Abstract
Purpose
Burkitt’s lymphoma (BL) is an aggressive lymphoma subtype with high 18F-FDG avidity at 18F-FDG-PET/CT, but no validated criteria for PET/CT in treatment evaluation or prediction of outcome in BL are available. The aim of our study was to investigate whether the metabolic baseline PET/CT parameters can predict treatment response and prognosis in BL.
Materials and methods
We retrospectively enrolled 65 patients who underwent baseline 18F-FDG-PET/CT, interim and end of treatment PET/CT. The PET images were analyzed visually and semi-quantitatively by measuring the maximum standardized uptake value body weight (SUVbw), the maximum standardized uptake value lean body mass (SUVlbm), the maximum standardized uptake value body surface area (SUVbsa), lesion to liver SUVmax ratio (L-L SUV R), lesion to blood-pool SUVmax ratio (L-BP SUV R), total metabolic tumor volume (tMTV) and total lesion glycolysis (TLG). Survival curves were plotted according to the Kaplan–Meier method.
Results
At a median follow-up of 40 months, the median PFS and OS were 34 and 39 months. MTV and TLG were significantly higher in patients with partial response compared to complete response group at end of treatment, while no significant differences were found at interim. Other metabolic PET/CT parameters were not related to treatment response. MTV and TLG were demonstrated to be independent prognostic factors for both PFS and OS; instead SUVbw, SUVlbm, SUVbsa, L-L SUV R and L-BP SUV R were not related to outcome survival.
Conclusions
Metabolic tumour features (MTV and TLG) were significantly correlated with response to treatment and long-term outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Burkitt’s lymphoma (BL) is a non-Hodgkin’s lymphoma (NHL) subtype with aggressive nature derived from germinal or postgerminal center B cells and may affect both nodal and extranodal sites. It is divided into three main forms: endemic, sporadic and immunodeficiency-associated variants. BL is extremely chemosensitive and survival ranges from over 80 to 50% related to the stage and age of diagnosis [1,2,3,4,5]. Conventional staging procedures usually include blood tests, bone marrow aspiration and biopsy, and imaging examinations as computed tomography (CT) [6]. The effective role of fluorine-18-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) in the evaluation of BL is not completely clear with several promising studies both in staging and restaging [7,8,9,10,11,12,13], but no shared evidence until now.
In addition to 18F-FDG PET/CT visual analysis and Deauville score scale [14] (which has demonstrated good results in prediction of response and prognosis in lymphomas), semiquantitative and quantitative PET/CT parameters may be obtained. The standardized uptake value (SUV) is the most widely used and generally accepted index in the current published literature for assessing disease activity in lymphoma, but many factors can affect the reliability of SUV, such as time between injection and imaging acquisition, partial volume effects, extravasation of administered 18F-FDG at the site of injection, residual activity in the syringe, decay of the injected dose, technological characteristic and parameters [15]. Moreover, in assessing tumor response using SUV, the quantification of small lesions dimensions could be underestimated. Thus, a different feature that takes into account the size, as well as the metabolic activity of the tumor is required. Metabolic tumour value (MTV) and total lesion glycolysis (TLG) are parameters which reflect both the size of a lesion and its aggressiveness [15]. Several studied demonstrated the prognostic role of MTV and TLG in survival outcome of many lymphomas, especially HL and DLBCL [16, 17].
Nowadays, no studies about the prognostic usefulness of these metabolic 18F-FDG PET/CT features in BL are available.
The aim of our study was to investigate whether the metabolic baseline PET/CT parameters can predict treatment response (at interim and end of treatment) and prognosis in BL.
Materials and methods
Patients
Between January 2007 and January 2017, 65 patients with histologically proven BL were retrospectively enrolled.
