Abstract
Objective
The recurrence rate of meningioma after surgery is high, and progression is often observed. The risk factors for recurrence and progression are not clear. We evaluated the risk factors for recurrence and progression in meningioma using 11C-methionine (MET) positron emission tomography (PET).
Methods
Thirty-seven patients (mean follow-up, 80 months) with an intracranial meningioma were enrolled. MET PET was performed before treatment between 1995 and 2010, and patients were followed up in an out-patient clinic. Surgery was performed in 33 patients, and a wait-and-see approach was taken in four patients. We evaluated the extent of tumor resection, location, WHO grade, Ki-67 labeling index, and lesion to normal ratio (LN ratio) of MET uptake.
Results
Six of the surgical cases had a recurrence, and two of the observation-only patients had tumor progression. A high LN ratio of MET uptake was a significant risk factor for recurrence and progression with univariate analysis. The area under the curve of receiver operating characteristic curve for the LN ratio of MET uptake was 0.754, and the optimal cutoff value was 3.18 (sensitivity 63 %, specificity 79 %). With multivariate analysis, a high LN ratio of MET uptake, non-gross total resection, and a high WHO grade were significant risk factors for progression and recurrence.
Conclusion
A high LN ratio of MET uptake was a risk factor for tumor progression and recurrence. The advantage of MET PET is that it is not invasive and can easily be used to evaluate the whole tumor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningioma is the most common primary brain tumor in adults. The frequency of meningioma among all types of brain tumors is 26.4 % in Japan [1] and 34.4 % in the United States. Many histopathological subtypes exist. Most meningiomas are benign, but World Health Organization (WHO) grade II and grade III meningiomas, which exhibit aggressive clinical behavior, are found in 10 % of patients with meningioma. We usually perform surgery for symptomatic cases or cases with large tumors. For small and asymptomatic cases, a wait-and-see approach is taken. However, gross total resection (GTR) is difficult in some surgical cases because of the tumor location and invasion into the brain tissue and the venous sinus. The residual tumor often recurs with malignancy, making the patient’s prognosis poor. Meningiomas that are only observed sometimes progress and require surgical resection. In previous papers, the recurrence rate after surgery was high. Even if the tumor is removed completely, the recurrence rate is between 7 and 32 %. After subtotal resection, the recurrence rate is between 19 and 50 % [2–4].
The risk factors for progression and recurrence in meningioma are not clear, and clarification of these factors is important for determining surgical indications and treatment strategies. We usually use the Ki-67 labeling index (LI) to evaluate the proliferative activity, but surgery is required to obtain a tissue specimen. Surgery is invasive for the patient, and evaluating the risk of recurrence with the Ki-67 LI is controversial because the tissue specimen sometimes does not reflect the whole tumor.
In this study, we evaluated 11C-methionine (MET) uptake of the whole tumor using MET positron emission tomography (PET) to investigate the risk factors for recurrence and progression.
Methods
Patients
From a database of patients who were examined with MET PET, we retrospectively retrieved data for all 73 patients who were diagnosed with intracranial meningioma between 1995 and 2010. These cases were not a consecutive series. We could not examine MET PET results for all meningioma cases because the number of cases that could be examined by MET PET per week in our facilities is limited. Thirty-seven patients fulfilled the inclusion criteria for this study: (1) patients were initially diagnosed with meningioma; (2) MET PET was performed before surgery or observation; (3) patients were followed at Osaka City University Hospital or affiliated hospitals; (4) during the follow-up period, no additional treatment was performed other than the first surgery. Thirty-three patients were excluded because of recurrence after surgery, and three patients dropped out during the follow-up period. Thus, 37 cases (23 females and 14 males) were enrolled in this study (Fig. 1). The mean age of the patients was 54.5 ± 12.9 years. All study participants provided informed consent, and the study design was approved by an ethics review board.
MET PET study
All patients underwent a MET PET scan with HEADTOME-IV (BGO, Shimadzu, Japan) between 1995 and 2005, Eminence-B (BGO) since 2005, and Biograph-16 (LSO, Siemens, Germany) since 2010. Twenty-six patients were examined with HEADTOME-IV. Axial and in-plane resolutions of the PET images were each 4.5 mm (in full width at half maximum), and the slice thickness was 4 mm. Twenty minutes after MET injection (4 MBq/kg), an emission scan of the brain was performed for 10 min. The emission scan was reconstructed to a matrix of 128 × 128 (using an iterative algorithm), and attenuation and scatter correction were done. The voxel size was 2 × 2 × 3.25 mm.
