Abstract
Objective
Mantle cell lymphoma (MCL) is an aggressive lymphoma sub-type with poor prognosis and high 18F-FDG avidity at PET/CT; nowadays, no validated criteria for PET/CT in treatment response evaluation and prediction of outcome are present. The aim of study was to investigate whether the metabolic PET/CT features may predict treatment evaluation and prognosis in MCL.
Methods
We retrospectively enrolled 87 patients who underwent baseline 18F-FDG PET/CT and 85 end-of-treatment (eot) PET/CT. The baseline PET images were analyzed visually and semi-quantitatively by measuring the maximum standardized uptake value body weight (SUVbw), lean body mass (SUVlbm), body surface area (SUVbsa), lesion-to-liver SUVmax ratio (L-L SUV R), lesion-to-blood pool SUVmax ratio (L-BP SUV R), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). EotPET/CT was visually interpreted according to the criteria of the Deauville 5-point scale (DC). Survival curves were plotted according to the Kaplan–Meier method.
Results
At a median follow-up of 40 months, relapse/progression occurred in 47 and death in 23 patients. Median PFS and OS were 30 and 41 months. Baseline MTV and TLG were significantly higher in patients with progressive metabolic response compared to complete/partial response group. EotPET/CT results using DC significantly correlated with PFS, not with OS. MTV and TLG were demonstrated to be independent prognostic factors for PFS; instead the other metabolic parameters were not related to outcome survival. Considering OS, no variable was significantly associated.
Conclusions
EotPET/CT results (using DC), MTV and TLG were significantly correlated with response to treatment and PFS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mantle cell lymphoma (MCL) is a B cell non-Hodgkin’s lymphoma (NHL) representing about 5–10% of all NHLs [1]. For a definitive diagnosis, the existence of a t(11;14) translocation must be demonstrated by fluorescence in situ hybridization. This translocation results in overexpression of cyclin D, which may be used as a marker for the disease [1, 2]. Despite recent improvements in therapy field, MCL have usually poor prognosis and only a minority of patients have a longer survival; moreover, the risk of relapse is very high [2]. At the moment, no biological, pathological, or imaging markers to identify this subset are available as well as prognostic index. There has been an attempt to create a prognostic index called Mantle Cell Lymphoma International Prognostic Index (MIPI) [3] to better classify patients with MCL and predict prognosis, but the validation of this index has been reported with conflicting results [4, 5]. Also other histopathological features, like Ki-67 score or blastoid variant, have been studied with promising results [6, 7]. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been demonstrated to have excellent sensitivity in the detection of nodal involvement in patients with MCL [8, 9]. However, the sensitivity for detection of disease in the bone marrow (BM) and gastrointestinal (GI) district is low and inadequate to replace routine BM biopsy and GI endoscopy in disease staging [10,11,12]. Moreover, the role of 18F-FDG PET/CT in restaging and treatment response evaluation in MCL seems to be good [5, 8, 13,14,15,16], while its role in follow-up is limited [17]. So far, no studies about the prognostic usefulness of 18F-FDG PET/CT metabolic features in MCL are available. An identification of validated prognostic criteria could be crucial because it could modify patient management and treatment. More sophisticated prognostic stratification are required to identify subgroups who might benefit from more aggressive treatments, or in whom the prognosis is already sufficiently good to obviate more conservative treatment approaches.
The aim of our study was to investigate whether the metabolic PET/CT parameters can predict treatment response and prognosis in MCL. Moreover, we verified the potential application of Deauville criteria at end-of-treatment PET/CT.
Materials and methods
Patients
We have retrospectively screened about 30,000 patients studied with 18F-FDG PET/CT in our Nuclear Medicine center from February 2007 until February 2018 using our institutional Radiology Information System (RIS). Among these patients, 97 patients with histologically proven MCL were retrospectively enrolled. Patients with concomitant malignancy or without baseline or eotPET/CT were excluded from the analysis. Finally, 87 patients were recruited; histopathological diagnosis revealed MCL in all the cases. We reviewed the medical records of these 87 patients analyzing epidemiological features (gender and age at diagnosis), morphological features (bulky disease and splenomegaly), clinical data (B symptoms, MIPI score, lactate dehydrogenase (LDH) level and β2-microglobulin level), histopathological data (Ki-67 score and blastoid variant) and metabolic features by 18F-FDG PET/CT. MIPI score, LDH level and β2-microglobulin were dichotomized using a cutoff value of 2, 245 U/L and 2.8 mg/L, respectively. Bulky disease was defined when the maximum width is equal or greater than one-third of the internal transverse diameter of the thorax or at an alternative site was defined as any mass measuring 10 cm or more by any imaging study. Splenomegaly was defined when cranial-caudal diameter was > 13 cm as suggested by Lugano recommendations [18]. Proliferative activity, measured by Ki-67 score, was available in 79 patients; the Ki-67 expression level was arbitrarily divided into two groups: ≤ 15% and > 15%.