The Ann Arbor system was used to classify these patients at the time of diagnosis. We reviewed the medical records and pathology reports of these patients: epidemiological features (age at diagnosis, gender, immune system condition), morphological features (extranodal or nodal localization, bulky disease), clinical data (B symptoms, IPI score, LDH level) and metabolic features by 18F-FDG PET/CT, treatment modality and follow-up data were collected and analyzed in all patients. International prognostic index (IPI) score and lactate-dehydrogenase (LDH) level were dichotomized using a cutoff value of 2 and 245 U/L, respectively, and tumor stage according to Ann Arbor classification in early (I and II) and advanced (III and IV) stage. Bulky disease was defined when the maximum width is equal or greater than one third of the internal transverse diameter of the thorax or at an alternative site was defined as any mass measuring 10 cm or more by any imaging study.
All patients were treated according to the institution’s standard protocol with a chemotherapy regimen. Fifty-five patients were treated according to the B-ALL/NHL 2002 protocol of the GMALL [18] with six cycles of immuno-chemotherapy including methotrexate, cytosine arabinoside, cyclophosphamide, etoposide, ifosphamide, vincristine, adriamycin and rituximab-corticosteroids alternating every 3 or 4 weeks followed by two additional courses of rituximab afterward. The remaining 10 patients received short-term intensified chemo-immunotherapy (rituximab, vincristine, etoposide, doxorubicin, methotrexate, cytarabine, prednisone) according to CARMEN regimen [19].
18F-FDG PET/CT imaging and interpretation
All patients underwent baseline 18F-FDG PET/CT before chemotherapy, a subsequent PET/CT after two cycles of chemotherapy (interim PET/CT) and then at completion of therapy after six cycles (end of treatment PET/CT). 18F-FDG-PET/CT was performed after at least 6 h fasting and with glucose level lower than 150 mg/dL. An activity of 3.5–4.5 MBq/Kg of 18F-FDG was administered intravenously and images were acquired 60 min after injection from the skull basis to the mid-thigh on a Discovery ST PET/CT tomograph (General Electric Company—GE®—Milwaukee, WI, USA) with standard parameters (CT: 80 mA, 120 Kv without contrast; 2.5–4 min per bed-PET-step of 15 cm); the reconstruction was performed in a 128 × 128 matrix and 60 cm field of view. Patients were instructed to void before imaging acquisition, no oral or intravenous contrast agents were administrated or bowel preparation used for any patient; written consent was obtained before studies. Baseline PET/CT was performed within 14 days before the first cycle of chemotherapy; interim PET/CT was made within the week before the third cycle (median 4 days before third cycle, range 1–7 days) and end of treatment PET/CT was done at least 3 weeks after the completion of therapy.
The PET images were analyzed visually and semi-quantitatively by measuring the maximum standardized uptake value body weight (SUVbw), maximum standardized uptake value lean body mass (SUVlbm), maximum standardized uptake value body surface area (SUVbsa), lesion to liver SUVmax ratio (L-L SUV R), lesion to blood-pool SUVmax ratio (L-BP SUV R), MTV and TLG. Readers had knowledge of clinical history, and every focal tracer uptake deviating from physiological distribution and background was regarded as suggestive of disease; it was defined as 18F-FDG activity higher than the surrounding tissue on visual analysis. We measured the SUV of the hypermetabolic lesions by drawing a region of interest (ROI) over the area of maximum activity and the SUVmax was calculated as the highest SUV of the pixels within the ROI. SUVmax of the liver was calculated at the VIII hepatic segment of transaxial PET images using a round-shape 10 mm ROI; SUVmax of the blood-pool was calculated at the aortic arch by use of transaxial PET images with a round-shape 10 mm ROI not involving the vessel wall. The lesion with the highest 18F-FDG uptake in each patient was taken as reference lesion and the SUVbw, SUVlbm, SUVbsa, L-L SUV R, L-BP SUV R of that lesion were calculated for analysis. MTV was measured from attenuation-corrected 18F-FDG-PET images using a SUV-based automated contouring program (Advantage Workstation 4.6, GE HealthCare) with an isocounter threshold method based on 41% of the SUVmax, as previously recommended by European Association of Nuclear Medicine because of its high inter-observer reproducibility [20]. Total MTV (tMTV) was obtained by the sum of all nodal and extranodal lesions. Bone marrow involvement was included in volume measurement only if there was focal uptake; splenic involvement was considered if there was focal uptake in spleen of diffuse uptake higher than 150% of the liver background. TLG was calculated as the sum of the product of MTV of each lesion and its SUVmean.