Ten patients were examined with Eminence-B. Axial and transaxial resolutions of the PET were each 4.5 mm (in full width at half maximum). The injection volume and timing of the scan were the same as HEADTOME-IV. The emission scan was reconstructed to a matrix of 128 × 128, and attenuation and scatter correction were done. The voxel size was 2 × 2 × 3.25 mm.
One patient was examined with Biograph-16. Axial and transaxial resolutions of the PET were 5.5 and 5.9 mm (in full width at half maximum), respectively. The injection volume and timing of the scan were the same as HEADTOME-IV. The emission scan was reconstructed to a matrix of 336 × 336, and attenuation and scatter correction were done. The voxel size was 1.02 × 1.02 × 2 mm.
All MET PET images were interpreted by an experienced neurosurgeon. The MET uptake was calculated by drawing a region of interest (ROI) using a freehand procedure. In all cases, MET uptake of the lesion was higher than in normal gray matter. In cases with a multiple meningioma, the lesion with the highest mass was evaluated. From the tumor lesion and normal reference region (frontal lobe of the normal side), the lesion to normal ratio (LN ratio) of mean MET uptake was calculated.
Surgical resection, pathological findings, and clinical follow-up
Thirty-three cases were treated with surgery, and four cases were observed. In surgical cases, GTR (Simpson grade I or II) was performed in 18 cases (55 %), and subtotal resection (Simpson grade III or IV) was performed in 13 cases (39 %). Partial resection (Simpson grade IV) was performed in one case (3 %), and a biopsy (Simpson grade V) was performed in one case (3 %). The pathological diagnosis and the WHO grade were determined by experienced pathologists according to the WHO classification updated in 2007. The Ki-67 LI was also calculated. All patients were followed up at our out-patient clinic without any additional treatment for the tumor during the follow-up period. For the surgical cases, gadolinium (Gd)-enhanced magnetic resonance imaging (MRI) was performed every 3–6 months in the first 2 years after surgery, and then every year during the follow-up period. For the observation cases, Gd-enhanced MRI was performed more than once a year. The mean follow-up period was 80 ± 52 months (range 4–180 months). In surgical cases, the lesion was defined as a ‘recurrence’ when a lesion was found at the same location or a residual lesion was obviously enlarged in the radiological examinations. In non-surgical observation cases, the lesion was defined as a ‘progression’ when the tumor size was obviously enlarged in the radiological examinations.
We evaluated the risk factors for recurrence and progression by age, gender, location (skull base or not), extent of resection (GTR or not), Ki-67 LI, and LN ratio of MET uptake.
Statistical analysis
We evaluated the risk factors for recurrence and progression using paired t tests. When the data were not normally distributed, Wilcoxon’s rank-sum test was used for continuous data. Fisher’s exact tests were used for categorical data. Cox proportional hazards regression analysis was used for the surgical cases to assess the predictors of recurrence and progression with duration of the recurrence-free period as the time variable. A receiver operating characteristics (ROC) curve was assessed to confirm the best cutoff value of the LN ratio for recurrence and progression. All statistical analysis was performed using JMP 9 software (SAS Institute Inc.).
Results
Characteristics and pathology
During the follow-up period, six surgical patients had a recurrence, and two observation patients progressed. The characteristics of the 37 cases are shown in Table 1. Summaries of the recurrence group and the non-recurrence group are shown in Table 2. The mean age of the recurrence group was 57.9 ± 11.8 years, and that of the non-recurrence group was 53.6 ± 13.2 years. We found no significant difference in the numbers of males and females in each group.
The tumor location is shown in Table 1. We classified the tumor location into two groups: skull base and non-skull base. The recurrence rate was not significantly different between these two groups.
Two patients died during the clinical follow-up period. One (case 20) died of thyroid cancer 51 months after PET examination, and another (case 21) died due to tumor progression 4 months after PET examination. The tumors were classified by pathology as follows. Ten were meningothelial (30 %), nine were fibrous (27 %), eight were transitional (24 %), two were angiomatous (6 %), two were chordoid (6 %), one was secretory (3 %), and one was atypical (3 %). Thirty cases were WHO grade I meningiomas, and three cases were WHO grade II meningiomas. The recurrence rate was not significantly different between WHO grade I (17 %, 5/30 cases) and grade II (33 %, 1/3 cases). The mean LN ratio of WHO grade I meningiomas was 2.99 ± 1.07, and the mean LN ratio of WHO grade II meningiomas was 2.35 ± 0.36. The LN ratio was not significantly different between WHO grade I and grade II.
