Abstract
Background
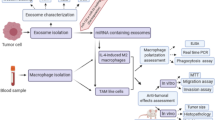
This review focuses on exosomes derived from various cancer cells. The review discusses the possibility of differentiating macrophages in alternatively activated anti-inflammatory pro-tumorigenic M2 macrophage phenotypes and classically activated pro-inflammatory, anti-tumorigenic M1 macrophage phenotypes in the tumor microenvironment (TME). The review is divided into two main parts, as follows: (1) role of exosomes in alternatively activating M2-like macrophages-breast cancer-derived exosomes, hepatocellular carcinoma (HCC) cell-derived exosomes, lung cancer-derived exosomes, prostate cancer-derived exosomes, Oral squamous cell carcinoma (OSCC)—derived exosomes, epithelial ovarian cancer (EOC)—derived exosomes, Glioblastoma (GBM) cell-derived exosomes, and colorectal cancer-derived exosomes, (2) role of exosomes in classically activating M1-like macrophages, oral squamous cell carcinoma-derived exosomes, breast cancer-derived exosomes, Pancreatic-cancer derived modified exosomes, and colorectal cancer-derived exosomes, and (3) exosomes and antibody-dependent cellular cytotoxicity (ADCC). This review addresses the following subjects: (1) crosstalk between cancer-derived exosomes and recipient macrophages, (2) the role of cancer-derived exosome payload(s) in modulating macrophage fate of differentiation, and (3) intracellular signaling mechanisms in macrophages regarding the exosome’s payload(s) upon its uptake and regulation of the TME.
Evidence
Under the electron microscope, nanoscale exosomes appear as specialized membranous vesicles that emerge from the endocytic cellular compartments. Exosomes harbor proteins, growth factors, cytokines, lipids, miRNA, mRNA, and DNAs. Exosomes are released by many cell types, including reticulocytes, dendritic cells, B-lymphocytes, platelets, mast cells, and tumor cells. It is becoming clear that exosomes can impinge upon signal transduction pathways, serve as a mediator of signaling crosstalk, thereby regulating cell-to-cell wireless communications.
Conclusion
Based on the vesicular cargo, the molecular constituents, the exosomes have the potential to change the fate of macrophage phenotypes, either M1, classically activated macrophages, or M2, alternatively activated macrophages. In this review, we discuss and describe the ability of tumor-derived exosomes in the mechanism of macrophage activation and polarization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eukaryotic cells release an array of membranous vesicles under physiological conditions as well as in disease states. There are two major classes of these membrane-derived extracellular vesicles (EVs), depending on their size: (a) the larger class of nanometer-sized vesicles are called microvesicles (100–1000 nm) and (b) the smaller class, exosomes (30–100 nm) (Fig. 1) [1, 2]. It is important to remember that these membranous vesicles are distinctly different from apoptotic bodies, which are released from cells undergoing programmed cell death, are of a different cellular origin and molecular composition and containing fragments of cell nuclei [2, 3].
Exosomes have been investigated in different types of diseases and found to have many effects from pathology to protection. Studies have increasingly indicated that exosomes can reflect their cellular origin and disease state through the bioactive cargoes they transport, making exosomes useful as potential biomarkers for diagnosis and prognosis of diseases [4, 5]. The formation of exosomes have been described to originate from hematopoietic cells, e.g., reticulocytes, B-lymphocytes, and T-cells, platelets, mast cells, dendritic cells, and macrophages [5]. However, exosomes are also produced by the cells of non-hematopoietic origin like epithelial cells (intestinal epithelial cells), Schwann cells, astrocytes, neurons, melanocytes, mesothelioma cells, adipocytes, fibroblasts, and tumor cells [4,5,6,7]. Exosomes have been shown to change the macrophage phenotype.
Based on the molecular constituents, exosomes can switch macrophage phenotype, either to M1, classically activated or to M2, alternatively activated macrophages (Fig. 1). M1 macrophages are described as the pro-inflammatory phenotype, play an important role in the mechanisms of direct host-defense against pathogens, such as phagocytosis and secretion of pro-inflammatory cytokines and microbicidal molecules [3, 8, 9]. In contrast, M2 macrophages are described to regulate the resolution phase of inflammation in the aftermath of tissue damage [10,11,12]. The imbalance of the macrophage phenotypes is associated with several immunity-related diseases. For example, it has been shown that increased M1/M2 ratio correlates with the development of inflammatory bowel disease, as well as osteoarthritis (OA) and obesity [13,14,15]. Paradoxically, in vitro experiments, implicated M2 macrophages as the primary mediators of tissue fibrosis [16]. Several studies have linked the fibrotic profile of M2 macrophages with the pathogenesis of systemic sclerosis [17, 18]. Here, we will describe and discuss the current knowledge on exosomes that can differentially activate macrophages, either to M1 or M2 states. The review will assemble the most recent information about the exosome-mediated macrophage phenotypic changes that are known to occur during various pathophysiological states.