Statistical analysis
All statistical analysis was carried out using Statistical Package for Social Science (SPSS) version 23.0 for Windows (IBM, Chicago, IL, USA) and MedCalc Software version 17.1 for Windows (Ostend, Belgium). The descriptive analysis of categorical variables comprised the calculation of simple and relative frequencies. The numeric variables were described as mean, minimum and maximum.
Mann-Whitney test was used to compare baseline metabolic features (SUVbw, SUVlbm, SUVbsa, L-L SUV R, L-BP SUV R, MTV and TLG) of the partial and complete response groups at interim and after treatment completion.
For the entire population, receiver operating characteristic (ROC) curve analysis was used to identify the optimal cutoff point of semiquantitative parameters in the light of which interpret the results of progression free survival (PFS) and overall survival (OS) (Table 2). Treatment response was defined according to the Lugano classification [21, 22]; Deauville score 3 at the end of treatment was considered negative scan. PFS was calculated from the date of baseline 18F-FDG PET/CT to the date of first disease progression, relapse, death or the date of last follow-up. OS was calculated from the date of baseline 18F-FDG PET/CT to the date of death from any cause or to the date of last follow-up. Survival curves were plotted according to the Kaplan–Meier method and differences between groups were analyzed by using a two-tailed log rank test. Cox regression was used to estimate the hazard ratio (HR) and its confidence interval (CI). A p value of <0.05 was considered statistically significant.
Results
Tumors characteristics
Among 65 patients with histological proven BL, 43 (66%) were male and 22 (34%) female; average age was 53 years with a range of 18–80 years. Patients were staged according to the Ann Arbor system as follows: stage I (n = 12), stage II (n = 5), stage III (n = 6) and stage IV (n = 42). B-symptoms were present in 29 patients; 49 (75%) patients were immunocompetent, while the remaining 16 patients (25%) were immune compromised, including 14 patients with HIV infection and two patients with post-transplant lymphoproliferative disease. Bulky disease was recognized in 21 cases (32%) while extranodal BL involvement was identified in 42 patients (65%). LDH level was high in 39 patients and IPI score was superior or equal to 2 in 43 cases. Baseline features of the patients are summarized in Table 1. Among immunocompetent and immunodeficient patients no significant differences were present considering the main epidemiological and clinical features (age, sex, LHD, IPI score, bulky disease, stage). Baseline PET/CT was positive in all 65 patients showing the presence of at least one hypermetabolic lesion. Average SUVbw of the lesion with higher 18F-FDG uptake was 16.1 (range 3.3–62); average SUVlbm was 12.1 (range 2.4–41), average SUVbsa was 4.4 (range 0.9–12), average L-L SUV R 7.8 (1.7–27.5), average L-BP SUV R 9.3 (1.85–29), average tMTV 408 cm3 (6.1–3000 cm3) and average TLG was 4153 (17–22,000) (Table 1).
Treatment response
Based on Lugano classification metabolic response categories [21], 44 patients had complete response and 21 patients had partial response at interim PET/CT, while 50 had complete response, 11 partial response and four progression of disease at end of treatment compared to baseline scan (Figs. 1 and 2).
