Abstract
Macrophages are the most abundant immune cells in primary and metastatic tumor tissues. Studies have shown that macrophages mainly exhibit a tumor-promoting phenotype and play a key role in tumor progression and metastasis. Therefore, many macrophage-targeted drugs have entered clinical trials. However, compared to preclinical studies, some clinical trial results showed that macrophage-targeted therapy did not achieve the desired effect. This may be because most of what we know about macrophages comes from in vitro experiments and animal models, while macrophages in the more complex human microenvironment are still poorly understood. With the development of technologies such as single-cell RNA sequencing, we have gained a new understanding of the origin, classification and functional mechanism of tumor-associated macrophages. Therefore, this study reviewed the recent progress of macrophages in promoting tumor progression and metastasis, aiming to provide some help for the formulation of optimal strategies for macrophage-targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although advances in traditional treatment methods (such as surgery, radiotherapy, chemotherapy, and gene-targeted therapy) have improved the prognosis of patients to a certain extent, local recurrence and distant metastasis are still the leading causes of death in cancer patients. In recent years, to solve this problem, researchers have gradually turned their attention from the study of tumor cells themselves to the study of tumor microenvironment (TME). With the in-depth study of TME, a new treatment method-immunotherapy has been successfully developed and applied in patients with refractory or metastatic cancer. Among them, immune checkpoint blockade (ICB) therapy targeting CD8+T cells has achieved particularly significant clinical effects in some cancer patients. Accordingly, many people believe that immunotherapy (including ICB, tumor vaccines, and adoptive immune cell therapy, etc.) will become the new pillar of cancer treatment. However, a substantial proportion of patients experience limited clinical benefit after ICB therapy, and almost all cancer patients eventually develop drug resistance. The reasons are analyzed as follows: on the one hand, tumor intrinsic factors such as tumor mutation burden limit the therapeutic effect of ICB therapy, on the other hand, TME produces ICB resistance by limiting the infiltration and activation of effector T cells. Fortunately, studies have found that the immunosuppressive network established by tumor-associated macrophages (TAMs) is one of the main mechanisms affecting the therapeutic effect of ICB therapy, and macrophage-targeted therapy can greatly improve the therapeutic effect of ICB therapy.
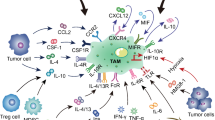
Macrophages (Mϕ) exist in almost all tissues and organs and play a crucial role in tissue homeostasis. As a pivotal component of the innate immune system, macrophages can recruit other immune cells to the site of infection, phagocytose and obliterate foreign pathogens, and activate the complement system and adaptive immunity [1]. In addition, macrophages play critical roles in development, disease (including cancer, infection, and inflammation), and tissue regeneration and remodeling. Actually, macrophages are the most pro-tumor immune cells in the TME. It has been demonstrated that tumors are significantly associated with the recruitment and polarization of macrophages. For example, tumor-secreted cytokines CSF-1 and CCL2 are the most important factors that promote the recruitment of macrophages. M1 exhibits pro-inflammatory, pro-immune and anti-tumor features, while M2 has anti-inflammatory, immunosuppressive and pro-tumor functions. In the TME, macrophages dynamically and continuously differentiate according to the changes in the current microenvironment. For example, IFN-γ, TNF-α, IL-12, GM-CSF, etc. induce the polarization of macrophages to M1, and IL-4, IL5, IL-10, IL-13, etc. induce their polarization to M2. Although the M1/M2 polarization dichotomy limits an accurate description of macrophage function, it is currently the most widely used classification of macrophages.
In addition to powerful immunosuppressive effects, macrophages have extensive tumor-promoting functions. For example, they can promote tumor progression and metastasis by participating in cancer stem cell activation, epithelial-mesenchymal transition (EMT), tumor angiogenesis, transendothelial migration, extracellular matrix (ECM) remodeling, and formation of pre-metastatic niches (PMN). Thus, therapies targeting macrophages may exert or improve anticancer therapeutic effects through broad and pleiotropic mechanisms. In conclusion, macrophages have emerged as one of the most promising therapeutic targets for controlling tumor recurrence and metastasis due to their powerful tumor-promoting characteristics.
Although preclinical studies have confirmed the good therapeutic effect of targeting macrophages, and many targeted drugs have entered clinical trials. We still lack a thorough understanding of macrophages due to their high heterogeneity and plasticity. Inaccurate targeted therapy will greatly reduce the effect of anti-tumor therapy, and even some serious complications will occur. Therefore, this review details the current status of research on the tumor-promoting mechanisms of macrophages, as detailed below.
Origin of macrophages
Macrophages is not only widely distributed in various healthy tissues, but also exists in most solid tumor tissues. Moreover, it is one of the most abundant immune cells in tumor tissue, and even accounts for up to 50% of immune cells [2]. So, where do these macrophages originate and how are they enriched in tumors? For more than half a century, the mainstream view has been that macrophages are derived from bone marrow hematopoietic stem cells. However, with the development of modern lineage tracing techniques and single-cell RNA sequencing (scRNA-seq), a large body of evidence has shown that they have different pathways of origin. First, in addition to myeloid-derived macrophages, the researchers identified embryonic-derived macrophages in adult mouse tissues [3]. Subsequently, further studies confirmed that embryonic-derived macrophages also exist in human organs and tissues [4]. Therefore, it is currently recognized that there are three origins of macrophages, which are: yolk sac-derived erythromyeloid progenitors (EMPs) produced embryonic macrophages, embryonic monocytes generated by EMPs colonization in the fetal liver and monocyte-macrophages differentiated from bone marrow hematopoietic stem cells. (Fig. 1) Actually, macrophages in most normal tissues, namely tissue-resident macrophages (TRMs), are dominated by embryonic macrophages with self-renewal and proliferation capacity, supplemented by the recruitment of myeloid-derived macrophages. However, not all TRMs have multiple origins. For example, TRMs in adult gastrointestinal and dermal tissues are almost exclusively derived from bone marrow, whereas TRMs in brain tissues are almost exclusively derived from embryos (yolk sac origin) [5].
Macrophage Origin and Polarization. a Macrophages have three origins (yolk sac, fetal liver, and bone marrow) and two forms (TRMs and myeloid-derived macrophages). b Unlike TRMs, which have a well-defined self-proliferation ability, the self-proliferation ability of bone marrow-derived TAMs in tumor tissues remains controversial. c TAMs mainly consisted of myeloid-derived macrophages, followed by TRMs. However, unlike TAMs, whether MAMs are partially derived from TRMs remains controversial. d TAMs can be further polarized into M1 and M2, while M1 and M2 can be converted into each other. TRMs tissue-resident macrophages, MAMs metastasis-associated macrophages, TAMs tumor-associated macrophages
Besides, there is currently controversy over the origin of TAMs. Lineage tracing studies in mouse models have shown that, in contrast to TRMs that are predominantly derived from embryonic precursors, the vast majority of TAMs are derived from myeloid-derived monocytes/monocyte-myeloid-derived suppressor cells (M-MDSCs) [6]. Therefore, it has been suggested that the “reservoir” of TAMs is mainly composed of circulating monocytes and M-MDSCs. However, recent studies have shown that TAMs have both embryonic and myeloid origins, at least in tumors such as lung cancer, pancreatic cancer, and glioblastoma [7]. These studies also found that TAMs of different origins have different phenotypic and functional characteristics. Among them, bone marrow-derived TAMs highly expressed genes related to immunosuppression and antigen presentation, while embryo-derived TAMs highly expressed genes related to tissue remodeling and wound healing [8]. Chow et al. found that Tim-4+ luminal resident macrophages sequestered and inhibited the proliferation of CD8+T cells, and blocking Tim-4 enhanced the efficacy of ICB therapy and adoptive T cell therapy in mice [9]. Ramos and colleagues found that FOLR2+TRMs are positively associated with good prognosis by interacting with CD8+T cells in breast cancer tissue [10]. In addition, the proportion of TAMs of different origins varied with tumor progression. Through scRNA-seq technology, it was found that TAMs in the early stage of tumors were mainly embryonic-derived TRMs. And they promote tumor growth and proliferation by establishing a pro-tumor niche by promoting tumor cell EMT, invasion, and protecting tumor cells from adaptive immunity [11]. Similarly, Huggins et al. identified a novel lipid-associated macrophage derived from alveolar macrophages (AMs) in an animal model of breast cancer lung metastasis. Interestingly, their study showed that the aggregation of these cells occurred before the formation of tumor metastases [12]. Therefore, we speculate that TRMs may be more inclined to proliferate and function in the early stage of tumors compared with myeloid-derived macrophages.