All the patients were treated according to the institution’s standard protocol with chemotherapy regimen. Twenty-one patients were treated according to R-BAC regimen up to six cycles of immuno-chemotherapy including Rituximab, Bendamustine and Cytarabine; 56 patients according to R-CHOP (Rituximab, Cyclophosphamide, Hydroxydoxorubicine, Oncovin and Prednisone) or alternating R-CHOP/R-DHAP (Rituximab, Dexamethasone, high-dose Ara-C cytarabine, and Cisplatin) regimen followed by autologous stem cell transplantation; and the remaining 10 patients were treated according to MCL 0208 trial which consisted of high-dose chemotherapy additioned with Rituximab, followed by autologous stem cell transplantation and Lenalidomide as maintenance therapy [19].
18F-FDG PET/CT imaging and interpretation
All the patients underwent baseline 18F-FDG PET/CT before chemotherapy and then at completion of therapy after six cycles (eotPET/CT). 18F-FDG PET/CT was performed after at least 4 h fasting and with glucose blood level lower than 150 mg/dl. An activity of 3.5–4.5 MBq/Kg of 18F-FDG was administered intravenously and scans were acquired about 60 min after radiotracer injection from the skull basis to the mid-thigh on a Discovery ST or Discovery 690 PET/CT tomograph (General Electric Company—GE®—Milwaukee, WI, USA) with standard parameters (CT: 80 mA, 120 kV without contrast; 2.5–4 min per bed-PET-step of 15 cm); the reconstruction was performed in a 128 × 128 matrix and 60 cm field of view. Time-of-flight (TOF) and point spread function (PSF) were used as reconstruction algorithms; filter cutoff 5 mm, 18 subsets; three iterations. Written informed consent was obtained before studies for every patient.
Baseline PET/CT was performed within 14 days before the first cycle of chemotherapy and eotPET/CT was done at least 3 weeks after the completion of therapy.
The PET images were analyzed qualitatively by visual analysis and semi-quantitatively by measuring the maximum standardized uptake value body weight (SUVbw), maximum standardized uptake value lean body mass (SUVlbm), maximum standardized uptake value body surface area (SUVbsa), lesion-to-liver SUVmax ratio (L-L SUV R), lesion-to-blood pool SUVmax ratio (L-BP SUV R), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Readers had knowledge of clinical history, and every focal tracer uptake deviating from physiological distribution and background was regarded as suggestive of disease; it was defined as 18F-FDG activity higher than the surrounding tissue on visual analysis. EotPET/CT was interpreted visually by two nuclear medicine physicians with experience in lymphoma imaging (FB, GB) using the Deauville five-point scale. In case of discordance, a third opinion was requested (DA). According to Deauville Criteria (DC), 18F-FDG PET was interpreted as follows: 1 = no uptake above background, 2 = uptake equal to or lower than mediastinum, 3 = uptake higher than mediastinum and lower than liver, 4 = uptake moderately increased compared to the liver and 5 = uptake markedly increased compared to the liver. With respect to the DC, 18F-FDG PET/CT scans were considered negative for scores 1–3 and positive for scores 4–5.