Extent of tumor resection and recurrence
Gross total resection was performed in 18 patients, and one patient (case 35) had a recurrence during clinical follow-up. In 15 patients, some tumor remained after the surgery. In this non-GTR group, recurrence of meningioma was observed in five patients. The recurrence rate was not significantly different between the non-GTR group and the GTR group (p = 0.053).
LN ratio of MET PET and Ki-67 LI for progression and recurrence
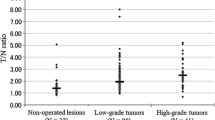
During the clinical follow-up, six cases of recurrence and two cases of progression were found. The average LN ratio of these eight cases was 3.67 ± 1.15 [95 % confidence interval (CI) 2.71–4.64] and that of the remaining 29 cases was 2.65 ± 0.86 (95 % CI 2.32–2.98). The average LN ratio of the cases with recurrence and progression was higher than that of the cases without recurrence or progression (p < 0.01, Fig. 2). The average Ki-67 LI of the recurrent six cases was 1.81 ± 1.21 (95 % CI 0.54–3.09), and that of the 27 cases without recurrence was 3.06 ± 3.84 (95 % CI 1.54–4.58). The Ki-67 LI was not significantly different between the recurrence group and the non-recurrence group (p = 0.44). No correlation was found between the LN ratio and the Ki-67 LI (Fig. 3). Risk factors evaluated with univariate analysis are summarized in Table 2. One illustrative case is shown in Fig. 4.
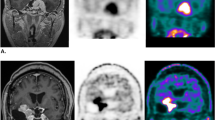
Case 36. a Preoperative Gadolinium (Gd)-enhanced T1-weighted image. The tumor is located at the clivus. b 11C-methionine was taken up into the tumor. The LN ratio was 4.35. c, d Photomicrograph of a sample of the lesion. Hematoxylin and eosin-stained section (c ×100) and Ki-67 staining (d ×200) of a meningioma tissue specimen. The diagnosis based on pathology was meningothelial meningioma. The Ki-67 LI was 0.5. e Postoperative Gd-enhanced T1-weighted image. In this case, the tumor was partially removed using a trans-sphenoidal approach. f Gd-enhanced T1-weighted image 15 months after surgery. The tumor had begun to grow again
In our study, the LN ratio was a significant risk factor for recurrence and progression with univariate analysis. We also evaluated risk factors using multivariate analysis. The results are summarized in Table 3. Multivariate analysis showed that the LN ratio, the extent of resection, and the WHO grade were significant risk factors for recurrence and progression. The hazard ratio of the LN ratio was 4.21. The LN ratio was the only factor examined preoperatively.
ROC curve analysis
A ROC curve was generated, and the area under the curve (AUC) was calculated to determine the best discriminating level of the LN ratio for predicting recurrence and progression. ROC analysis confirmed 3.18 as the best predictive cutoff value of the LN ratio for recurrence and progression. The AUC was 0.754. Using the best cutoff value of 3.18, the sensitivity and specificity were 63 and 79 %, respectively (Fig. 5).
Discussion
The risk factors for recurrence and progression in meningioma have been reported in many previous studies. They include age [5, 6], gender [7], tumor size [8], calcification [7, 9], brain invasion [10], location [11], vascular density [12], Ki-67 LI [8, 13–15], extent of the resection [12, 16, 17], and WHO grade [11]. In our study, age, gender, tumor location, Ki-67 LI, and the LN ratio of MET PET were investigated. A high LN ratio was significantly correlated with tumor recurrence and progression. However, age, gender, tumor location, and KI-67 LI were not significantly correlated with tumor recurrence. These risk factors remain controversial.