Moreover, Antunes et al. found that myeloid-derived TAMs also have the ability to self-renew and proliferate, and inhibiting the recruitment of myeloid-derived macrophages only activates the proliferation compensatory mechanism of TAMs, but does not reduce the total number of TAMs [13]. Wang and colleagues found that granulocyte-macrophage colony-stimulating factor (GM-CSF) enhanced A2A receptor expression on TAMs and synergized with adenosine to induce proliferation of TAMs in liver cancer tissues [14]. These may be one of the reasons for the poor efficacy of pan-macrophage-targeted therapies, such as anti-CSF-1 antibody. In conclusion, it is still unclear how TAMs of different origins regulate the TME and cancer progression. Further clarification of this issue is of great significance for elucidating the diversity of TAMs functional phenotypes and targeted therapy strategies.
Recruitment and polarization of TAMs
Tumor promotes the recruitment and polarization of macrophage. Ali N and colleagues [2] summarized the five recruitment axes of monocyte-macrophages, including CCL2/CCR2, CXCL12/CXCR4, VEGF/VEGFR, complement components (especially C5a/C5a receptors) and CSF-1 /CSF-1R. Studies have found that tumors can also recruit macrophages/M-MDSCs through CCL3, CCL5, CX3CL1, IL34, and periostin. In addition, recent studies on tumor metabolism have shown that the hypoxic environment in tumors plays a key role in promoting the recruitment and differentiation of TAMs [15]. Metabolites in the TME may also be important factors in tumor recruitment of TAMs. For example, Barrio et al. found that adenosine can promote the recruitment of monocytes through a self-amplifying mechanism that is further adenosine production by M2 or MDSCs recruited by adenosine [16].
Traditionally, TAMs have been classified into M1 (classically activated macrophages) and M2 (alternatively activated macrophages) polarized phenotypes based on their functional characteristics. M1 exhibits pro-inflammatory, pro-immune, and anti-tumor features, while M2 has anti-inflammatory, immunosuppressive, pro-angiogenic, and metastasis-promoting functions [17]. Recently, some scholars have further subdivided M2 cells into four subgroups: M2a, M2b, M2c and M2d [1, 18]. The present findings suggest that IFN-γ, TNF-α, Toll-like receptor (TLR) agonists (such as bacterial lipopolysaccharide (LPS)), IL-12, GM-CSF can induce TAMs polarization to M1 [19,20,21,22], while IL-4, IL5, IL-10, IL-13, IL-33, CSF-1, TGF-β, FGF2, PGE2, Sonic Hedgehog (SHH), BMP4 and Sema3A induce TAMs polarization to M2 [20, 23,24,25,26,27,28]. Besides, accumulating evidence suggests that TAMs can promote their polarization toward M1 or M2 by ingesting tumor cell-derived exosomes [29,30,31,32,33,34]. In addition, metabolic reprogramming of tumors [20], hypoxic state of the TME [35], and remodeling of the ECM [36, 37] can promote TAMs polarization toward M2. However, TAMs differentiate dynamically and continuously according to changes in the current microenvironment, resulting in their high degree of heterogeneity and plasticity. On the one hand, their functional phenotypes have the characteristics of spatiotemporal distribution [11, 38]. That is, TAMs show different phenotypic and functional characteristics between different patients, between different tumors in the same patient, and even in different sites or different stages of the same tumor. For example, despite the overall predominance of an anti-tumor phenotype, TAMs mainly exhibit an anti-tumor M1 phenotype in early tumor stage and a pro-tumor M2 phenotype in tumor progression [18, 39,40,41]. Besides, unlike M1 that are predominantly enriched in normoxic tumor areas, M2 are predominantly enriched in hypoxic, necrotic and perivascular tumor areas [20]. On the other hand, continued dynamic differentiation also results in a large number of intermediate transition states in TAMs, or TAMs with both M1 and M2 phenotypes [42,43,44,45]. Moreover, the M1/M2 polarization dichotomy is a taxonomy to describe the activation state of TAMs in vitro, limiting an accurate description of the multifunctional complexity of TAMs in humans [46,47,48]. Therefore, some scholars have tried to use scRNA-seq technology to classify TAMs subsets. For example, Zhang L divided TAMs in colorectal cancer into subgroups of C1QC+ and SPP1+TAMs. Among them, C1QC+TAMs highly expressed genes involved in phagocytosis and antigen presentation, while SPP1+TAMs preferentially expressed genes involved in angiogenesis. This study further found that anti-CSF-1R treatment preferentially reduced C1QC+TAMs with inflammatory features and did not affect SPP1+TAMs expressing pro-angiogenic/tumorigenic genes [49]. In a pan-cancer scRNA-seq study, TAMs were further divided into four subgroups: SPP1+TAMs, C1QC+TAMs, ISG15+TAMs and FN1+TAMs [43]. In addition, Obradovic found that TREM2+/APOE+/C1Q+ TAMs were associated with postoperative recurrence of clear cell renal cell carcinoma [50]. We believe that scRNA-seq technology will become a new classification method of macrophages and influence the formulation of targeted therapy strategies for macrophages. Despite its obvious limitations, the M1/M2 polarization dichotomy is currently the most commonly used method for classifying TAMs in medical research.
TAMs and tumor growth
To the body, the tumor is like an endless wound that keeps on starting to heal, but never completely heals [51]. In the process of tumorigenesis, precancerous lesions or oncogenic inflammation should eliminate abnormal cells by recruiting pro-inflammatory immune cells such as macrophages and dendritic cells, thereby inhibiting tumorigenesis. At this point, the recruited macrophages (M1) exert anti-tumor functions. The specific manifestations are: (1) phagocytosing and killing tumor cells; (2) presenting neoantigens to CD8+T cells to remove tumor cells and start the "immune editing" program; (3) producing cytotoxic factors (such as NO and ROS) to directly kill tumor cells; (4) release of pro-inflammatory factors (such as IFN-γ, TNF-α, IL-1β, IL-2, IL-6, IL-12, IL-18, IL-23 and CXCL9) to further activate the anti-tumor immune response [52, 53]. In addition, M1 increases the expression of major histocompatibility complex class II (MHC II) molecules, resulting in a higher degree of activation of tumor-associated antigen-presenting cells (APCs) such as monocytes, macrophages, and dendritic cells (DCs) [54]. However, while exerting pro-inflammatory and anti-tumor functions, M1 also increases the gene mutation burden of precancerous cells by releasing IFN-γ, TNF-α, TGF-β, IL-1β, and reactive oxygen species/reactive nitrogen (ROS/NOS) [55, 56]. An increased gene mutation load is required for tumorigenesis. Gradually, the accumulation of gene mutational burden in precancerous cells leads to tumorigenesis, and tumor cells further promote macrophage recruitment and polarization toward a pro-tumor phenotype by regulating the TME. Importantly, M2 plays a multifaceted tumor-promoting role during tumor progression, as detailed below (Fig. 2).