We measured the SUV, using both algorithms TOF and PSF, of the hypermetabolic lesions by drawing a region of interest (ROI) over the area of maximum activity and the SUVmax was calculated as the highest SUV of the pixels within the ROI. SUVmax of the liver was calculated at the VIII hepatic segment of axial PET images using a round-shape ROI of 10 mm; SUVmax of the blood pool was calculated at the aortic arch by not involving the vessel wall in a similar way. The lesion with the highest 18F-FDG uptake in each study was taken as reference lesion, and the SUVbw, SUVlbm, SUVbsa, L-L SUV R, and L-BP SUV R of that lesion were included for analysis. MTV was measured from attenuation-corrected PET images using a SUV-based automated contouring program (Advantage Workstation 4.6, GE HealthCare) with an isocounter threshold method based on 41% of the SUVmax, as previously recommended by European Association of Nuclear Medicine because of its high inter-observer reproducibility [20]. Total MTV (tMTV) was obtained by the sum of all nodal and extranodal lesions. Bone marrow involvement was included in volume measurement only if there was focal uptake; splenic involvement was considered if there was focal uptake in spleen or diffuse uptake higher than 150% of the liver background. TLG was calculated as the sum of the product of MTV of each lesion and its SUVmean. Semiquantitative analysis was performed by the same nuclear medicine physicians with long experience in lymphoma and in the use of Advantage Workstation 4.6, GE HealthCare for contouring.
Statistical analysis
All statistical analyses were carried out using Statistical Package for Social Science (SPSS) version 23.0 for Windows (IBM, Chicago, Illinois, USA). The descriptive analysis of categorical variables comprised the calculation of simple and relative frequencies. The numeric variables were described as mean, minimum and maximum.
Mann–Whitney test was used to compare baseline metabolic features (SUVbw, SUVlbm, SUVbsa, L-L SUV R, L-BP SUV R, MTV and TLG) of the response (partial or complete response) and progressive metabolic response (progression of disease or relapse) groups at end of treatment.
For the entire population, receiver operating characteristic (ROC) curve analysis was used to identify the optimal cutoff point of semiquantitative parameters in the light of which interpret the results of progression-free survival (PFS) and overall survival (OS) (Table 1). Treatment response was defined according to the Lugano classification [18, 21]. PFS was calculated from the date of baseline 18F-FDG PET/CT to the date of first disease progression, relapse, death or the date of last follow-up detected by subsequent imaging techniques (CT, PET/CT). Progression/relapse was considered when a dimensional and/or numerical increase of lesion or an increase of FDG uptake after baseline PET/CT or the appearance of new lesions was demonstrated. OS was calculated from the date of baseline 18F-FDG PET/CT to the date of death from any cause or to the date of last follow-up. Survival curves were plotted according to the Kaplan–Meier method and differences between groups were analyzed using a two-tailed log rank test. Cox regression was used to estimate the hazard ratio (HR) and its confidence interval (CI). A P value of < 0.05 was considered statistically significant.
Results
Tumor characteristics
Among 87 patients with histological proven MCL, 65 (75%) were male and 22 (25%) female; average age was 64.8 years with a range of 29–88 years. Patients were staged according to the Ann Arbor system as follow: stage I (n = 1), stage II (n = 5), stage III (n = 9) and stage IV (n = 72). B symptoms were present in 23 patients. Bulky disease was recognized in 12 cases (14%) while splenomegaly was identified in 37 patients (43%). LDH level was high in 27 patients and MIPI score was inferior or equal to 2 in 41 cases. Baseline features of the patients are summarized in Table 2. Pathological 18F-FDG uptake could be identified in all 87 patients showing the presence of at least one hypermetabolic lesion. Average SUVbw of the lesion with higher 18F-FDG uptake was 11 (range 3.3–26.2); average SUVlbm was 6.7 (range 2.4–23.2), average SUVbsa was 2.4 (range 0.8–7.2), average L-L SUV R 4.7 (1.3–45), average L-BP SUV R 5.5 (1.5–54), average tMTV 628 cm3 (2–4000 cm3) and average TLG was 3417 (6–23,000) (Table 2). All the patients had 18F-FDG-avid nodal disease; extranodal involvement included bone marrow in 39 (45%) patients and gastrointestinal system in 14 (16%).
Treatment response
Based on Lugano classification metabolic response categories [18], at eotPET/CT 60 (69%) patients had complete response, 17 (20%) patients had partial response and 8 (9%) patients had progression of disease (Fig. 1).Two patients died before performing eotPET/CT (Fig. 2).
