stiles AR, Guo C, Liu CZ (2014) Microalgae cultivation in photobioreactors: an overview of light characteristics. Eng Life Sci 14(6):550–559. https://doi.org/10.1002/elsc.201300170
Wang X, Chang VWC, Tang CY (2016) Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: advances, challenges, and prospects for the future. J Membr Sci 504:113–132. https://doi.org/10.1016/j.memsci.2016.01.010
Wu YH, Hu HY, Yu Y, Zhang TY, Zhu SF, Zhuang LL, Zhang X, Lu Y (2014) Microalgal species for sustainable biomass/lipid production using wastewater as resource: a review. Renew Sust Energ Rev 33:675–688. https://doi.org/10.1016/j.rser.2014.02.026
Xu M, Bernards M, Hu Z (2014) Algae-facilitated chemical phosphorus removal during high-density Chlorella emersonii cultivation in a membrane bioreactor. Bioresour Technol 153(0):383–387. https://doi.org/10.1016/j.biortech.2013.12.026
Xu M, Li P, Tang TY, Hu ZQ (2015) Roles of SRT and HRT of an algal membrane bioreactor system with a tanks-in-series configuration for secondary wastewater effluent polishing. Ecol Eng 85:257–264. https://doi.org/10.1016/j.ecoleng.2015.09.064
Xue WC, Tobino T, Nakajima F, Yamamoto K (2015) Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: effect of membrane properties and feed solution chemistry. Water Res 69:120–130. https://doi.org/10.1016/j.watres.2014.11.007
Zhuang LL, Wu YH, Espinosa VMD, Zhang TY, Dao GH, Hu HY (2016) Soluble algal products (SAPs) in large scale cultivation of microalgae for biomass/bioenergy production: a review. Renew Sust Energ Rev 59:141–148. https://doi.org/10.1016/j.rser.2015.12.352
Funding
This research was funded by the Singapore National Research Foundation under its Competitive Research Program for the project entitled, “Advanced FO Membranes and Membrane Systems for Wastewater Treatment, Water Reuse and Seawater Desalination” (Grant number: R-279-000-338-281).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Praveen, P., Loh, KC. Nutrient removal in an algal membrane photobioreactor: effects of wastewater composition and light/dark cycle. Appl Microbiol Biotechnol 103, 3571–3580 (2019). https://doi.org/10.1007/s00253-019-09696-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09696-0