Exosomes in the activation of M2-like macrophages
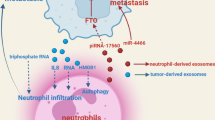
Highly transformed cancer cells are usually anchorage-independent cells, and their cytoskeletal proteins are less defined than well-differentiated cells [19, 20]. It is, therefore, not surprising that colorectal cancer cell-derived exosomes that release cytoskeleton protein fragments can induce conversion of M1–M2 phenotypes [21]. Rearrangement of cytoskeletal proteins is a primordial characteristic feature of macrophage activation and also the maturation of macrophages [21]. The tumor microenvironment (TME) is known to support tumor growth [22]. Moreover, tumor progression is associated with several immune cells, mainly macrophages. Exosome activated macrophages are now considered to be key players in cancer progression, as macrophages stimulate accessory signaling pathways to promote tumor growth, invasion, tumor-associated angiogenesis, tissue inflammation, and immunologic remodelling [23, 24]. Macrophages are key modulators for the activation of cancer-associated fibroblasts, pro-angiogenic factors, and metastatic factors; together these participate in the formation of the TME [24,25,26].
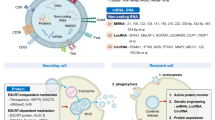
Tumor-derived exosomes play key roles in immune modulation and other physiological processes, and all types of immune cells have been reported to respond to exosomes. However, exosomes can induce the extent and the magnitude of macrophage polarization; after that, activated M1/M2 macrophages act according to the TME they sense. It has been shown that TME can educate macrophages, called tumor-associated macrophages (TAM) that display M2 phenotype, secrete pro-angiogenic factors and cytokines, responsible for angiogenesis, tumor growth and metastasis [23]. Cellular crosstalk is essential for maintaining tissue homeostasis and disease development [27, 28]. Intercellular communication occurs via direct cell-to-cell contact (short distance) or through chemokines/cytokines (long distances). In the recent past, the third mechanism for cellular information exchange has emerged, which involves intracellular migration of extracellular vesicles (EVs) [29, 30]. This mode of cellular interaction has the potential to deliver diverse molecular cargo to recipient cells, thereby modulating their phenotype and expression [31]. Tumor-derived exosomes are considered important mediators of cellular communication between the immune cell and cancerous cells. Within the cancer microenvironment, immune cells often display altered phenotypes capable of contributing to tumor progression, including the promotion of tumor growth, migration, pre-metastatic niche formation, and metastasis [32, 33]. Recent studies have shown more evidence on the role of tumor-derived exosomes in macrophage M2 polarization to promote tumor progression as described in Table 1.
Breast cancer-derived exosomes
The role of exosomes derived from a cancer cell in macrophage polarization is currently unclear. While some studies have shown that exosomes have the potential to lead macrophages towards the M1 phenotype [34, 35], other studies have shown the polarization of macrophages to the M2 phenotype [36, 37]. Contrary to these two opposing views, some studies suggest the inability of tumor-derived exosomes to sufficiently evoke macrophage plasticity. For instance, Ham et al., (2018) revealed that the IL-6 receptor gp130 was found in breast cancer cell-derived exosomes which could stimulate STAT3 signaling in bone marrow-derived macrophages (BMDMs) (Table 1). In response to exosome exposure, these BMDMs could upregulate pro- and anti-inflammatory cytokines and survive longer [38]. Exposure of macrophages to tumor-derived exosomes was shown to significantly decrease the mRNA levels of IFNγ, an M1 macrophage marker, whereas IL-1β is upregulated. Other M1 markers, such as iNOS and TNFα, showed no change in expression. The expression of Arg1, and TGF-β, which were indicative of M2 macrophage phenotype was similarly not altered [37]. Together, these results suggest that cancer-derived exosomes alone are insufficient to generate a strong, distinct M1 or M2 macrophage phenotype (Table 1). However, they help in maintaining the TME to mediate pro-survival and proliferative phenotypes [38]. Additionally, another study by Piao et al., (2018) revealed that triple-negative breast cancer (TNBC)-derived exosomes could promote M2 polarization of macrophages, thus creating favourable niche for lymph node metastasis. Co-culture of TNBC-derived exosomes with macrophages exhibited appreciably elongated morphology of macrophages after exosomes were internalized by these macrophages. Quantitative real-time PCR analysis showed a significant increase in M2 markers (Fizz1, CD206, Arg-1) of macrophages co-cultured with cancer-derived exosomes. Thus, TNBC-derived exosomes can act as cellular messengers to orchestrate M2-type macrophage polarization, as evidenced by the enhanced tumor growth and axillary lymph node metastasis in an orthotropic TNBC model [37].
Hepatocellular carcinoma (HCC) cells derived exosomes
HCC is the fourth most common cancer type and mostly associated with chronic liver disease [39, 40]. Growing evidence suggests the ability of HCC to secrete a large number of exosomes into their local environment. Mass spectroscopic analysis and other molecular analytical techniques revealed exosomes as small packages, rich in varied content, which can influence the local or distant environment [41, 42]. Li et al. [43] reported that HCC cells–derived exosomes contain elevated levels of the long non-coding RNA (lncRNA) TUC339, which could crosstalk with neighbouring macrophages to modulate the M1/M2 phenotype. The biological function of HCC derived exosomes rich in lncRNA-TUC339 was dissected using ThP1 as a model system which can take up the HCC derived exosomes. Suppression of lncRNA-TUC339 using specific siRNA showed an enhanced M1 phenotype and increased pro-inflammatory cytokine production by macrophages [43]. Overexpression of TUC339 in ThP1 macrophages led to decreased levels of TNFα and IL1β in response to LPS stimulation compared to the empty vector-transfected cells [43]. This suggests that the lncRNA TUC339 can be transported from HCC tumor cells to neighbouring macrophages to modulate macrophage polarization to an M2 phenotype, which has the potential to dampen an anti-tumor immune response (Table 1).