Recently, the MET PET method has been used in gliomas and other intracranial tumors to evaluate the malignancy of the tumor and the proliferative activity. In previous studies of gliomas, MET uptake correlated with the WHO grade, Ki-67 LI, and patient survival [18–21]. However, the role of MET PET in meningioma is not clear. In a previous study using 18F-fluorodeoxyglucose (FDG) PET, 18F-FDG uptake was correlated with the Ki-67 LI but not with recurrence of the meningioma [22, 23]. Using kinetic analysis with 18F-FDG PET, Tsuyuguchi [24] showed that the kinetic rate constant of glucose metabolism is related to the Ki-67 LI. However, that analysis requires frequent arterial blood samplings and dynamic PET scanning. The procedure is very complicated and not practical for clinical use. Moreover, the results of 18F-FDG PET are influenced by blood glucose. In patients with hyperglycemia, the results may lead to overestimation [25]. Iuchi et al. [26] showed that MET uptake is significantly correlated with the count of nuclear organizer regions, which is a histological index of protein synthesis, the Ki-67 LI, and a histological index of proliferative activity. In that study, 18F-FDG uptake showed no significant correlation with the Ki-67 LI or clinical malignancy. The uptake of methionine reflects amino acid transport and metabolism, but this does not mean that methionine uptake is correlated with protein synthesis and proliferation [27]. Some previous studies have shown that MET uptake correlates with microvessel density in glioma cases [28, 29], but in meningioma cases, MET uptake does not correlate with microvessel density [30]. This observation may reflect the fact that meningioma has multiple pathological subtypes and, thus, microvessel density may be different in each subtype. To evaluate the correlation between the LN ratio and microvessel density in meningioma, many cases of each subtype would be necessary.
Arita et al. [30] showed that the LN ratio of MET uptake is not significantly correlated with tumor doubling time. In this study, many asymptomatic patients were enrolled, and the mean tumor doubling time was very long (174 ± 270 months) despite a short follow-up period (26.7 ± 16.7 months). Thus, evaluation of recurrence and progression in meningioma using tumor doubling time appeared to be difficult because most meningiomas progress slowly.
Compared with 18F-FDG PET, the contrast between a meningioma lesion and normal brain tissue is clear in MET PET and, thus, we can correctly define the ROI using MET PET. Recently, we have evaluated MET uptake more correctly by fusing PET images with computed tomography or MR images.
In this study, we calculated the LN ratio using the mean MET uptake of the lesion and the normal brain tissue. The methionine uptake in the tumor depends not only on the metabolic rate, but also on the vascular bed [31]. The vascular bed of the meningioma is different within the various pathological types of meningiomas [32], and the vascular bed may be variable in the same specimen. Biological activity is heterogeneous in the same meningioma lesion [33, 34]. Thus, partially high MET uptake does not always indicate a high metabolic rate of the whole tumor. In this study, we used the mean MET uptake, not the maximum MET uptake, to reduce the influence on the heterogeneity of MET uptake.
In this study, tumor progression and recurrence were not significantly different between the GTR and non-GTR groups. However, in some cases with a high Ki-67 LI, GTR was performed, and the recurrence rate was low. GTR was a factor that strongly influenced the recurrence rate. We evaluated the recurrence and progression in the non-GTR group. The LN ratio in the group with recurrence and progression was also significantly higher than that in the group without recurrence and progression (p < 0.05). The Ki-67 LI was not significantly different (p = 0.18). Our observations are summarized in Table 4.
In our study, the LN ratio was a significant risk factor for recurrence and progression. The LN ratio of MET PET may indicate the proliferative activity of meningioma. Using ROC analysis, the AUC was 0.754, and the best cutoff value was 3.18, resulting in a sensitivity and specificity of 63 and 79 %, respectively. The sensitivity and specificity of the LN ratio were not less than those of the Ki-67 LI, as described in a previous study [35, 36].
In our study, the Ki-67 LI was not significantly different between the patients with recurrence and those without. We also found no correlation between the LN ratio and the Ki-67 LI. Some previous papers have reported that the correlation between the Ki-67 LI and tumor recurrence is controversial [4, 33, 37, 38]. Meningioma is characterized by heterogeneous biological activity within the same tumor tissue [33, 34]. It is doubtful that the Ki-67 LI obtained from a small tumor specimen can adequately evaluate the proliferative potential of the whole tumor. In fact, MET uptake is heterogeneous in a large tumor and may reflect the heterogeneity of the Ki-67 LI. The MET PET method is useful for evaluating the whole tumor. The Ki-67 LI overlaps within each grade of meningioma [39–41]. Evaluating the proliferative activity of the whole tumor and providing an accurate prognosis may be difficult with only one index.
The extent of resection was a significant risk factor as shown in a previous study [12, 16, 17]. However, the location of the tumor was not a significant risk factor in this study. Sixteen cases of skull base meningioma were included. In these cases, total resection without complications is difficult. A GTR of the tumor would reduce the risk of recurrence. This result may indicate that additional treatments are necessary for a residual tumor in which the LN ratio is higher than 3.18.
The WHO grade of meningioma was also a significant risk factor. In this study, we investigated preoperative cases and, thus, most cases were WHO grade I; only three cases were WHO grade II. Cases with WHO grade III meningioma are relatively infrequent at initial diagnosis. Almost all cases of meningioma are pathologically benign. Thus, we have to follow patients for a long time to investigate malignant changes and the prognosis. We must investigate additional consecutive cases to evaluate the largest number and the widest variety of cases.