Promote cancer stem cells activation
Cancer stem cells (CSCs) are tumor cell subsets with stem cell-like properties that play a key role in tumorigenesis, immune evasion, metastasis and therapy resistance. Several studies have demonstrated that the acquisition and maintenance of CSCs activity can be regulated by TAMs through the secretion of cytokines such as IL-6, IL-8, CD51 and GPNMB [57,58,59,60]. Sharma et al. found that TAMs could activate the Notch1-Jagged1/2 signaling pathway through direct contact with tumor cells, thereby inducing stemness in cancer cells [61]. Additionally, TAMs can promote tumor growth and proliferation by secreting collagen [62] and insulin-like growth factor-1 (IGF-1) [63]. In addition, M2 increases the iron absorption rate of tumor cells by up-regulating the expression of ferroportin, which in turn promotes their growth and proliferation [64].
Promote angiogenesis
The unlimited proliferation of tumor cells requires continuous consumption of a large amount of oxygen and nutrients. When the TME fails to meet the metabolic demands of the tumor, solid tumors turn on a program called an “angiogenesis” switch This procedure replenishes the tumor with nutrients and removes waste by triggering the formation of a dense network of blood vessels. Importantly, TAMs play a key role in turning on the “angiogenic switch”. It can secrete various cytokines (such as VEGFA, platelet-derived growth factor (PDGF), angiopoietin, placental growth factor (PlGF), adrenomedullin (AMD), VEGFC, TNF, IL-1β, IL-6, CCL18, CXCL8, CXCL12, FGF2, WNT7B and signaling protein 4D (Sema4D)) to promote angiogenesis [65,66,67]. Among them, VEGFA mainly comes from TAMs and promotes the recruitment, proliferation and maturation of endothelial cells by binding to VEGF receptor 2. In addition, TAMs degrade ECM by expressing membrane-bound or soluble proteases such as matrix metalloproteinases, urokinase-type plasminogen activator (uPA), thymidine phosphorylase, and cathepsin, assisting endothelial cell migration and formation of new vascular buds, and mobilizing free VEGFA in ECM [68]. TAMs also degrade collagen in the ECM by endocytosis in a mannose receptor-dependent manner [69]. Interestingly, TIE2+TAMs aggregated around tumor blood vessels, where they supported vascular sprouting or anastomosis in a paracrine fashion [70].
TAMs are also associated with high permeability of neovascularization within tumors. On the one hand, TAMs promote the transendothelial migration of tumor cells by secreting inflammatory factors (such as VEGFA and TGF-β) that cause endothelial cells to lose adherent junctions, expand the vascular endothelial cell space and increase permeability. On the other hand, overexpression of these angiogenic growth factors produces immature new blood vessels that appear to be barely covered by pericytes and loosely bound to the basement membrane [71]. When tumor cells migrate around blood vessels, TIE2+TAMs open a door for tumor cells to enter the vasculature by assisting their transendothelial migration [72]. Ginter and colleagues found that the tumor metastasis microenvironment (TMEM), involving tumor cells, TAMs, and endothelial cells, is the gateway for hematogenous dissemination of the primary tumor and lymph nodes of breast cancer [73]. Additionally, preclinical studies have demonstrated that targeted killing or depletion of TAMs can reduce the number of circulating tumor cells [74].
In addition to participating in angiogenesis, TAMs may be associated with increased infiltration and density of lymphatic vessels. It has been found that TAMs can promote lymphangiogenesis and lymph node metastasis by secreting VEGF-C/D [75] and expressing S1PR1 [76] and PDPN [77]. Furthermore, TAMs can differentiate into lymphatic endothelial progenitors and integrate with existing lymphatic vessels to induce new lymphatic sprouting [60].
Suppress immune response
We conclude that TAMs mainly exert their immunosuppressive abilities in the following four ways.
Membrane proteins
TAMs highly express T cell immune checkpoint ligands on the membrane surface, including PD-L1 (also known as B7H1 or CD274), PDL2 (also known as B7-DC or CD273), CD80 (also known as B7-1), and CD86 (also known as B7-2) [78]. PD-L1 and PD-L2 expression of TAMs is up-regulated by stimulation of cytokines and hypoxia, and they induce effector T cell exhaustion by binding to the inhibitory receptor PD-1 on the membrane surface of effector T cells. However, CD80 and CD86 inhibit the activity of effector T cells by binding to CTLA-4 on effector T cells. Indeed, TAMs themselves also express high levels of PD-1, which has the ability to reduce their antigen presentation and increase the expression of immunosuppressive factors such as ARG1, IL-10, and TGF-β [2, 79]. Kryczek et al. found that another member of the B7 superfamily (B7-H4) expressed by TAMs is also involved in suppressing the immune activity of effector T cells [80]. In addition, the V-type immunoglobulin domain-containing suppressor of T cell activation (VISTA) expressed on the membrane of TAMs is a novel immunosuppressive checkpoint and is often considered to be an indicator of poor prognosis in various cancers [81]. Furthermore, SIRPα [82], LILRB1 [83], Siglec-10 [84] expressed by TAMs transmit the signal of “don’t eat me” by binding to CD47, β2M and CD24 on the surface of tumor cell membranes, respectively, thereby protecting cancer cells from being phagocytosed by TAMs.
Scavenger receptors are composed of a structurally diverse group of membrane proteins (including A-H classes) that have important effects on inflammatory responses, tissue repair and remodeling, and innate immune responses by recognizing a broad range of ligands [79]. Among the various scavenger receptors expressed by macrophages, CD206, SR-A (also known as CD204) and CD163 have been widely used as biomarkers for M2. Many scavenger receptors, including SR-A [85], MARCO [86], and Clever-1 (also known as stabilin-1 or FEEL1) [87], contribute to the expression of tumor-promoting activity in macrophages through ligand binding. Fleur et al. found that MARCO+TAMs in non-small cell lung cancer promoted Treg cell proliferation and IL10 production, and decreased CD8+T cell activity [88]. Masetti et al. found that MARCO+TAMs can maintain the growth and invasion of prostate cancer by promoting lipid metabolism and lipid accumulation [89]. Recent studies have found that TREM2+TAMs are associated with exhaustion of effector T cells and resistance to anti-PD-1 therapy. Targeted TREM2 therapy inhibits tumor growth and improves ICB efficacy while remodeling TAMs [90]. However, TREM2 plays different roles in the occurrence and development of different malignant tumors, and its specific biological function needs to be further confirmed by a large number of studies [91].