Lung-cancer derived exosomes
Lung cancer is one of the leading causes of cancer-related deaths, with the survival of only 17.7% of the patients for 5 years after diagnosis [44, 45]. Studies show that hypoxia stimulates lung cancer to secrete exosomes rich in microRNAs like miR-103a whereby the lung cancer TME shows enhanced angiogenesis and blood vessel permeability, resulting in increased metastasis [46]. Tumor-secreted miR-103a in exosomes was shown to directly regulate the macrophage polarization protein PTEN by binding to its 3′-UTRs and eventually decreasing its expression. Downregulation of PTEN causes activation of PI3K/Akt and STAT3 signaling pathways finally leads to the enhanced accumulation of cancer-promoting factors like IL10, CCL2, and VEGF-A and diminishes the antitumor immune response [47]. Thus, tumor hypoxia can switch macrophages from tumor suppressing to the tumor-promoting characteristics (Table 1).
Prostate-cancer derived exosomes
Several clinical studies provide evidence of involvement of hypoxia-related signaling pathways in prostate tumor growth, progression, and metastasis [48,49,50,51,52]. A recent report by Panigrahi et al. [36] showed increased concentrations of exosomes under hypoxic conditions compared to normoxia in prostate cancer. These hypoxic prostate cancer cells were shown to secrete exosomes rich in lactate (product of glycolysis) which could promote neighboring macrophages such as TAM towards M2 like characteristics, eventually developing an immunosuppressive environment for tumor proliferation and progression [36]. Colegio et al. [53] provided a detailed mechanistic view on the functional polarization of macrophages by tumor-derived lactate. Conditioned medium obtained from tumor cells was rich in lactate that could upregulate the mRNA expression of VEGF and Arg1 in macrophages. VEGF mediates neovascularization of the tumor, and its expression is regulated by induction of hypoxia (HIF1α) or by nutrient deprivation [54]. Tumor-derived lactate was shown to induce VEGF and Arg1 expression in macrophages via stabilization of transcription factor HIF1α in macrophages [53]. HIF1α-deficient macrophages stimulated with lactic acid failed to upregulate VEGF or Arg1, thus confirming the involvement of HIF1α. Arg1 is an essential enzyme in the synthesis of polyamines which regulate cell proliferation and thus promote tumor growth [55]. Additionally, enhanced production of Arg1 by macrophages can lead to a weak cytotoxic response to a growing tumor cell. Thus, high levels of Arg1 can act as a key component in providing a positive feedback loop to promote immune suppression at a tumor site: ARG1 enables the generation of polyamines in macrophages which promote tumor cell development which secrete lactate thereby maintaining the HIF-1α in an active state in M2 macrophages which in turn secrete ARG1 (Table 1) [55].
Oral squamous cell carcinoma (OSCC) derived exosomes
Oral squamous cell carcinoma (OSCC) belongs to head and neck squamous cell carcinoma (HNSCC) and accounts for 24% of all head and neck cancers. Additionally, more than 50% of OSCC patients exhibit lymph node metastasis which is one of the most common adverse prognostic factors in OSCC patients [56,57,58]. The OSCC matrix is rich in immune cells, such as macrophages, which can differentiate into M1 or M2 subtype depending on the TME. Recent investigations show that OSCC cells release a high amount of circulating exosomes rich in microRNAs [57, 59,60,61,62,63]. One of such study showed higher expression of miR-29 (including miR-29a-3p, miR-29b-3p, and miR-29c-3p) in the developing OSCC tissue [60, 64]. Out of these three microRNA subtypes, OSCC secreted exosomes showed high levels of miR-29a-3p [59]. Co-culture of OSCC derived exosomes with naïve macrophages showed elevated expression of macrophage M2 markers (CD206, Arg1, and IL10) with no significant change in M1 markers (IL1β and CXC10), thus emphasizing that exosome-enclosed miR-29a-3p can promote macrophage polarization towards the immunosuppressive M2 phenotype. Transfection of miR-29a-3p mimics into macrophages resulted in a decrease in SOCS1 mRNA and protein expression which ultimately increased the phosphorylation of STAT1, an important transcription factor for M2 markers (Table 1). Thus, this study revealed that miR-23a-3p rich tumor-secreted exosomes could act as cellular transmitters to modulate the naïve macrophage towards M2 phenotype by activating SOCS1/STAT1 signaling that subsequently enhances the proliferation and invasion of OSCC cells [59]. In the recent past, it was established that the expression of microRNA in solid tumors is highly dependent on the TME. Hypoxia, the master regulator of cancer metastasis, can stabilize and activate hypoxia-inducible factors (HIF), especially HIF1α and HIF2α, which ultimately activate a set of genes that facilitate tumor growth, angiogenesis, and metastasis [65]. Many lines of evidence suggest that hypoxia may promote the release of functional exosomes by cancer cells in a HIF1α dependent manner [65,66,67]. Li et al. [65], determined the miRNA profiles of exosomes derived from normoxic and hypoxic OSCC using miRNA-seq and reported that a high number of common miRNAs (approximately 214) existed in these two groups. Out of these 214 miRNAs, 105 miRNAs were differentially upregulated in hypoxic exosomes