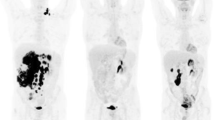
A representative case of a 62-year-old female with stage IVB MCL and both nodal and extranodal (splenic) disease. Baseline maximum intensity projection (MIP, a) showing diffuse hypermetabolic lesion in many cervical, axillar (b), abdominal (c) and pelvic (d) nodes, and splenomegaly. Considering the lesion with higher uptake, SUVbw was 9.6, SUVlbm 6.3, SUVbsa 2.4, L-L SUV R 4.8, L-BP SUV R 5.6 and tMTV 625 and TLG 2447. End-of-treatment MIP (h) revealed no increased 18F-FDG uptake (Deauville score 1) in all body and previous hypermetabolic lesions disappeared (e, f, g)
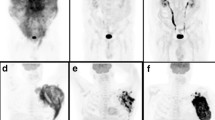
A representative case of a 80-year-old male with stage IV MCL with nodal and splenic disease FDG-positive at MIP (a); axial PET and PET/CT fused images showing FDG-avid lymph nodes in right axillar and mediastinum (b, c) and near to right common iliac artery (d, e). A subsequent PET/CT after 6 cycles of chemotherapy showing progression of disease (Deauville score 5) with new hypermetabolic lesions in left axillar (f, g) and near to left common iliac artery (h, i) and confirming by MIP (l)
There was a statistically significant difference between tMTV and TLG of response group (complete or partial response) and progressive metabolic response group at end-of-treatment PET/CT (p 0.040 and 0.002, respectively; Table 3).
Other metabolic PET/CT features, as SUVbw, SUVlbm, SUVbsa, L-L SUV R and L-BP SUV R showed no statistically significant difference among these two groups. No significant difference was demonstrated comparing only partial response and complete response groups.
Role of 18F-FDG PET/CT in predicting PFS and OS
At a median follow-up of 40 months, relapse or progression of disease occurred in 47 patients with an average time of 20.2 months (range 3–62 months) from the baseline PET/CT and death occurred in 23 patients with an average time of 30.5 months (range 3–125). Among patients who had relapse/progression, negative eotPET/CT scans according to DC were present in 32 cases and positive in 13 (Deauville score 4 in five cases and Deauville score 5 in eight). Among patients who died during the course of the disease, considering eotPET/CT 9 was positive using DC (score 4 in four cases and score 5 in five) and 12 were negative. The median PFS was 30 months (range 3–130 months) and the median OS was 41 months (range 3–130 months). The estimated 2-year PFS and OS rates were 52% and 82%, respectively, while 3-year PFS and OS rates were 48% and 72%, respectively. The description of the baseline functional PET/CT parameters studied is resumed in Table 2, which summarizes the results of the ROC analysis used to identify optimal cutoff points. In univariate analysis, SUVbw, SUVlbm, SUVbsa, L-L SUV R and L-BP SUV R were not related to outcome survival, both for PFS and OS (Fig. 3). Instead, tMTV and TLG were significantly correlated with prognosis considering PFS, while no significant association was discovered analyzing OS (Fig. 4). Also, the presence of bulky disease, the presence of splenomegaly and high Ki-67 score were significantly related to PFS, but not with OS. Other clinical/pathological features (sex, age, MIPI score, LDH level, β2-microglobulin level and blastoid variant) were not related to outcome (Table 4).
PFS was statistically significantly shorter in patients with eotPET/CT DC positive compared to negative, whereas eotPET/CT results according to DC did not predict OS. In fact, patients with a negative eotPET/CT (Deauville score 1–3) had a significantly better outcome, with a 2-year PFS of 70% and a 3-year PFS of 51% versus 33% and 19% (p < 0.001) for patients with positive eotPET (Deauville score 4–5). At multivariate analysis, only metabolic features (Deauville score, MTV and TLG) were confirmed to be independent prognostic factors for PFS (p < 0.001; = 0.049; = 0.019, respectively) (Table 4). Patients with a high tMTV (> 78 cm3) had a significantly shorter PFS, with a 3-year PFS of 36% versus 58% (p < 0.001) for patients with a lower tMTV. The same evidence was not confirmed considering OS: in patients with high tMTV, 3-year OS was 67% versus 75% (p NS) in patients with low MTV. Also, considering patients with high TLG (> 1322), PFS and OS was significantly shorter compared to low-TLG groups, with a 3-year PFS of 32% compared to 55% (p < 0.001); also, for TLG no significant differences in OS are demonstrated (3-year OS 67% vs. 72%; p NS).