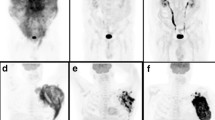
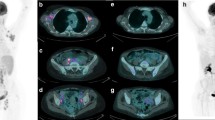
A representative case of a 57-year-old male with stage IVB BL and both nodal and extranodal disease. Baseline maximum intensity projection (MIP, a) showing diffuse hypermetabolic lesion in many abdominal nodes, liver, kidney and laterocervical nodes. Considering the lesion with higher uptake, SUVbw was 25, SUVlbm 19, SUVbsa 7, L-L SUV R 16, L-BP SUV R 21 and tMTV 270 and TLG 4102. Interm PET/CT (b) after two cycles after chemotherapy showing a complete metabolic response with no 18F-FDG uptake; end of treatment MIP (c) revealed new increased 18F-FDG uptake in abdomen, considering a partial response compared to baseline scan. This patient died 24 months after baseline PET/CT
Another example of a 31-year-old male, stage IA with left axillary adenopathies at baseline MIP (a) and SUVbw of 12, SUVlbm of 9.7, SUVbsa of 4, L-L SUV R of 7.5, L-BP SUV R 11, tMTV of 127 cm3and TLG of 980. Subsequent interim MIP (b) demonstrating only weak uptake in the left axel and last MIP (c) at the end of therapy showing complete metabolic response. After a follow-up time of 72 months, this patient did not develop relapse and was alive
There was statistically significant difference between tMTV and TLG of complete response and partial response group at end of treatment PET/CT (p= 0.018 and 0.027, respectively; Table 3), while no significant difference was found at interim between complete response and partial response groups (p = 0.979 and p = 0.510, respectively).
Other metabolic PET/CT features, as SUVbw, SUVlbm, SUVbsa, L-L SUV R and L-BP SUV R showed no statistically significant difference among complete response and partial response group both on interim and end of treatment.
Role of 18F-FDG PET/CT in predicting the progression-free survival and overall survival
At a median follow-up of 40 months, relapse or progression of disease occurred in 21 patients with an average time of 16.7 months (range: 4–82 months) from the baseline PET/CT and death occurred in 15 patients with an average time of 10.5 months (range 6–55). The median PFS was 34 months (range 4–138 months) and the median OS was 39 months (range 6–138 months). The estimated 3-year PFS and OS rates were 62 and 70%, respectively, while 5-year PFS and OS rates were 52 and 60%, respectively. The description of the baseline functional PET/CT parameters studied is resumed in Table 2, which summarizes the results of the ROC analysis used to identify optimal cutoff points (Table 3).
In univariate analysis, SUVbw, SUVlbm, SUVbsa, L-L SUV R and L-BP SUV R were not related to outcome survival, both for PFS and OS (Fig. 3). Instead, tMTV and TLG were significantly correlated with prognosis (Fig. 4). Other clinical/pathological features (sex, tumor stage, immune system condition, IPI score, LDH level and bulky disease) were not related to outcome. Also in multivariate analysis, tMTV and TLG were confirmed to be independent prognostic factors for both PFS (p = 0.008 and p = 0.009) and OS (p = 0.005 and p = 0.012) (Table 4).
Patients with a high tMTV (≥231 cm3) had a significantly worse outcome, with a 3-year PFS of 27% versus 89% (p < 0.001) for patients with a lower tMTV. The same evidence resulted considering OS in patients with high tMTV (≥228 cm3) with a 3-year OS of 32% versus 93% (p < 0.001) in patients with MTV < 228 cm3. Only two patients with low-tMTV values died and three had relapse in the follow-up time.
Also, considering patients with high TLG (≥2891 for PFS and ≥ 1089 for OS) PFS and OS was significantly shorter compared to low-TLG groups, with a 3-year PFS of 30% and 3-year OS of 38% compared to 84 and 91% (p < 0.001). Among patients with low-TLG only two died and five relapsed.
Discussion
In addition to the standard qualitative visual analysis, there is increasing evidence of the prognostic value of semiquantitative parameters from initial staging 18FDG-PET/CT in patients with high-grade NHL and HL [14, 15]. Initially, SUV was the most widely studied parameter with promising results [23,24,25], but SUV has many limitations, as explained in the introduction section, and its prognostic role is not yet established. More recently, volume-based metabolic assessment, including MTV and TLG, has emerged and seems useful in evaluation of treatment response and outcome both in HL [26,27,28] and NHL [28,29,30,31]. However, so far, no specific studies have addressed BL as a distinct subtype, focusing on the possible prognostic role of 18F-FDG PET/CT parameters.