compared to normoxic exosomes. Among all the upregulated miRNAs, HIF1 dependent activation and stabilization resulted in significant high-level expression of miR-21 in the hypoxic exosomes (Table 1). Thus, this study postulates that hypoxic OSCC cells released exosome rich in miR-21, which, when recognized by TLRs of normoxic immune cells promote their premetastatic behavior by activating immune-suppressive genes like IL10 and also an enhanced expression of Snail1 and vimentin, the key epithelial-mesenchyme transition (EMT) regulatory factors [65, 68]. TME remodelling by EMT is a major event for regulating epithelial plasticity to ensure cancer progression. The key EMT transcription factor Snail can undergo CBP mediated acetylation at Lysine 146, and Lysine 187, leading to its stabilization [69]. Over-expression of Snail in cancer cells can activate the expression of different cytokines such as IL-6, IL-8, CCL2, and CCL5, which act as chemoattractants for the recruitment of TAMs [69, 70]. Additionally, Snail expressing cancer cells were also shown to secrete exosomes rich in miR-21 into the TME. Mechanistically, chromatin immunoprecipitation studies revealed that Snail1 could directly bind to the promoter of miR-21 and increase its expression at the transcriptional and translational level [70]. Exosomal miR-21 is known to polarize TAMs to M2-Like phenotype to promote tumor progression by utilizing two signaling events. In one event, miR-21 favors pre-metastatic niche formation by inhibiting the expression of a variety of tumor suppressors such as PTEN, PDCD4, and IGFBP3 through direct binding to the 3′-UTR of target transcripts in macrophages and cancer [71,72,73,74,75,76]. miR-21 mediated downregulation of intracellular kinases like PTEN and PDCD4 which contribute to a tumor-promoting microenvironment. PTEN and PDCD4 are important regulators of PI3K/AKT pathways which activate pro-survival signals by enhancing the expression of classical M2 markers, Arg1, and IL10 [68, 77, 78]. A second mechanism by which miR-21 contributes to cancer progression is by increasing the secretion of chemokines like CCL2, CCL5, and IL-8. These lead to the recruitment of TAMs which further recognize exosomal miR-21 leading to the up-regulation of M2 markers like MRC1, CD163, and IL10, thus providing an immunosuppressive microenvironment for cancer cell survival and invasion [69, 70, 79]. Taken together, these studies suggest that cancer cell-derived exosomes containing microRNAs (mir-21, miR-29, and others) provide a positive feedback loop to amplify the TME signals to facilitate tumor progression.
Epithelial ovarian cancer (EOC) derived exosomes
Because of lack of early clinical symptoms and progressive development of drug resistance, EOC remains the 5th most common cause of malignant death in women [80]. Communication between the cancer cells and TAM within the TME is considered to be essential for the progression and development of EOC [81]. These communications can be mediated through exosomes released from the tumor cells [82]. High expression of exosome markers (CD63 and CD81) and M2 markers (CD163) was reported to be associated with poor clinical outcome [83]. A recent study by Chen et al., (2017) demonstrated the secretion of a large number of exosomes from EOC cells into the TME that were taken up by TAM-like macrophages. Gene expression analyses in the same study also showed a significant increase in cancer-derived exosomal microRNAs from EOC cells under the hypoxic conditions as compared to normoxic conditions.
In a microRNAs array analysis, exosomes isolated from patients with benign ovarian cancer showed a higher level of miR-940 [84, 85]. A co-culture of non-polarized macrophages with normoxic exosomes or hypoxic exosomes isolated from epithelial ovarian cancer cells showed macrophage polarization towards tumor supportive M2 phenotypes confirmed by the higher expression of CD163 and CD206 (M2 type markers). Transwell migration and MTS proliferation analyses revealed that M2-phenotype macrophages from miR-940 conditioned medium could augment cancer cell proliferation and migration [84]. The same group showed that under hypoxic conditions, the expression of 3 microRNAs, namely miR-21-3p, miR-125 b-5p, and miR-181 d-5p were increased in the EOC-derived exosomes [86]. The co-culture of the naïve macrophage with hypoxia-induced microRNA containing exosomes could lead to a significant increase in CD163, CD206, and IL10 (M2 markers) at the translational level, thus demonstrating macrophage polarization towards M2 phenotype. These microRNA-rich exosomes recognized by macrophage and were found to regulate the SOCS/STAT pathway in the latter cells. Transfection of macrophages with these three microRNA mimics resulted in decreased SOCS4 expression and a subsequent increase in p-STAT3, which promoted macrophage M2 polarization, along with cancer cell proliferation and distant migration [86]. Similarly, EOC-derived exosomes enriched with miR-222-3p was shown to inhibit the macrophage SOCS3 expression and induce phosphorylation of STAT3 which ultimately increased CD206, Arg1, and IL10 expression and decreased TNFα and IL12 expression in macrophages, promoting them to develop into the M2 phenotype (Table 1). These cancer supportive M2 macrophages could promote the growth and metastasis of ovarian cancer cells [87].