Besides, Hu et al. found that DC-SIGN+TAMs were associated with inactivation of CD8+T cells, and anti-DC-SIGN treatment enhanced the therapeutic effect of PD-1 inhibitors [92]. Qi et al. found that Gal-9+TAMs are associated with effector T cell exhaustion [93]. Chow et al. found that Tim-4+luminal resident macrophages could sequester and inhibit the proliferation of CD8+T cells, and blocking Tim-4 enhanced the efficacy of ICB and adoptive T cell therapy in mice [9]. TAMs also negatively regulate NK and T cell activity by expressing atypical major histocompatibility complex class I (MHC-I) molecules such as HLA-E and HLA-G, thereby exerting immunosuppressive effects [72]. TAMs also express ligands for the death receptors FAS and TRAIL, which bind to their related receptors on T cells and lead to T cell apoptosis by activating the caspase 3 and caspase 8 pathways [60, 72]. In conclusion, the specific membrane-expressed receptors of TAMs play an important role in promoting tumor immunosuppression, and blocking them can significantly improve the outcome of immunotherapy in patients. Compared with pan-macrophage-targeted therapy, we believe that targeting receptors on the surface of macrophage membranes is a precise, safe, and more effective therapeutic strategy.
Cytokines
TAMs can suppress the function of effector T cells by secreting a series of cytokines to recruit natural regulatory T cells (nTreg) and induce the differentiation of adaptive regulatory T cells (iTreg) [72]. For example, TAMs-derived chemokines CCL2, CCL3, CCL4, CCL5, CCL20, and CCL22 promote the recruitment of immunosuppressive nTregs to tumors [19, 78, 94]. TGFβ, IL-10 and exosomes derived from TAMs can induce the differentiation and expansion of CD4+T cells to iTreg [72, 95]. Besides, TAMs can inhibit the cytotoxic activity of effector T cells and NK cells through the expression of IL-10 and TGFβ, thereby promoting tumor progression [78, 96]. Specifically, secreted TGFβ can directly inhibit CD8+ T cell function through transcriptional repression of genes encoding functional cytokines such as perforin, granzymes, and cytotoxins [97]. Alternatively, secreted TGFβ indirectly inhibits effector T cells by upregulating the expression of indoleamine 2,3-dioxygenase (IDO) in plasma cell-like dendritic cells (pDC) and CCL22 in myeloid DC (mDC) [98]. Membrane-bound TGF-β can also affect NK cell phenotype and cytotoxicity through direct cell-to-cell contact [99]. IL-10, which mainly comes from TAMs, can indirectly attenuate the activity of CD8+T cells by inhibiting DC secretion of IL-12 [100]. Finally, the lysosomes of TAMs inhibit the cross-presentation of antigens due to their highly active cysteine protease activity, thereby inhibiting the activity of CD8+T cells [101].
Cellular metabolism
M2 participates in the metabolism of L-arginine through high expression of arginase. On the one hand, arginine depletion of M2 leads to "arginine starvation" of effector T cells, which in turn inhibits the proliferation and activation of effector T cells by blocking the cell cycle [102]. On the other hand, ornithine (M2-related arginine metabolite), as a precursor of proline and polyamines, can remodel the ECM by promoting collagen synthesis, remodeling of damaged tissues, and formation of fibrosis. Moreover, TAMs can degrade tryptophan in TME through high expression of IDO, thereby inhibiting the cytotoxic activity of T cells and NK cells. A large amount of kynurenine (a metabolite of tryptophan mediated by IDO) can play an immunosuppressive function directly or by binding to AhR on the surface of Treg cells, NK cells and DC cell membranes [103]. In addition, TAMs indirectly exert pleiotropic immunosuppressive functions by promoting the hydrolysis of extracellular ATP to adenosine by expressing high levels of membrane-bound nucleotidase CD39 and CD73, or by initiating NAD+ extracellular adenosine synthesis by CD38 [20, 104]. Recently, Hinshaw et al. found that TAMs can regulate metabolic processes through the Hedgehog (Hh) signaling pathway, thereby promoting their immunosuppressive activity [105].
ECM remodeling
It is well known that one of the key factors in the failure of ICB therapy is T cell exclusion, that is, the dense fibrotic structure of the ECM hinders the physical contact of CD8+T cells with cancer cells, thereby rendering them unable to perform the function of killing cancer cells. Therefore, overcoming this physical barrier imposed by the ECM is a necessary precondition to guarantee the success of ICB therapy. Increasing evidence has confirmed that TAMs play an important role in the formation of ECM fibrosis. The traditional view is that TAMs mainly activate tumor-associated fibroblasts by releasing inflammatory factors, and then indirectly induce ECM fibrosis [106]. However, studies have shown that TAMs can modulate tumor-associated fibrosis by directly participating in ECM remodeling [107]. Not only that, there is evidence that M2 cells have the potential to transform into tumor-associated fibroblasts [108].
TAMs, MAMs and distant metastasis
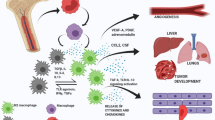
Tumors metastasize mainly through the following steps: (1) establishment of a pre-metastatic niche (PMN), (2) metastasis and seeding of primary tumor cells (including invasion, migration, vascular infiltration, circulation, vascular extravasation and seeding), (3) growth and proliferation of metastatic tumor cells (including micrometastases, dormancy and secondary growth). And TAMs play an important role in almost all processes. (Fig. 3).
Macrophages promote distant metastasis of tumors. a The formation process of distant metastasis of tumor includes: invasion, migration and vascular infiltration of primary tumor cells, survival and vascular extravasation of circulating tumor cells, and seeding and proliferation of metastatic tumor cells. b During the whole process of tumor metastasis, macrophages play an important promoting role through different molecular mechanisms. EMT Epithelial-mesenchymal transition, PMN pre-metastatic niche
Establishment of PMN
Tumor-secreted factors including EVs, cytokines and chemokines, and other molecular components contribute to tumor cell metastasis. Before the primary tumor cells spread into the blood circulation, the soluble factors (including CCL-2, TNFα, VEGF-A, TGFβ and CXCL1) [109] and EVs [110] released by the primary tumor cells are first released into the blood circulation and recruit macrophages/MDSCs to pre-metastatic organs [56]. Furthermore, tumor cell-derived tissue factor (TF) [111] and exosomes [112] can promote the recruitment of macrophages by promoting the formation of microthrombi in pre-metastatic organs. The recruited macrophages/MDSCs, together with neutrophils, establish a PMN by remodeling ECM, creating an immunosuppressive environment, stimulating neovascularization, and secreting inflammatory factors [56]. Blocking the recruitment of these myeloid cells to the PMN can significantly reduce the incidence of metastasis and improve disease-free survival [113]. It is worth mentioning that although it was initially thought that TRMs such as alveolar macrophages (AMs) were not involved in tumor metastasis, recent studies have found that AMs can function as metastasis-associated macrophages (MAMs) [12, 114, 115]. For example, Sharma et al. showed that AMs can promote the formation of pre-metastatic niches by inhibiting antitumor immunity [114]. Nosaka et al. found that monocyte-derived AMs promote the progression of lung metastases via leukotriene B4 (LTB4) [115]. In addition, Huggins et al. discovered a novel pro-metastatic lipid-associated macrophage subset (Lgals3, Trem2) in breast cancer lung metastases, and suggested that this cell population was derived from AMs [12].