Discussion
The molecular hallmark of MCL is the t(11;14) translocation, which results in cyclin D1 overexpression and consequently increased cell proliferation [22, 23]. The high proliferation rate may contribute to MCL aggressiveness and poor clinical outcomes [24]. Despite the introduction of more aggressive treatments, relapses or progression of disease are still frequent, and we need further research into innovative management approaches. Moreover, there is a group of MCL patients with a more indolent course [25] and better identification of these patients may help to streamline management algorithms. Treatment of MCL is a challenge and often stays unsatisfactory and complete responses to the standard chemotherapy regimens are rare. Considering the aggressiveness of this disease and the absence of validated and shared prognostic factors, there will be increased need for pre- and post-therapy PET scans for evaluation of treatment response and prognosis. MCL lymphoma seems to be an FDG-avid lymphoma at presentation, as already described by several authors [9,10,11, 26], especially at nodal site of disease. Instead BM and GI localization seem to be less 18F-FDG avid with value of sensitivity reported in literature low [10,11,12]. Also, in our study, we found that all PET/CT were positive showing the presence of at least a hypermetabolic lesion consistent of MCL. In 2009, it was proposed a five-point scale called Deauville criteria (DC) to evaluate PET/CT results after treatment in lymphoma [27]; this scale using the mediastinum and liver activity as the reference standard has been recommended for reporting both interim and end-of-treatment PET for HL and several NHL [18, 21, 28]. In this study, we tried to validate this score also in MCL and we reached positive evidences; patients with negative DC at end of treatment PET had significantly longer PFS than patients with positive DC. This result was not confirmed considering OS where DC seems not to influence survival. Like us, the other authors [29, 30] showed that eotPET/CT results evaluated with DC are correlated with PFS. Mato et al. [5] studied this issue in non-transplanted subjects treated with the R-Hyper-C-VAD (fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone) regimen, and found results of the end-of-therapy PET scan to be highly predictive of long-term PFS. Instead, Czuczman et al. [31] in a multicenter open-label single-arm phase II study showed that the complete metabolic response at PET/CT was a good predictor of survival. In addition to 18F-FDG PET/CT visual analysis and DC, also semiquantitative and quantitative PET/CT parameters have been studied as prognostic factors and predictor of treatment response. SUV is the most widely used and accepted index in the current published literature for assessing disease activity in lymphoma, but many factors can affect the reliability of this variable, such as time between injection and imaging acquisition, extravasation of administered radiotracer at the site of injection, residual activity in the syringe, partial volume effects, decay of the injected dose, and technological features [32]. Instead, MTV and TLG can be considered a subsequent step because of features which take into account the dimension as well as the metabolic activity [32]. Several studies have shown the prognostic role of MTV and TLG in prognostic field of many lymphomas, both in HL and NHL [33,34,35,36]. However, so far, no specific studies have addressed MCL as a distinct sub-type, focusing on the possible prognostic role of all these 18F-FDG PET/CT parameters. Karam et al. [37] suggested that lower FDG avidity at presentation (SUVmax < 5) predicts an improved OS and disease-free survival (DFS) in patients with MCL. Also, Bodet-Milin et al. [38] stratified patients into high-risk versus low-risk groups using SUVmax cutoff of six. In our study, we did not find any relationship between SUVmax and outcome survival in agreement with other studies [10, 11]; probably due to the differences in the study populations as well as different types of scanners and image acquisition techniques. Other metabolic PET/CT features (SUVlbm, SUVbsa, lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio) were not previously described and so comparison is not possible.
In this study, we demonstrated that baseline total MTV and TLG, which represent a combination of tumor volume and metabolism, were robust predictor of outcome, only for PFS. These parameters include both morphological and metabolic features and may be considered an expression of tumor aggressiveness together with tumor size. Other studies demonstrated significant prognostic value of MTV and TLG in different lymphoma sub-types [39,40,41,42,43,44]. The use of MTV and TLG in clinical practice could probably be premature, because of the lack of a standardized method for their measurement. Different methods are proposed and a wide range of threshold levels have been used to calculate the volume-based PET/CT parameters. We estimated the MTV using an isocontour threshold method based on 41% of the SUVmax, like proposed by Boellard et al. [20]. The real effectiveness of MTV and TLG in risk stratification and the possibility to combine these with other clinical/histological parameters should be evaluated in future studies and could be the key to better stratify MCL patients. In our paper, the main epidemiological (gender, sex), histopathological (Ki-67 score, LDH level, β2-microglobulin level, and blastoid variant) and morphological (splenomegaly and bulky disease) features were not correlated at multivariate analysis with outcome, despite positive correlation for Ki-67 score, bulky disease and splenomegaly at univariate analysis. Many authors agree that the blastoid variant of MCL has a more aggressive clinical behavior with a very poor prognosis [10, 45, 46]. Instead in our paper, blastoid variant seems not to be related with outcome.