Glioblastoma (GBM) cell-derived exosomes
Glioblastoma represents an aggressive form of cancer affecting the adult primary central nervous system and accounting for 52% of all primary brain tumors. In untreated patients diagnosed with this aggressive neoplasm, the median survival period is only three months [88, 89]. Moreover, the situation remains grim in patients undergoing surgical, chemo-radiotherapeutic and anti-angiogenic therapy with a median survival of only 15–16 months [90, 91]. One of the major reasons for the low efficacy of GBM immunotherapy could be the development of tumor-mediated immunosuppression by the release of anti-inflammatory cytokines and expansion of regulatory T-cells [92,93,94]. Recent work done by Manda et al. [95] showed the abundant presence of exosomes secreted by glioblastoma cells in the peripheral blood of glioblastoma patients. Moreover, these researchers also identified that glioblastoma-derived exosomes were rich in tumor markers like epidermal growth factor receptor (EGFR), EGFR variant III (EGFRvIII), and IDH1-R132H which ultimately fuel glioma transformation/progression. Several studies have revealed that patients with glioblastoma show an elevated number of monocytes relative to healthy donors [96,97,98,99]. Tumor-derived factors, such as TGF-β, can recruit macrophages and preferentially skew them to adopt the immune-suppressive phenotype capable of mediating tumor growth and promoting invasion [100, 101]. Mass spectroscopy analysis performed by Gabrusiewicz et al. [102] revealed that GBM-derived stem cells (GSCs) derived exosomes (GDEs) were enriched with EIF2, mTOR, and ephrin-B signaling related pathway proteins. Analysis of the cargo carried in the GDEs showed elevated levels of phospho-STAT3, which has macrophage polarizing activity. GDE-treated monocytes showed elevated expression of programmed death-ligand 1 (PD-L1), which was accompanied by increased p-STAT3 levels. This finding is in agreement with the observation that CD14+ monocyte/macrophages derived from the GBM tissue of patients showed upregulated PD-L1 expression, whereas no significant expression was seen in CD14+ cells isolated from the blood of GBM patients and control counterparts. Macrophages exposed to GDEs showed increased expression of CD163 and CD206 (M2 markers). Thus, the study demonstrates that GDEs could modulate macrophage M2 polarization and STAT3 mediated induction of immune-suppressive PD-L1 expression [102] (Table 1).
Colorectal cancer-derived exosomes
Colorectal cancer (CRC), the second-highest death-causing cancer disease worldwide, is one of the most prevalent malignant tumors where metastasis account for around 90% of total CRC-related deaths. Metastasis primarily occurs in the liver [35, 103]. CRC is common in the aging population in developed countries and the risk of developing CRC increases in those with unfavorable dietary habits, low physical activity, obesity and smoking [104]. Globally, it is the third most common cancer in men and second in women [105, 106]. CRC is accompanied by immune responses at all stages. TAMs, conventionally considered as pro-tumorigenic, are present abundantly in both primary and metastatic sites of CRC [107, 108]. These macrophages create a favourable TME by providing cytokines, chemokines, and angiogenic factors in the CRC. However, the mechanism of cellular communication between CRC cells and macrophages or other cells in the TME is unclear with exosomes been strongly implicated. Infiltrating monocytes in the TME may differentiate to either anti or pro-inflammatory macrophages upon priming with cancer cell’s secretion. CRC derived exosomes have the potential to educate macrophages to differentiate into anti-inflammatory pro-tumoral M2 phenotype [106, 108]. Being a messenger in cellular communication and associated with various processes like tumor invasion, angiogenesis, cell death and immune evasion, exosomes are the key initiators of pre-metastatic niche formation in multiple cancer types including CRC [21, 109]. SILAC based mass spectrometry was used to trace the proteome of the cargo transported by CRC-exosomes. CT-26 cells derived exosomes were shown to educate macrophages to secrete significantly more TNF and MCP-1, both known to promote CRC cell growth, while no significant differences in IL-6, IL-1β, IL-12p70, and IL-10 expression were seen. Additionally, the transported proteins from these exosomes were abundant in proteins involved in cytoskeleton rearrangement, mediating elongation and F-actin polarization in macrophages. These exosomes were recognized by FcR of macrophages as confirmed by FcR-blocking experiments that diminished cytoskeleton rearrangement in macrophages [21]. In another study, colon cancer cell lines, HCT8 or HCTI16 when co-cultured with THP-1, were shown to contribute to M2 polarization as confirmed by high expression of Arg-1 and Il-10 level and reduced expression of IL-6 and IL-1β [110]. Furthermore, the conditioned medium from THP-1 was reported to contain a high level of TGF-β, HGF (hepatocyte growth factor) and EGF (epidermal growth factor) which can promote M2 polarization of macrophages via activation of the EGFR/PI3K/AKT/mTOR pathway [110]. In addition to above study, miR-203 was identified as the signaling messenger between tumor cells and monocytes in metastatic colorectal cancer (CRC) patients [111]. In vitro studies suggest that CRC cell-derived exosomes with miR-203 cargo could promote higher