BL is a lymphoma with high cell turnover and with high 18F-FDG avidity, as previously demonstrated in some studies [8, 9, 13, 32], and it may be considered an 18F-FDG-avid lymphoma as HL, diffuse large B-cell lymphoma and follicular lymphoma. In the literature, average SUVmax of BL ranges from 12.1 to 18.3 [8, 11, 12]; in our study, we had a mean SUVmax of 16.1, a value similar to that reported in other articles; this could explain the high aggressiveness of this NHL subtype. Other metabolic PET/CT features (SUVlbm, SUVbsa, lesion to liver SUVmax ratio and lesion to blood pool SUVmax ratio) were not previously described.
A standardized system to evaluate treatment response in patients affected by lymphoma is clinically important for many different reasons; the Lugano recommendations routinely recommend 18F-FDG PET/CT before and after treatment in tracer avid lymphoma demonstrating good prognostic value of interim and end of treatment PET/CT scan; instead, the role of PET/CT is less clear in those subtypes considered to have variable 18F-FDG avidity as mucosa associated lymphatic tissue lymphoma [33]. There are very limited available data on the role and prognostic value of interim and post-therapy 18F-FDG PET/CT in Burkitt’s lymphoma [10, 12] and there is no validated criteria for evaluation of PET/CT response in these patients. Albano et al. [13] showed that both Deauville score and IHP criteria results at end of treatment significantly predicted outcome (OS and PFS) in BL while no significant correlation were demonstrated considering interim PET/CT results. Also, in our study, we founded a positive correlation between PET/CT and end of treatment response considering tMTV and TLG. In fact tMTV and TLG were significantly higher in patients with partial response than complete response after the end of chemotherapy. Some articles evaluated the prognostic role of 18F-FDG PET/CT in BL considering ΔSUVmax, a parameter which represents the reduction in the maximum standardizes uptake value between basal and after therapy scans. Wei et al. [12] compared three different ΔSUVmax threshold (25%, 50 and 75%) to predict OS and PFS and they demonstrated that the optimal cutoff ΔSUVmax from the baseline PET/CT to post-therapy PET/CT that could predict significantly OS was 50%. Carrillo-Cruz et al. [11] founded ΔSUVmax<66% as the better cutoff for evaluation of treatment response with positive predictive value and negative predictive value of 100%. Also, in a recent multicenter study ΔSUV demonstrated to be a prognostic parameter in aggressive non-Hodgkin lymphoma, including Burkitt’s lymphoma [26].
In this study, we demonstrated that baseline total MTV and TLG, which represent a combination of tumor volume and metabolism, were the most robust predictor of outcome, both for PFS and OS. These parameters include both morphological and metabolic features and may be considered an expression of tumor aggressiveness together with tumor size characteristic. Other studies demonstrated significant prognostic value of MTV and TLG in different lymphoma subtypes [27,28,29,30,31,32] and confirmed our results. The use of MTV and TLG in clinical practice could probably be premature, because of the lack of a standardized methodology for their estimation. Different methods are available and a wide range of threshold levels have been proposed to calculate the volume-based PET/CT parameters. Moreover, there are no published technical references about the methodology for the calculation of the volume based PET parameters and definition of specific cutoff values in BL. We estimated the MTV of BL using an isocontour threshold method based on 41% of the SUVmax, such as proposed by Boellard et al. [20] for the evaluation of tumoral lesions and tested by several authors both in HL and NHL [32, 34]. But also other thresholds are studied with positive impact and many open questions stay [35,36,37]. Despite this, promising results concerning the predictive role of metabolic tumour parameters are present, especially in DLBCL [36, 37]. Probably the integration of metabolic parameters together with genetic/histological features could help to better classify and evaluate these lymphoma [37]. Only one study [38] described MTV and TLG in BL; in that study 67 patients with primary extranodal lymphoma were evaluated, and among them four BL were recognized with an average MTV of 156.9 cm3 and TLG of 1065.3; these values were lower than ours but difficult to compare considering the different sample analyzed and the different clinical and morphological presentation of disease. In our study, MTV seem to be more significant than TLG; this is unexpected because TLG is simply the sum of the SUV of the different voxels included in the total MTV calculation and we have demonstrated that SUV metrics were not prognostic.