Tumor metastasis
Tumor cell reprogramming is a hallmark of tumor invasion initiation. In epithelial cell carcinoma, epithelial-mesenchymal transition (EMT) is the main manifestation of tumor cell reprogramming. EMT encompasses dynamic changes in cellular organization from epithelial to mesenchymal phenotypes, which leads to functional changes in cell migration and invasion [116]. EMT can enhance the metastatic ability of tumor cells, which is manifested by remodeling of the cytoskeleton, changes in cell polarity, loosening of intercellular and cell-matrix junctions, individualization of cells, acquisition of cell motility, and enhancement of cell invasive capacity [117]. In addition, EMT is associated with stem cell properties, cell viability, metabolic changes, and drug resistance [117, 118]. Importantly, TAMs can promote EMT of tumor cells by releasing various cytokines, such as TGF-β, Wnt-1, EGF, IL-6, IL-8, IL-10, TNF-α, COX-2 and heme oxygenase-1 (HO-1). Mechanistically, these cytokines promote EMT by increasing the expression and activation of EMT-related transcription factors such as Zeb, Snail, Twist, and FoxQ1, which in turn downregulates the expression of E-cadherin and epithelial cell adhesion molecule (EpCAM).
In addition to promoting EMT, TAMs can participate in tumor invasion and metastasis by producing a variety of proteolytic enzymes that degrade basement membrane and ECM. These enzymes include metalloproteinases (MMP2, MMP7, MMP9), urokinase-type plasminogen activator (uPA), COX2, and cathepsins. TAMs also produce several other molecules to advance tumor cell invasion. One example is osteoadhesin (also known as SPARC), which promotes migration by increasing tumor cell-ECM interactions through integrins [119]. When migrating to the vicinity of blood vessels, tumor cells infiltrate into the vasculature with the assistance of TAMs (see above for details).
In the process of tumor metastasis, some paracrine circuits between TAMs and tumor cells play an important role. For example, preclinical studies have found that breast cancer cells can recruit TAMs by releasing CSF-1 and promote their secretion of EGF and Wnt-1, thereby further supporting tumor cell migration and infiltration into blood vessels [120]. In addition, neuregulin (NRG1) produced by breast cancer cells can promote the secretion of Jag-1 (ligand of Notch receptor) by TAMs, and Jag-1 further promotes the transendothelial migration and infiltration of tumor cells through the Notch signaling pathway [121]. In squamous cell carcinoma, tumor-initiating cells (TICs) induce FcεRIα+TAMs to accumulate in their vicinity by overexpressing IL-33. In turn, FcεRIα+TAMs promoted the low differentiation and invasiveness of TICs through the expression of TGF-β, and further up-regulated the expression of IL-33 [122].
During hematogenous metastasis, tumor cells must overcome challenges such as immune monitoring, fluid shear stress, and oxidative stress. Although Bernabé et al. demonstrated that TAMs promote the survival of tumor cells in the blood circulation, the specific mechanism remains unclear [111]. In addition, previous studies have found that macrophages can activate the PI3K/Akt signaling pathway in tumor cells through the interaction of α4 integrin and vascular cell adhesion molecule-1 (VCAM-1), thereby supporting the survival of cancer cells in the circulation [123]. Afterwards, tumor cells reside in the capillary bed of the PMN and infiltrate into the parenchyma within hours of their entry into the blood. Intact lung imaging reveals that macrophages are required for tumor cell extravasation [124]. Qian et al. found that inflammatory monocytes can promote tumor cell extravasation at least by secreting VEGF [124]. Häuselmann et al. showed that monocytes can promote transendothelial extravasation of tumor cells by inducing E-selectin-dependent endothelial cell retraction and regulating tight junctions through dephosphorylation of VE-cadherin [125].
MAMs derived from inflammatory monocytes are one of the most abundant immune cell types in the metastatic tumor niche [126]. They are recruited by CCL2, which is highly expressed by tumor metastatic cells and endothelial cells [127], and adhere to cancer cells via autocrine CCL-3, thus leading to prolonged residence of many MAMs at metastatic sites [128]. That is, CCL2 is mainly responsible for the recruitment of macrophages, while CCL3 is mainly responsible for the retention of macrophages. MAMs play an important role in maintaining the immunosuppressive microenvironment and promoting the growth of metastatic tumors. For example, MAMs can inhibit the activity of effector T cells by secreting CCL20 and expressing CD80/86 and PDL-1/2 [56, 129], and inhibit the antitumor activity of NK cells by releasing HGF and expressing membrane-bound TGF-β [130, 131]. In addition, MAMs can promote the growth of metastatic tumors through high expression of VEGFR-1 and IL-4R [126, 132]. In a model of pancreatic ductal adenocarcinoma liver metastases, granulin secreted by MAMs promotes the transformation of hepatic stellate cells into myofibroblasts, which secrete periostin, thereby creating a fibrotic microenvironment that sustains metastatic tumor growth [133]. Moreover, because the recruitment of inflammatory monocytes is faster than the rate of differentiation into MAMs, a large number of metastasis-associated macrophage precursors (MAMPCs) are also present at tumor metastatic sites. Ultimately, immunosuppressive MAMPCs can differentiate into MAMs within hours [56].
Conclusion
In this review, we summarize the latest research progress of macrophages, including its origin, classification, functional roles and molecular mechanisms in different stages of tumors. Based on our research, we believe that macrophages are very potential anti-cancer therapeutic targets, and the development and application of macrophage-targeted drugs has important clinical significance for improving the prognosis of cancer patients. But our current understanding of TAMs is still relatively limited due to their high heterogeneity and plasticity, as well as the intricate interactions between cells within the TME. Therefore, we strongly recommend further research in macrophage-targeted therapy in the future. In this context, the application of new technologies (including scRNA-seq, mass cytometry, multiplex fluorescence immunohistochemistry, TAMs in vivo imaging, and spatial transcriptome sequencing) and the development of clinical trials will be very helpful for our comprehensive understanding of TAMs. In conclusion, TAMs-targeted therapy is a very promising antitumor immunotherapy strategy. However, to obtain the optimal-targeted macrophages therapy, that is, to deliver specific macrophage-targeted drugs dynamically and efficiently in real time, we still need to conduct more in-depth research on TME and TAMs.
References
Li J, Jiang X, Li H, Gelinsky M, Gu Z. Tailoring materials for modulation of macrophage fate. Adv Mater. 2021;33(12): e2004172. https://doi.org/10.1002/adma.202004172.
Chamseddine AN, Assi T, Mir O, Chouaib S. Modulating tumor-associated macrophages to enhance the efficacy of immune checkpoint inhibitors: a TAM-pting approach. Pharmacol Ther. 2021. https://doi.org/10.1016/j.pharmthera.2021.107986.
Bleriot C, Chakarov S, Ginhoux F. Determinants of resident tissue macrophage identity and function. Immunity. 2020;52(6):957–70. https://doi.org/10.1016/j.immuni.2020.05.014.
Bian Z, Gong Y, Huang T, Lee CZW, Bian L, Bai Z, et al. Deciphering human macrophage development at single-cell resolution. Nature. 2020;582(7813):571–6. https://doi.org/10.1038/s41586-020-2316-7.
Prinz M, Masuda T, Wheeler MA, Quintana FJ. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol. 2021;39:251–77. https://doi.org/10.1146/annurev-immunol-093019-110159.
Haist M, Stege H, Grabbe S, Bros M. The functional crosstalk between myeloid-derived suppressor cells and regulatory T cells within the immunosuppressive tumor microenvironment. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13020210.
Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643-60.e17. https://doi.org/10.1016/j.cell.2020.05.007.
Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47(2):323-38.e6. https://doi.org/10.1016/j.immuni.2017.07.014.
Chow A, Schad S, Green MD, Hellmann MD, Allaj V, Ceglia N, et al. Tim-4(+) cavity-resident macrophages impair anti-tumor CD8(+) T cell immunity. Cancer Cell. 2021;39(7):973-88.e9. https://doi.org/10.1016/j.ccell.2021.05.006.
Nalio Ramos R, Missolo-Koussou Y, Gerber-Ferder Y, Bromley CP, Bugatti M, Nunez NG, et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell. 2022. https://doi.org/10.1016/j.cell.2022.02.021.
Casanova-Acebes M, Dalla E, Leader AM, LeBerichel J, Nikolic J, Morales BM, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595(7868):578–84. https://doi.org/10.1038/s41586-021-03651-8.
Huggins DN, LaRue RS, Wang Y, Knutson TP, Xu Y, Williams JW, et al. Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 2021;81(20):5284–95. https://doi.org/10.1158/0008-5472.Can-21-0101.
Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24(4):595–610. https://doi.org/10.1038/s41593-020-00789-y.
Wang J, Wang Y, Chu Y, Li Z, Yu X, Huang Z, et al. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol. 2021;74(3):627–37. https://doi.org/10.1016/j.jhep.2020.10.021.
Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–9. https://doi.org/10.1172/jci84427.
Montalbán Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wöckel A, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages-a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016;4:49. https://doi.org/10.1186/s40425-016-0154-9.
Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–26. https://doi.org/10.1016/j.it.2011.12.001.
Hourani T, Holden JA, Li W, Lenzo JC, Hadjigol S, O’Brien-Simpson NM. Tumor associated macrophages: origin, recruitment, phenotypic diversity, and targeting. Front Oncol. 2021;11: 788365. https://doi.org/10.3389/fonc.2021.788365.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):78. https://doi.org/10.1186/s12929-019-0568-z.
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50. https://doi.org/10.1016/j.cmet.2019.06.001.
Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 2015;53(5):676–88. https://doi.org/10.1165/rcmb.2015-0012OC.
Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, et al. M-CSF and GM-CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res. 2016;76(1):35–42. https://doi.org/10.1158/0008-5472.Can-15-0869.
Martinez VG, Rubio C, Martinez-Fernandez M, Segovia C, Lopez-Calderon F, Garin MI, et al. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder cancer. Clin Cancer Res. 2017;23(23):7388–99. https://doi.org/10.1158/1078-0432.CCR-17-1004.
Im JH, Buzzelli JN, Jones K, Franchini F, Gordon-Weeks A, Markelc B, et al. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat Commun. 2020;11(1):4064. https://doi.org/10.1038/s41467-020-17914-x.
Faas M, Ipseiz N, Ackermann J, Culemann S, Grüneboom A, Schröder F, et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. 2021;54(11):2531-46.e5. https://doi.org/10.1016/j.immuni.2021.09.010.
Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24(6):695–709. https://doi.org/10.1016/j.ccr.2013.11.007.
Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129(12):5151–62. https://doi.org/10.1172/jci128644.
Solís-Martínez R, Cancino-Marentes M, Hernández-Flores G, Ortiz-Lazareno P, Mandujano-Álvarez G, Cruz-Gálvez C, et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol Lett. 2018;196:140–8. https://doi.org/10.1016/j.imlet.2018.02.009.
Chen Q, Li Y, Gao W, Chen L, Xu W, Zhu X. Exosome-mediated crosstalk between tumor and tumor-associated macrophages. Front Mol Biosci. 2021;8: 764222. https://doi.org/10.3389/fmolb.2021.764222.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977.
Baig MS, Roy A, Rajpoot S, Liu D, Savai R, Banerjee S, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69(5):435–51. https://doi.org/10.1007/s00011-020-01318-0.
Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G, et al. Colorectal cancer-derived small extracellular vesicles promote tumor immune evasion by upregulating PD-L1 expression in tumor-associated macrophages. Adv Sci (Weinh). 2022. https://doi.org/10.1002/advs.202102620.
Yang C, Dou R, Wei C, Liu K, Shi D, Zhang C, et al. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol Ther. 2021;29(6):2088–107. https://doi.org/10.1016/j.ymthe.2021.02.006.
Rabe DC, Walker ND, Rustandy FD, Wallace J, Lee J, Stott SL, et al. Tumor extracellular vesicles regulate macrophage-driven metastasis through CCL5. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13143459.
Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–20. https://doi.org/10.1007/82_2010_74.
Zhang G, Guo L, Yang C, Liu Y, He Y, Du Y, et al. A novel role of breast cancer-derived hyaluronan on inducement of M2-like tumor-associated macrophages formation. Oncoimmunology. 2016;5(6): e1172154. https://doi.org/10.1080/2162402x.2016.1172154.
Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110(2):587–95. https://doi.org/10.1182/blood-2007-01-068031.
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47. https://doi.org/10.1146/annurev-pathmechdis-012418-012718.
Braun DA, Street K, Burke KP, Cookmeyer DL, Denize T, Pedersen CB, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. 2021;39(5):632-48.e8. https://doi.org/10.1016/j.ccell.2021.02.013.
Trinh A, Gil Del Alcazar CR, Shukla SA, Chin K, Chang YH, Thibault G, et al. Genomic alterations during the in situ to invasive ductal breast carcinoma transition shaped by the immune system. Mol Cancer Res. 2021;19(4):623–35. https://doi.org/10.1158/1541-7786.MCR-20-0949.
Koh MY, Sayegh N, Agarwal N. Seeing the forest for the trees-single-cell atlases link CD8(+) T cells and macrophages to disease progression and treatment response in kidney cancer. Cancer Cell. 2021;39(5):594–6. https://doi.org/10.1016/j.ccell.2021.03.008.
Chong BF, Tseng LC, Hosler GA, Teske NM, Zhang S, Karp DR, et al. A subset of CD163+ macrophages displays mixed polarizations in discoid lupus skin. Arthritis Res Ther. 2015;17:324. https://doi.org/10.1186/s13075-015-0839-3.
Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184(3):792-809.e23. https://doi.org/10.1016/j.cell.2021.01.010.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. https://doi.org/10.1038/nri2448.
Wang L, Sfakianos JP, Beaumont KG, Akturk G, Horowitz A, Sebra RP, et al. Myeloid cell-associated resistance to pd-1/pd-l1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin Cancer Res. 2021;27(15):4287–300. https://doi.org/10.1158/1078-0432.Ccr-20-4574.
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs functional differentiation. Front Immunol. 2014;5:514. https://doi.org/10.3389/fimmu.2014.00514.
Mantovani A. Reflections on immunological nomenclature: in praise of imperfection. Nat Immunol. 2016;17(3):215–6. https://doi.org/10.1038/ni.3354.
Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17(1):34–40. https://doi.org/10.1038/ni.3324.
Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020. https://doi.org/10.1016/j.cell.2020.03.048.
Obradovic A, Chowdhury N, Haake SM, Ager C, Wang V, Vlahos L, et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell. 2021. https://doi.org/10.1016/j.cell.2021.04.038.