expression of M2 markers thereby switching monocyte polarization toward M2 TAMs. Both co-culture of miR-203 transfected CRC cell lines (RKO and CaR-1), as well as isolated exosomes from miR-203 transfected CRC cell lines with monocyte like cell line THP-1, resulted in higher expression of M2 markers (CD163 and STAT3) and downregulation of M1 markers (CXCL-10 and NOS2) (Table 1). Injection of miR-203 transfected RKO cells along with THP-1 cells into the splenic vein of a xenograft mouse model showed a nodule generation in all mice when compared to injection of miR-203 transfected RKO cells without THP-1 [111]. Similarly miR-145 carrying EVs (exosomes and shed microvesicles) were also capable of inducing M2 polarization of monocytes in CRC [112]. Mechanistically, miR-145 targeted HDAC11 and TLR4 to upregulate the expression of anti-inflammatory cytokines such as IL-10. CRC cell line DLD-1 derived exosomes were shown to carry miR-145, which, when cultured with THP-1 or NOMO-1 cells, increased the transcription of IL-10 and VEGF-A while the expression of IL-12p40 and TNF-α were reduced (Table 1). The population of surface markers CD11b+, CD68+, and CD206+ on the macrophages also increased in the presence of DLD-1 derived exosomes. Previous studies suggested that miR-145 could target the histone deacetylase HDAC11 and regulate IL-10 production [113]. Disruption of HDAC11 expression was shown to enhance the acetylation of histone H3 and H4 followed by the recruitment of Sp1 and STAT3 transcription factors, which transcriptionally upregulated IL-10. DLD-1 derived exosomes carrying miR-145 could target HDAC11 and promote M2 polarization. Conversely, the overexpression of HDAC11 could reverse the process and downregulate IL-10 expression significantly. Meanwhile, overexpression of miR-145 in DLD-1 cells highly promoted the surrounding monocytes to acquire a M2 phenotype [112,113,114].
Pancreatic-cancer derived exosomes
One of the hallmarks of pancreatic cancer is the presence of a persistent, mild inflammatory TME with high numbers of TAMs, which is directly related to patient mortality [115, 116]. In the TME, the TAMs receive multiple signals from the tumor as well as surrounding cells. Exosomes from PDAC (Pancreatic Ductal Adenocarcinoma) cells were shown to play a significant role in this cellular communication [117]. Linton et al. [116] demonstrated that various pancreatic cancer cell-derived exosomes could influence macrophage polarization and function. These macrophages further facilitated metastasis by producing and secreting several soluble factors. A comparative analysis of exosomes derived from multiple pancreatic cancer cell lines (like AsPC-1, BxPC-3, PANC-1, and MIA PaCa-2) and healthy pancreatic epithelial cells (H6c7) revealed that AsPC-1 (ascites-derived metastatic human PDAC cell line) derived exosomes had a higher expression of surface proteins involved in cell-to-cell interaction and a stronger pro-tumorigenic phenotype [116]. AsPC-1 derived exosomes were rich in ICAM-1 and known to mediate cell–cell interaction [118] and arachidonic acid (AA) (Table 1). Both were found to contribute to the interaction and fusion of exosomes with macrophages. AsPC-1 derived exosomes incubated with non-polarized macrophages (THP-1 cells) caused higher expression of surface proteins CD14, CD163 and CD206 as well as higher secretion of pro-tumorigenic factors like VEGF, MCP-1, IL-6, IL-1β, MMP-9 and TNF-α corroborating with its polarization to a M2-like phenotype [116, 119]. ICAM-1 derived from AsPC-1 exosomes was shown to mediate docking with non-polarized PMA treated differentiated THP-1, which have higher surface exposed CD11c, when compared to undifferentiated THP-1 which has undetectable levels of CD11c [120]. This elevated interaction between the ICAM from exosomes and differentiated THP-1 was indicative of the uptake of exosomes by the macrophages [121]. However, very low rate of interaction and uptake was also reported in the exosomes with low or undetectable levels of ICAM-1 which suggest the involvement of other methods of interaction between exosomes and macrophages. Linton et al. [116] also suggested that the AA content of the exosomes contributed to the fusogenicity of these exosomes with THP-1 macrophages. High AA-containing phosphatidylinositol membranes in colorectal cancer cells [122] as well as high levels of phosphatidylserine, phosphatidylinositol, and phosphatidylcholine containing membranes in metastatic MDA-MB-231 breast cancer cells contribute to enhanced fusion of exosomes with the cancer cells [123]. The high AA content in the phospholipids of the AsPC-1 derived exosomes similarly enhance their fusion to the target macrophages. M2 macrophages in the PDAC secrete higher level of the eicosanoid metabolizing enzymes Microsomal prostaglandin E synthase-1 (mPGES-1) and 5-Lipoxygenase (5-LOX) both of which are considered to be oncogenic. Phospholipase A2 (PLA2) activity releases AA from membrane phospholipids; the free AA can then be converted into PGE2 by the catalytic activity of 5-LOX. PGE2 is considered to impart pro-tumorigenic effect to cancer cells. AsPC-1 cells secrete negligible levels of PGE2; however, exosomes derived from the cells when fused with macrophages triggered the secretion of significantly increased amount of PGE2. Interestingly, pre-treatment of AsPC-1 derived exosomes with PLA2 was shown to cleave out the AA and hence decreased the fusogenicity with macrophages [116].