Considering OS, no significant prognostic factors were found. Probably, there is unknown factor or combination of several features which influence the risk of death; moreover, the low sensitivity of PET/CT in detecting bone marrow and gastrointestinal disease could explain the absence of prognostic significance of PET/CT parameters considering OS.
The limitations of our study are the retrospective nature of the study design, the relatively low number of patients analyzed, also due to the rarity of the disease, the SUV values calculated using mixed algorithms (TOF and PSF), and the heterogeneous management of patients (as different chemotherapy regimens). Despite this, so far, the present study represents the first series of MCL investigated with an analysis of 18F-FDG PET/CT semiquantitative parameters and their possible prognostic role. In conclusion, in our study, we demonstrated that eotPET/CT results (evaluated according to Deauville criteria) and the metabolic tumor features (MTV and TLG) were significantly correlated with PFS. Moreover, baseline TLG and MTV were significantly lower in patient with response compared to patients without response at the end of therapy.
References
Swerdlow SH, Campo E, Harris NL, et al. World health organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008.
Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132:1647–56.
Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65.
Shah JJ, Fayad L, Romaguera J. Mantle cell international prognostic index (MIPI) not prognostic after R-hyper-CVAD. Blood. 2008;112:2583 author reply 2583-2584.
MatoAr Svodoba J, Feldman T, et al. Post-treatment (not interim) positron emission tomography-computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R-HyperCVAD. Cancer. 2012;118:3565–70.
Katzenberger T, Petzoldt C, Holler S, et al. The Ki67 proliferation index is a quantitative indicator of clinical risk in mantle cell lymphoma. Blood. 2006;107:3407.
Tiemann M, Schrader C, Klapper W, Dreyling MH, Campo E, Norton A, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol. 2005;131:29–38.
Gill S, Wolf M, Miles Prince H, et al. 18F Fluorodeoxyglucose positron emission tomography scanning for staging, response assessment, and disease surveillance in patients with Mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2008;8:158–65.
Alavi A, Shrikanthan S, Aydin A, et al. Fluorodeoxyglucose-positron-emission tomography findings in mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2011;11:261–6.
Brepoels L, Stroobants S, De Wever W, et al. Positron emission tomography in mantle cell lymphoma. Leuk Lymphoma. 2008;49:1693–701.
Hosein PJ, Pastorini VH, Paes FM, et al. Utility of positron emission tomography scans in mantle cell lymphoma. Am J Hematol. 2011;86:841–5.
Cohen JB, Hall NC, Ruppert AS, et al. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transplant. 2013;48:1212–7.
Tateishi U, Tatsumi M, Terauchi T, et al. Relevance of monitoring metabolic reduction in patients with relapsed or refractory follicular and mantle cell lymphoma receiving bendamustine: a multicenter study. Cancer Sci. 2011;102:414–8.
Kedmi M, Avivi I, Ribakovsky E, et al. Is there a role for therapy response assessment with 2-[fluorine-18] fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography in mantle cell lymphoma? Leuk Lymphoma. 2014;55:2484–9.
Magnusson E, Cao Q, Linden MA, et al. Hematopoietic cell transplantation for mantle cell lymphoma: predictive value of pretransplant positron emission tomography/computed tomography and bone marrow evaluations for outcomes. Clin Lymphoma Myeloma Leuk. 2014;14:114–21.
Mei MG, Cao TM, Chen L, et al. Long-term results of high-dose therapy and autologous stem cell transplantation for mantle cell lymphoma: effectiveness of maintenance rituximab. Biol Blood Marrow Transpl. 2017;23:1861–9.
Guidot DM, Switchenko JM, Nastoupil LJ, et al. Surveillance imaging in mantle cell lymphoma in first remission lacks clinical utility. Leuk Lymphoma. 2018;59:888–95.
Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Bomben R, Ferrero S, D’Agaro T, et al. A B-cell receptor-related gene signature predicts survival in mantle cell lymphoma: results from the Fondazione Italiana Linfomi MCL-0208 trial. Hemtaologica. 2018;103:849–56.
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–58.
Fernandez V, Hartmann E, Ott G, et al. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–9.
Leonard JP, Schattner EJ, Coleman M. Biology and management of mantle cell lymphoma. Curr Opin Oncol. 2001;13:342–7.
Garcia M, Romaguera JE, Inamdar KV, et al. Proliferation predicts failure-free survival in mantle cell lymphoma patients treated with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab plus high-dose methotrexate and cytarabine. Cancer. 2009;115:1041–8.
Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–13.
Weiler-Sagie M, Bushelev O, Epelbaum R, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25–30.
Meignan M, Gallamini A, Haioun C, Polliack A. Report on the second international workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma. 2010;51:2171–80.
Albano D, Bosio G, Re A, et al. Metabolic behaviour and prognostic value of early and end of treatment 18F-FDG PET/CT in adult Burkitt lymphoma: role of Deauville and IHP criteria. Leuk Lymphoma. 2018;3:1–8.
Klener P, Fronkova E, Belada D, et al. Alternating R-CHOP and R-cytarabine is a safe and effective regimen for transplant-ineligible patients with a newly diagnosed mantle cell lymphoma. HematolOncol. 2018;36:110–5.
Lamonica D, Graf DA, Munteanu MC, Czuczman MS. 18F-FDG PET for measurement of response and prediction of outcome to relapsed or refractory mantle cell lymphoma therapy with bendamustine-rituximab. J Nucl Med. 2017;58:62–8.
Czuczman MS, Goy A, Lamonica D, et al. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann Hematol. 2015;94:2025–32.
Kostakoglu L, Chauvie S. Metabolic tumour volume metrics in lymphoma. Semin Nucl Med. 2018;48:50–66.
Xie M, Wu K, Liu Y, et al. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol. 2015;32:446.
Albano D, Bertoli M, Battistotti M, et al. Prognostic role of pretreatment 18F-FDG PET/CT in primary brain lymphoma. Ann Nucl Med. 2018;32:532–41.
Procházka V, Klugar M, Bachanova V, et al. Comparing the accuracy of quantitative versus qualitative analyses of interim PET to prognosticate Hodgkin lymphoma: a systematic review protocol of diagnostic test accuracy. BMJ Open. 2016;5:6.
Albano D, Bosio G, Pagani C, et al. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in Burkitt lymphoma. Eur J Nucl Med Mol Imaging. 2019;46:87–96.
Karam M, Ata A, Irish K, et al. DG positron emission tomography/computed tomography scan may identify mantle cell lymphoma patients with unusually favorable outcome. Nucl Med Comm. 2009;30:770–8.
Bodet-Milin C, Touzeau C, Leux C, et al. Prognostic impact of 18F-fluorodeoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from GOELAMS group. Eur J Nucl Med Mol Imaging. 2010;37:1633–42.
Meignan M, Cottereau AS, Versari A, et al. Baseline metabolic tumor volume predicts outcome in high–tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J ClinOncol. 2016;34:3618–26.
Kanoun S, Rossi C, Berriolo-Riedinger A, et al. Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1735–43.
Moskowitz AJ, Schoder H, Gavane S, et al. Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood. 2017;130:2196–203.
Sasanelli M, Meignan M, Haioun C, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:2017–22.
Mikhaeel NG, Smith D, Dunn JT, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43:1209–19.
Albano D, Bosio G, Camoni L, et al. Prognostic role of baseline 18F-FDG PET/CT parameters in MALT lymphoma. Hematol Oncol. 2019;37:39–46. https://doi.org/10.1002/hon.2563.
Bernard M, Gressin R, Lefrère F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15:1785–91.
Weigert O, Unterhalt M, Hiddemann W, Dreyling M. Current management of mantle cell lymphoma. Drugs. 2007;67:1689–702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albano, D., Bosio, G., Bianchetti, N. et al. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in mantle cell lymphoma. Ann Nucl Med 33, 449–458 (2019). https://doi.org/10.1007/s12149-019-01354-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01354-9