The real effectiveness of MTV and TLG in risk stratification and the possibility to combine TLG with other clinical or imaging parameters should be evaluated in future studies.
Other metabolic baseline 18F-FDG PET/CT parameters (SUVbw, SUVlbm, SUVbsa, L-L SUV R and L-BP SUV R) seemed not to be related to survival outcome of BL patients.
The limitations of our study are the retrospective nature of the study design and the relatively low number of patients analyzed, also due to the rarity of the disease. Despite this, so far, the present study represents the first series of BL investigated with an analysis of 18F-FDG PET/CT semiquantitative parameters and their possible prognostic role. In conclusion, in our study we demonstrated that metabolic tumour features (MTV and TLG) were significantly correlated with outcome survival, both for PFS and OS. Moreover TLG and MTV were significantly lower in patient with complete response compared to partial response group at the end of therapy.
References
Swerdlow SH, Campo E, Harris NL, et al. World Health Organization classification of Tumours of Haematopoietic and lymphoid tissues. Lyon: IARC press; 2008.
Bishop PC, Rao VK, Wilson WH. Burkitt’s lymphoma: molecular pathogenesis and treatment. Cancer Investig. 2000;18:574–83.
Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104:3009–20.
Wästerlid T, Brown PN, Hagberg O, Hagberg H, Pedersen LM, D’Amore F, et al. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic lymphoma group. Ann Oncol. 2013;24:1879–86.
Molineux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt’s lymphoma. Lancet. 2012;379:1234–44.
Sandlund JT. Burkitt lymphoma: staging and response evaluation. Br J Hematol. 2012;156:761–5.
Just PA, Fieschi C, Baillet G, Galicier L, Oksenhendler E, Moretti JL. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in AIDS-related Burkitt lymphoma. AIDS Patient Care STDs. 2008;22:695–700.
Zeng W, Lechowicz MJ, Winton E, Cho SM, Galt JR, Halkar R. Spectrum of FDG PET/CT findings in Burkitt lymphoma. Clin Nucl Med. 2009;4:355–8.
Karantanis D, Durski JM, Lowe VJ, Nathan MA, Mullan BP. Georgiou E, et al. 18F-FDG PET and PET/CT in Burkitt’s lymphoma. Eur J Radiol. 2012;75:e68–73.
Bailly C, Eugène T, Couec ML, Strullu M, Frampas E, Campion L, et al. Prognostic value and clinical impact of (18)FDG-PET in the management of children with Burkitt lymphoma after induction chemotherapy. Front Med (Lausanne). 2014;16(1):54.
Carrillo-Cruz E, Mar ın-Oyaga VA, Rodrıguez MS, Borrego-Dorado I, de la Cruz Vicente F, Quiroga Cantero E, et al. Role of 18F-FDG-PET/CT in the management of Burkitt lymphoma. Eur J Haematol. 2015;94:23–30.
Wen WX, Huang JJ, Li WY, Zhang X, Xia Y, Jiang WQ, et al. Prognostic values of interim and post-therapy 18F-FDG PET/CT scanning in adult patients with Burkitt’s lymphoma. Chin J Cancer. 2015;2(34):608–13.