Exosomes in the activation of M1-like macrophages
Johnstone and Stahl group in the 1970s described the concept of exosomes as nano-sized vesicles involved in cellular communication of various types of cells [124]. The three main EVs naturally formed in a variety of cells and secreted by exocytosis or engulfed by endocytosis are apoptotic bodies, microvesicles, and exosomes, whose sizes range from as large as 5 μm to as small as 10 nm [125, 126]. Cellular and molecular analyses have provided evidence that EVs can be found in many different cell types and mainly characterized by their sizes, constituents, biogenesis, and cellular functions [125]. Extensive studies carried out with exosomes in the recent past have revealed that exosomes can be released by both normal and diseased cells (e.g., cancer cells) [127, 128]. Most cells of the immune system (B-cells, T-cells, dendritic cells, mast cells, neutrophils, macrophages, etc.) originating from progenitor hematopoietic cells release exosomes in the extracellular environment [129]. These exosomes which release either from macrophages themselves or from other immune cells, or other normal or diseased cells, have shown the potential to polarize the naïve macrophages toward a M1 phenotype. Exosomes released from cells can serve as a vehicle to transport both biological constituents as well as signals for various intracellular communications. Cancer cell derived exosomes are rich in multiple proteins and RNAs which stimulate the macrophages to classically activated form and lead to increased pro-inflammatory cytokine production via different signaling pathways [34, 63, 130].
The tumor stroma also referred to as the tumor microenvironment (TME) is a complex junction of different cell types where cancer cells participate in intricate interaction with these surrounding cells. The important component of this tumor stroma is Tumour-associated macrophages (TAMS) which are the most abundant crucial non-neoplastic immune cells and account for around 30–50% of the total tumor mass [131]. The fundamental event in the establishment of tumor microenvironment as pro-tumorigenic or anti-tumorigenic highly depends on the polarization of macrophages in either anti-inflammatory pro-tumorigenic M2 phenotype or pro-inflammatory anti-tumorigenic M1 macrophages which majorly depend on microenvironmental signals or stimuli [132]. Briefly, TAMs are the collection of different macrophages, including infiltrating and resident macrophages originated from various cellular sources. It is suggested through many studies that TAMs have polarization plasticity and can exhibit either of the phenotypes. The majority of studies provide ample evidence that TAMs acquire M2-like polarized macrophage phenotype which promotes the tumor progression by facilitating angiogenesis, immunosuppression and growth factors eventually leading to metastasis [131, 132]. Potential strategies to counter the tumor growth has been studied and designed, major one of these include the strategy to use the TAM-associated immunotherapy. Several studies have confirmed the depletion in TAMs survival and suppression in M2 polarization hold as a potential therapeutic strategy [133]. TAMs targeted immunotherapy involves three effective strategies for cancer treatment—(a) repolarization of M2-like TAMs to anti-tumorigenic M1-phenotype, (b) inhibiting the infiltrating macrophages recruitment and (c) interfering with the TAMs survival. The most straightforward method is inducing or re-polarizing TAMs towards the M1-like phenotype in tumor stroma. The re-polarization of M2-like TAMs can be done through various means such as by using a; (a)TLR agonist, M1-stimulating agents and cytokines, (C)by application of antagonistic antibodies to shut down M2-related pathways to further force macrophages to adopt pro-inflammatory M1 phenotype, (d) by employing antisense miRNA (e.g., miR-155 and miR-125) to inhibit M2-related pathways or (e) by using small inhibiting molecules (e.g., COX2 (cyclooxygenase) inhibitor, histone deacetylase (HDAC) inhibitor, etc.) [130, 132, 134,135,136,137].
Interestingly, as delineated below in detail, the exosomes alone carry all such components and features mentioned above to stimulate and remodel the TAMs in the tumor microenvironment against cancer. Due to their complex interaction with diverse cargo, they are accepted as key players in TAMs remodelling and hence are also considered for extensive study to understand their role as a nanocarrier to stimulate an immune cell response against cancer. Apart from their natural potential, the therapeutic strategy is to load exosomes with proteins guiding them to target site-specific cancer to create anti-tumorigenic pro-inlammatory M1 macrophage environment. This can be effectively done by studying exosome donor cells. The benefits of such exosomes over any other drug-carrying nanoparticles is its non-toxic and non-immunogenic nature due to their endogenous origin [132, 138,139,140]. Hence, we believe that exosome-based immunotherapy to immunomodulate macrophage phenotype from immunosuppressive pro-tumorigenic to M1-phenotype can be a great prospect in controlling tumor growth.