Dvorak HF. Tumors: wounds that do not heal similarities between tumor stroma generation and wound healing. N Engl J Med. 1986. https://doi.org/10.1056/nejm198612253152606.
Marcovecchio PM, Thomas G, Salek-Ardakani S. CXCL9-expressing tumor-associated macrophages: new players in the fight against cancer. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-002045.
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. 2020;11:1731. https://doi.org/10.3389/fimmu.2020.01731.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96. https://doi.org/10.1038/ni.1937.
Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32(6):869-83.e5. https://doi.org/10.1016/j.ccell.2017.11.004.
Guc E, Pollard JW. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity. 2021;54(5):885–902. https://doi.org/10.1016/j.immuni.2021.03.022.
Zhang B, Ye H, Ren X, Zheng S, Zhou Q, Chen C, et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019;459:204–15. https://doi.org/10.1016/j.canlet.2019.06.005.
Chen P, Hsu WH, Han J, Xia Y, DePinho RA. Cancer stemness meets immunity: from mechanism to therapy. Cell Rep. 2021;34(1): 108597. https://doi.org/10.1016/j.celrep.2020.108597.
Liguori M, Digifico E, Vacchini A, Avigni R, Colombo FS, Borroni EM, et al. The soluble glycoprotein NMB (GPNMB) produced by macrophages induces cancer stemness and metastasis via CD44 and IL-33. Cell Mol Immunol. 2021;18(3):711–22. https://doi.org/10.1038/s41423-020-0501-0.
Nasrollahzadeh E, Razi S, Keshavarz-Fathi M, Mazzone M, Rezaei N. Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol Immunother. 2020;69(9):1673–97. https://doi.org/10.1007/s00262-020-02616-6.
Sharma VP, Tang B, Wang Y, Duran CL, Karagiannis GS, Xue EA, et al. Live tumor imaging shows macrophage induction and TMEM-mediated enrichment of cancer stem cells during metastatic dissemination. Nat Commun. 2021;12(1):7300. https://doi.org/10.1038/s41467-021-27308-2.
Qiu S, Deng L, Liao X, Nie L, Qi F, Jin K, et al. Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci. 2019;110(7):2110–8. https://doi.org/10.1111/cas.14078.
Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016. https://doi.org/10.1126/science.aad3018.
Mertens C, Mora J, Ören B, Grein S, Winslow S, Scholich K, et al. Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology. 2018;7(3): e1408751. https://doi.org/10.1080/2162402x.2017.1408751.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74. https://doi.org/10.1038/nrc.2017.51.
Lin L, Chen YS, Yao YD, Chen JQ, Chen JN, Huang SY, et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015;6(33):34758–73. https://doi.org/10.18632/oncotarget.5325.
Yeo EJ, Cassetta L, Qian BZ, Lewkowich I, Li JF, Stefater JA 3rd, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74(11):2962–73. https://doi.org/10.1158/0008-5472.Can-13-2421.
Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. https://doi.org/10.3389/fonc.2019.01370.
Rømer AMA, Thorseth ML, Madsen DH. Immune modulatory properties of collagen in cancer. Front Immunol. 2021;12: 791453. https://doi.org/10.3389/fimmu.2021.791453.
Cendrowicz E, Sas Z, Bremer E, Rygiel TP. The Role of macrophages in cancer development and therapy. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13081946.
Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 2015;5(9):932–43. https://doi.org/10.1158/2159-8290.Cd-15-0012.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. https://doi.org/10.1016/j.immuni.2014.06.010.
Ginter PS, Karagiannis GS, Entenberg D, Lin Y, Condeelis J, Jones JG, et al. Tumor microenvironment of metastasis (TMEM) doorways are restricted to the blood vessel endothelium in both primary breast cancers and their lymph node metastases. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11101507.
Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23(5):1239–48. https://doi.org/10.1016/j.celrep.2018.04.007.
Chen C, He W, Huang J, Wang B, Li H, Cai Q, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9(1):3826. https://doi.org/10.1038/s41467-018-06152-x.
Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J Exp Med. 2017;214(9):2695–713. https://doi.org/10.1084/jem.20160392.
Bieniasz-Krzywiec P, Martín-Pérez R, Ehling M, García-Caballero M, Pinioti S, Pretto S, et al. Podoplanin-expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metab. 2019;30(5):917-36.e10. https://doi.org/10.1016/j.cmet.2019.07.015.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. https://doi.org/10.1038/nrd.2018.169.
Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):1–12. https://doi.org/10.1038/s41423-019-0306-1.
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7–H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–81. https://doi.org/10.1084/jem.20050930.
ElTanbouly MA, Schaafsma E, Noelle RJ, Lines JL. VISTA: coming of age as a multi-lineage immune checkpoint. Clin Exp Immunol. 2020;200(2):120–30. https://doi.org/10.1111/cei.13415.
Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα Immune Checkpoint. Immunity. 2020;52(5):742–52. https://doi.org/10.1016/j.immuni.2020.04.011.
Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, et al. Engagement of MHC class i by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19(1):76–84. https://doi.org/10.1038/s41590-017-0004-z.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6. https://doi.org/10.1038/s41586-019-1456-0.
Neyen C, Plüddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, et al. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol. 2013;190(7):3798–805. https://doi.org/10.4049/jimmunol.1203194.
Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15(9):2000–11. https://doi.org/10.1016/j.celrep.2016.04.084.
Viitala M, Virtakoivu R, Tadayon S, Rannikko J, Jalkanen S, Hollmen M. Immunotherapeutic blockade of macrophage clever-1 reactivates the CD8(+) T-cell response against immunosuppressive tumors. Clin Cancer Res. 2019;25(11):3289–303. https://doi.org/10.1158/1078-0432.CCR-18-3016.
La Fleur L, Botling J, He F, Pelicano C, Zhou C, He C, et al. Targeting MARCO and IL37R on immunosuppressive macrophages in lung cancer blocks regulatory T cells and supports cytotoxic lymphocyte function. Cancer Res. 2021;81(4):956–67. https://doi.org/10.1158/0008-5472.Can-20-1885.
Masetti M, Carriero R, Portale F, Marelli G, Morina N, Pandini M, et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J Exp Med. 2022. https://doi.org/10.1084/jem.20210564.
Binnewies M, Pollack JL, Rudolph J, Dash S, Abushawish M, Lee T, et al. Targeting TREM2 on tumor-associated macrophages enhances immunotherapy. Cell Rep. 2021;37(3): 109844. https://doi.org/10.1016/j.celrep.2021.109844.
Esparza-Baquer A, Labiano I, Sharif O, Agirre-Lizaso A, Oakley F, Rodrigues PM, et al. TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms. Gut. 2021;70(7):1345–61. https://doi.org/10.1136/gutjnl-2019-319227.
Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang T, et al. Blockade of DC-SIGN(+) tumor-associated macrophages reactivates antitumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res. 2020;80(8):1707–19. https://doi.org/10.1158/0008-5472.CAN-19-2254.
Qi Y, Chang Y, Wang Z, Chen L, Kong Y, Zhang P, et al. Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol Immunother. 2019;68(12):2067–80. https://doi.org/10.1007/s00262-019-02429-2.
Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics. 2021;11(8):3839–52. https://doi.org/10.7150/thno.53749.
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, et al. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6(12):1578–92. https://doi.org/10.1158/2326-6066.Cir-17-0479.
Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–40. https://doi.org/10.1016/j.immuni.2019.03.024.