Our study showed that the MET PET method has useful sensitivity and specificity for evaluation of recurrence and progression in meningioma. The most beneficial point is that 11C-methionine PET is not invasive, whereas analysis of the Ki-67 LI requires surgery. Thus, without surgery, we can evaluate the risk of progression and recurrence and consider the treatment strategy. We can determine the risk of progression and recurrence before deciding on observation or surgery. In asymptomatic cases, high LN ratio of MET PET may be the decisive factor for determining surgical treatment. We did not evaluate a large number of cases, and thus continued collection of cases and evaluation of the data are necessary.
Conclusion
The results of our study showed that MET uptake by the meningioma was a significant prognostic factor. MET uptake was significantly higher in cases with recurrence or progression. The AUC of the LN ratio for recurrence or progression was 0.754, and the best cutoff value was 3.18. The greatest advantage associated with the MET PET method is its non-invasive nature.
References
Committee of Brain Tumor Registry of Japan. Report of Brain Tumor Registry of Japan (1984-2000) Neurol Med Chir. 2009;49 Suppl.
Crompton MR, Gautier-Smith PC. The prediction of recurrence in meningiomas. J Neurol Neurosurg Psychiatry. 1970;33(1):80–7.
Jellinger K, Slowik F. Histological subtypes and prognostic problems in meningiomas. J Neurol. 1975;208(4):279–98.
Roser F, Samii M, Ostertag H, Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir. 2004;146(1):37–44 (discussion).
McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88(5):831–9.
Nakamura M, Roser F, Michel J, Jacobs C, Samii M. The natural history of incidental meningiomas. Neurosurgery. 2003;53(1):62–70 (discussion-1).
Niiro M, Yatsushiro K, Nakamura K, Kawahara Y, Kuratsu J. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68(1):25–8.
Kasuya H, Kubo O, Tanaka M, Amano K, Kato K, Hori T. Clinical and radiological features related to the growth potential of meningioma. Neurosurg Rev. 2006;29(4):293–6 discussion 296-297.
Kuratsu J, Kochi M, Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92(5):766–70.
Alvernia JE, Dang ND, Sindou MP. Convexity meningiomas: study of recurrence factors with special emphasis on the cleavage plane in a series of 100 consecutive patients. J Neurosurg. 2011;115(3):491–8.
McGovern SL, Aldape KD, Munsell MF, Mahajan A, DeMonte F, Woo SY. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. J Neurosurg. 2010;112(5):925–33.
Guevara P, Escobar-Arriaga E, Saavedra-Perez D, Martinez-Rumayor A, Flores-Estrada D, Rembao D, et al. Angiogenesis and expression of estrogen and progesterone receptors as predictive factors for recurrence of meningioma. J Neurooncol. 2010;98(3):379–84.
Matsuno A, Fujimaki T, Sasaki T, Nagashima T, Ide T, Asai A, et al. Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol. 1996;91(5):504–10.
Takeuchi H, Kubota T, Kabuto M, Kitai R, Nozaki J, Yamashita J. Prediction of recurrence in histologically benign meningiomas: proliferating cell nuclear antigen and Ki-67 immunohistochemical study. Surg Neurol. 1997;48(5):501–6.
Lanzafame S, Torrisi A, Barbagallo G, Emmanuele C, Alberio N, Albanese V. Correlation between histological grade, MIB-1, p53, and recurrence in 69 completely resected primary intracranial meningiomas with a 6 year mean follow-up. Pathol Res Pract. 2000;196(7):483–8.
Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58(1):51–6.
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18–24.
Ribom D, Eriksson A, Hartman M, Engler H, Nilsson A, Langstrom B, et al. Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer. 2001;92(6):1541–9.
Torii K, Tsuyuguchi N, Kawabe J, Sunada I, Hara M, Shiomi S. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Ann Nucl Med. 2005;19(8):677–83.
Kim S, Chung JK, Im SH, Jeong JM, Lee DS, Kim DG, et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005;32(1):52–9.
Kato T, Shinoda J, Oka N, Miwa K, Nakayama N, Yano H, et al. Analysis of 11C-methionine uptake in low-grade gliomas and correlation with proliferative activity. AJNR Am J Neuroradiol. 2008;29(10):1867–71.