Albano D, Bosio G, Re A, Pagani C, Giubbini R, Bertagna F. Metabolic behaviour and prognostic value of early and end of treatment 18F-FDG PET/CT in adult burkitt lymphoma: role of Deauville and IHP criteria. Leuk Lymphoma. 2018. https://doi.org/10.1080/10428194.2018.1482541.
Meignan M, Gallamini A, Haioun C, Polliack A. Report on the second international workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma. 2010;51:2171–80.
Kostakoglu L, Chauvie S. Metabolic tumour volume metrics in lymphoma. Semin Nucl Med. 2018;48:50–66.
Xie M, Wu K, Liu Y, Jiang Q, Xie Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol. 2015;32:446.
Procházka V, Klugar M, Bachanova V, Klugarová J, Tučková D, Papajík T. Comparing the accuracy of quantitative versus qualitative analyses of interim PET to prognosticate Hodgkin lymphoma: a systematic review protocol of diagnostic test accuracy. BMJ Open. 2016;5:6.
Hoelzer D, Walewski J, Döhner H, Viardot A, Hiddemann W, Spiekermann K, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124:3870–9.
Ferreri AJ, Bruno Ventre M, Donadoni G, Cattaneo C, Fumagalli L, Foppoli M, et al. Safety and activity of a new intensive short-term chemoimmunotherapy in HIV-positive patients with Burkitt lymphoma. Br J Haematol. 2012;159:252–5.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–58.
Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50:527–33.
Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, et al. Groupe d’etude des lymphomes de l’adulte (GELA). SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118:37–43.
Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40:1312–20.
Dührsen U, Müller S, Hertenstein B, Thomssen H, Kotzerke J, Mesters R, et al. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): a multicenter, randomized phase III trial. J Clin Oncol. 2018;36:2024–34.
Kanoun S, Rossi C, Berriolo-Riedinger A, Dygai-Cochet I, Cochet A, Humbert O, et al. Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1735–43.
Moskowitz AJ, Schoder H, Gavane S, Thoren KL, Fleisher M, Yahalom J, et al. Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood. 2017;130:2196–203.
Sharma P, Gupta A, Patel C, Bakhshi S, Malhotra A, Kumar R. Pediatric lymphoma: metabolic tumor burden as quantitative index for treatment response evaluation. Ann Nucl Med. 2012;26:58–66.
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:2017–22.
Mikhaeel NG, Smith D, Dunn JT, Phillips M, Møller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43:1209–19.
Meignan M, Cottereau AS, Versari A, Chartier L, Dupuis J, Boussetta S, et al. Baseline metabolic tumor volume predicts outcome in high–tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;34:3618–26.
Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25–30.
Cottereau AS, Becker S, Broussais F, Casasnovas O, Kanoun S, Roques M, et al. Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL). Ann Oncol. 2016;27:719–24.
Cottereau AS, Buvat I, Kanoun S, Versari A, Casasnovas O, Chauvie S, et al. Is there an optimal method for measuring baseline metabolic tumor volume in diffuse large B cell lymphoma? Eur J Nucl Med Mol Imaging. 2018;450:1463–4.
Cottereau AS, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H, et al. Molecular profile and FDG-PET/CT Total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22:3801–9.
Toledano MN, Desbordes P, Banjar A, Gardin I, Vera P, Ruminy P, et al. Combination of baseline FDG PET/CT total metabolic tumour volume and gene expression profile have a robust predictive value in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2018;45:680–8.
Okuyucu K, Ozaydin S, Alagoz E, Ozgur G, Ince S, Oysul FG, et al. Prognosis estimation under the light of metabolic tumor parameteres on initial FDG-PET/CT in patients with primary extranodal lymphoma. Radiol Oncol. 2016;50:360–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participant included in the study.
Rights and permissions
About this article
Cite this article
Albano, D., Bosio, G., Pagani, C. et al. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in Burkitt lymphoma. Eur J Nucl Med Mol Imaging 46, 87–96 (2019). https://doi.org/10.1007/s00259-018-4173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4173-2