Quaranta V, Schmid MC. Macrophage-mediated subversion of anti-tumour immunity. Cells. 2019. https://doi.org/10.3390/cells8070747.
Hanks BA, Holtzhausen A, Evans KS, Jamieson R, Gimpel P, Campbell OM, et al. Type III TGF-β receptor downregulation generates an immunotolerant tumor microenvironment. J Clin Invest. 2013;123(9):3925–40. https://doi.org/10.1172/jci65745.
Regis S, Dondero A, Caliendo F, Bottino C, Castriconi R. NK cell function regulation by TGF-β-induced epigenetic mechanisms. Front Immunol. 2020;11:311. https://doi.org/10.3389/fimmu.2020.00311.
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–37. https://doi.org/10.1016/j.ccell.2014.09.006.
Cui C, Chakraborty K, Tang XA, Schoenfelt KQ, Hoffman A, Blank A, et al. A lysosome-targeted DNA nanodevice selectively targets macrophages to attenuate tumours. Nat Nanotechnol. 2021;16(12):1394–402. https://doi.org/10.1038/s41565-021-00988-z.
Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829-42.e13. https://doi.org/10.1016/j.cell.2016.09.031.
Labadie BW, Bao R, Luke JJ. Reimagining ido pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin Cancer Res. 2019;25(5):1462–71. https://doi.org/10.1158/1078-0432.Ccr-18-2882.
d’Almeida SM, Kauffenstein G, Roy C, Basset L, Papargyris L, Henrion D, et al. The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer tumor-associated macrophages: regulatory role of IL-27. Oncoimmunology. 2016;5(7): e1178025. https://doi.org/10.1080/2162402x.2016.1178025.
Hinshaw DC, Hanna A, Lama-Sherpa T, Metge B, Kammerud SC, Benavides GA, et al. Hedgehog signaling regulates metabolism and polarization of mammary tumor-associated macrophages. Cancer Res. 2021;81(21):5425–37. https://doi.org/10.1158/0008-5472.CAN-20-1723.
Sun X, He X, Zhang Y, Hosaka K, Andersson P, Wu J, et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut. 2022;71(1):129–47. https://doi.org/10.1136/gutjnl-2020-322744.
Inagaki T, Fujiwara K, Shinohara Y, Azuma M, Yamazaki R, Mashima K, et al. Perivascular macrophages produce type I collagen around cerebral small vessels under prolonged hypertension in rats. Histochem Cell Biol. 2021;155(4):503–12. https://doi.org/10.1007/s00418-020-01948-9.
Tang PC, Chung JY, Xue VW, Xiao J, Meng XM, Huang XR, et al. Smad3 promotes cancer-associated fibroblasts generation via macrophage-myofibroblast transition. Adv Sci (Weinh). 2022;9(1): e2101235. https://doi.org/10.1002/advs.202101235.
Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77(13):3655–65. https://doi.org/10.1158/0008-5472.Can-16-3199.
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33(10):2040-58.e10. https://doi.org/10.1016/j.cmet.2021.09.002.
Gil-Bernabé AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–75. https://doi.org/10.1182/blood-2011-08-376426.
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–48. https://doi.org/10.1016/j.ccell.2016.10.009.
Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang H, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. 2020;579(7798):284–90. https://doi.org/10.1038/s41586-020-2054-x.
Sharma SK, Chintala NK, Vadrevu SK, Patel J, Karbowniczek M, Markiewski MM. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194(11):5529–38. https://doi.org/10.4049/jimmunol.1403215.
Nosaka T, Baba T, Tanabe Y, Sasaki S, Nishimura T, Imamura Y, et al. Alveolar Macrophages drive hepatocellular carcinoma lung metastasis by generating leukotriene b(4). J Immunol. 2018;200(5):1839–52. https://doi.org/10.4049/jimmunol.1700544.
Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21(6):341–52. https://doi.org/10.1038/s41580-020-0237-9.
Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16(6):488–94. https://doi.org/10.1038/ncb2976.
Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21(5):325–38. https://doi.org/10.1038/s41568-021-00332-6.
Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. https://doi.org/10.1186/s13045-019-0760-3.
Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9(1):21. https://doi.org/10.1038/s41467-017-02481-5.
Cabrera RM, Mao SPH, Surve CR, Condeelis JS, Segall JE. A novel neuregulin-jagged1 paracrine loop in breast cancer transendothelial migration. Breast Cancer Res. 2018;20(1):24. https://doi.org/10.1186/s13058-018-0960-8.
Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, Oshimori N. Tumor-initiating cells establish an IL-33-TGF-β niche signaling loop to promote cancer progression. Science. 2020. https://doi.org/10.1126/science.aay1813.
Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor vcam-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–49. https://doi.org/10.1016/j.ccr.2011.08.025.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. https://doi.org/10.1038/nature10138.
Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, et al. Monocyte induction of e-selectin-mediated endothelial activation releases ve-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res. 2016;76(18):5302–12. https://doi.org/10.1158/0008-5472.Can-16-0784.
Ma RY, Zhang H, Li XF, Zhang CB, Selli C, Tagliavini G, et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J Exp Med. 2020. https://doi.org/10.1084/jem.20191820.
Keklikoglou I, Cianciaruso C, Güç E, Squadrito ML, Spring LM, Tazzyman S, et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2019;21(2):190–202. https://doi.org/10.1038/s41556-018-0256-3.
Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212(7):1043–59. https://doi.org/10.1084/jem.20141836.
Kfoury Y, Baryawno N, Severe N, Mei S, Gustafsson K, Hirz T, et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell. 2021;39(11):1464-78.e8. https://doi.org/10.1016/j.ccell.2021.09.005.
Kitamura T, Kato Y, Brownlie D, Soong DYH, Sugano G, Kippen N, et al. Mammary tumor cells with high metastatic potential are hypersensitive to macrophage-derived HGF. Cancer Immunol Res. 2019;7(12):2052–64. https://doi.org/10.1158/2326-6066.Cir-19-0234.
Brownlie D, Doughty-Shenton D, Yh Soong D, Nixon C, N OC, L MC, et al. Metastasis-associated macrophages constrain antitumor capability of natural killer cells in the metastatic site at least partially by membrane bound transforming growth factor β. J Immunother Cancer. 2021;9(1). https://doi.org/10.1136/jitc-2020-001740.
Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212(9):1433–48. https://doi.org/10.1084/jem.20141555.
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18(5):549–60. https://doi.org/10.1038/ncb3340.
Funding
This work was supported by the key research and development (R&D) projects of Gansu Province (Grant no. 17YF1FA126); Lanzhou Science and Technology Bureau Medical and Health Project (Grant no. 2021–90); Special fund project for doctoral training program of Lanzhou University Second Hospital (Grant no. YJS-BD-25); and CuiYing Science and Technology Innovation plan project of Lanzhou University Second Hospital (Grant no. CY2017-BJ05)
Author information
Authors and Affiliations
Contributions
S-PF had the idea for the article. S-PF and H-HL performed the literature search and data analysis. Z-XX, B-WX, H-LS, D-YL, X-W, W-HB and S-PF drafted and/or critically revised the work. Z-XX, B-WX and H-LS drawed figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Bai, W., Hu, L. et al. The pleiotropic mode and molecular mechanism of macrophages in promoting tumor progression and metastasis. Clin Transl Oncol 25, 91–104 (2023). https://doi.org/10.1007/s12094-022-02932-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02932-6