Lee JW, Kang KW, Park SH, Lee SM, Paeng JC, Chung JK, et al. 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36(10):1574–82.
Lippitz B, Cremerius U, Mayfrank L, Bertalanffy H, Raoofi R, Weis J, et al. PET-study of intracranial meningiomas: correlation with histopathology, cellularity and proliferation rate. Acta Neurochirurgica Suppl. 1996;65:108–11.
Tsuyuguchi N. Kinetic analysis of glucose metabolism by FDG-PET versus proliferation index of Ki-67 in meningiomas–comparison with gliomas. Osaka City Med J. 1997;43(2):209–23.
Cremerius U, Bares R, Weis J, Sabri O, Mull M, Schroder JM, et al. Fasting improves discrimination of grade 1 and atypical or malignant meningioma in FDG-PET. J Nucl Med. 1997;38(1):26–30.
Iuchi T, Iwadate Y, Namba H, Osato K, Saeki N, Yamaura A, et al. Glucose and methionine uptake and proliferative activity in meningiomas. Neurol Res. 1999;21(7):640–4.
Gudjonsson O, Blomquist E, Lilja A, Ericson H, Bergstrom M, Nyberg G. Evaluation of the effect of high-energy proton irradiation treatment on meningiomas by means of 11C-l-methionine PET. Eur J Nucl Med. 2000;27(12):1793–9.
Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of l-methyl-11C-methionine (MET) uptake with l-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99(2):217–25.
Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A, et al. Methyl-[11C]- l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging. 2003;30(6):868–73.
Arita H, Kinoshita M, Okita Y, Hirayama R, Watabe T, Ishohashi K, et al. Clinical characteristics of meningiomas assessed by 11C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neurooncol. 2012;107(2):379–86.
Abe Y, Matsuzawa T, Itoh M, Ishiwata K, Fujiwara T, Sato T, et al. Regional coupling of blood flow and methionine uptake in an experimental tumor assessed with autoradiography. Eur J Nucl Med. 1988;14(7–8):388–92.
Kimura H, Takeuchi H, Koshimoto Y, Arishima H, Uematsu H, Kawamura Y, et al. Perfusion imaging of meningioma by using continuous arterial spin-labeling: comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol. 2006;27(1):85–93.
Siegers HP, Zuber P, Hamou MF, van Melle GD, de Tribolet N. The implications of the heterogeneous distribution of Ki-67 labelled cells in meningiomas. Br J Neurosurg. 1989;3(1):101–7.
Abramovich CM, Prayson RA. Histopathologic features and MIB-1 labeling indices in recurrent and nonrecurrent meningiomas. Arch Pathol Lab Med. 1999;123(9):793–800.
Bruna J, Brell M, Ferrer I, Gimenez-Bonafe P, Tortosa A. Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology. 2007;27(2):114–20.
Kim YJ, Ketter R, Henn W, Zang KD, Steudel WI, Feiden W. Histopathologic indicators of recurrence in meningiomas: correlation with clinical and genetic parameters. Virchows Archiv. 2006;449(5):529–38.
Moller ML, Braendstrup O. No prediction of recurrence of meningiomas by PCNA and Ki-67 immunohistochemistry. J Neurooncol. 1997;34(3):241–6.
Nakaguchi H, Fujimaki T, Matsuno A, Matsuura R, Asai A, Suzuki I, et al. Postoperative residual tumor growth of meningioma can be predicted by MIB-1 immunohistochemistry. Cancer. 1999;85(10):2249–54.
Abramovich CM, Prayson RA. MIB-1 labeling indices in benign, aggressive, and malignant meningiomas: a study of 90 tumors. Hum Pathol. 1998;29(12):1420–7.
Kolles H, Niedermayer I, Schmitt C, Henn W, Feld R, Steudel WI, et al. Triple approach for diagnosis and grading of meningiomas: histology, morphometry of Ki-67/Feulgen stainings, and cytogenetics. Acta Neurochir. 1995;137(3–4):174–81.
Langford LA, Cooksley CS, DeMonte F. Comparison of MIB-1 (Ki-67) antigen and bromodeoxyuridine proliferation indices in meningiomas. Hum Pathol. 1996;27(4):350–4.
Acknowledgments
The authors appreciate the technical support of the radiological technologist at our institute.
Conflict of interest
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikeda, H., Tsuyuguchi, N., Kunihiro, N. et al. Analysis of progression and recurrence of meningioma using 11C-methionine PET. Ann Nucl Med 27, 772–780 (2013). https://doi.org/10.1007/s12149-013-0747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0747-z