Oral squamous cell carcinoma (OSCC)-derived exosomes
The oral squamous cell carcinoma (OSCC) belongs to head and neck squamous cell carcinoma (HNSCC) which is characterized by its transformation of normal epithelium cells to epithelial precancerous and hence to the cancerous lesion. The secretory products from OSCC cells were reported to educate macrophages via paracrine loops [141,142,143]. Exosomes isolated and identified from the conditioned medium of the OSCC cell lines SCC25 and Cal27 were reported to be taken up by the human macrophage cell line THP-1 [63]. PBMC-derived macrophages treated with CM of OSCC, with and without exosomes, revealed that CM with exosomes played a significant role in M1 polarization and increased expression of M1 signature cytokines (such as TNF-α, IL-1β, and IL-6). However, CM from normal epithelium or leukoplakia cells failed to do so. Mechanistically, significant activation of p38 MAPK, Akt and SAPK/JNK were observed at the early time points after exosome stimulation. Proteomic analysis by mass spectrometry of OSCC cells derived exosome indicated the presence of around 891 proteins, which are believed to contain effector proteins that mediate macrophage activation. Among this pool of proteins, THBS1 (thrombospondin 1) was highly enriched and was considered a potent regulator of macrophage activation. THBS1, which is also produced by macrophages, is considered to induce potent pro-inflammatory signals for macrophages. Knock down of THBS1 in OSCC cells significantly reduced the expression of TNFα, IL-1β, and IL-6 in exosome stimulated THP-1 macrophages [63] (Table 2).
Breast cancer-derived exosomes
Considerable advancement has taken place in the last 5 decades about diagnosis, prognosis, and treatment of breast cancer patients, leading to a remarkable 40% decrease in breast cancer deaths [144]. The molecular analysis of breast cancer provided insights into their biologically driven heterogeneous subtypes [145]. This heterogeneous nature of breast cancer is the result of tumor metastasis to secondary sites in the body. The 5-year survival rates are 99% for localized breast cancer, 84% for regional stage (nearby lymph nodes), and 23% for metastases (distant organs and lymph nodes) [146]. EGCG (Epigallocatechin gallate) has been reported to have anticancer activity by inhibiting cell proliferation and induction of apoptosis. Jang et al. [34] demonstrated that EGCG reduced tumor progression by switching macrophages from M2 like TAM to M1. These authors also revealed that EGCG upregulated cellular and exosomal microRNA levels in breast cancer cells. Further, quantitative real-time PCR analyses provided evidence of up-regulation of miR-16 in EGCG-treated breast cancer-derived exosomes [34]. These secretory exosomes rich in miR-16 could skew the TAM phenotype, either towards tumor-suppressive immunological type, referred to as M1, or tumor-promoting inflammatory/immune-suppressive population. miR-16 was shown to reduce the expression of IKKα expression, a well-known negative regulator of NF-κB signal transduction pathway in macrophages [147]. EGCG-treated cancer cell-derived miR-16 containing exosomes resulted in decreased IKKα protein expression and subsequent accumulation of the IκB in TAM leading to the suppression of IL-6 and TGF-β (M2 associated cytokines) and increased production of TNF-α (M1 associated cytokine) (Table 2). Moreover, breast cancer cells treated with EGCG showed reduced expression of two significant attractants and growth factors for TAM, namely colony-stimulating factor-1 (CSF-1) and Chemokine (C–C motif) ligand 2 (CCL-2). Thus, EGCG-treated cancer cell-derived miR-16 exosome had the potential to suppress macrophage infiltration; promote TAM polarization towards immunosuppressive subtype M1, ultimately leading to reduced tumor growth [34].
Pancreatic-cancer derived modified exosomes
In the United States, pancreatic cancer is projected to claim the second leading cause of death from cancer in the next 10 years [148]. Globally, pancreatic cancer is the cause of 7th highest mortality [149]. Pancreatic Ductal Adenocarcinoma (PDAC), an aggressive malignancy, constitutes almost 80–90% of total pancreatic cancer cases leading to 4th highest death rate in the United States and is predicted to rise at 2nd in between 2020 to 2030 [130, 150, 151]. The non-transformed cells such as fibrotic stromal cells and excessive myeloid cells infiltration are a hallmark in PDAC which make interaction amongst each other and hence create TME. Tumor-associated macrophages (TAMs) are the most abundant among these myeloid cells whose interaction and communication with pancreatic tumor cells results in M1 or M2 phenotypic polarization [152, 153]. Recent studies have shown that these tumor cells secrete exosomes in excess as compared to untransformed normal cells and are involved in cell-to-cell communication [154,155,156]. PDAC cells overexpressing certain microRNAs were reported to change their exosomal payload, which in turn could alter the macrophage polarization from M2 to M1 phenotype [130]. Macrophages infiltrating tumor stroma mostly differentiate into TAMs, which are predominantly of the M2 phenotype [157]. Moreover, wild type Panc-1 cells co-cultured with classically activated M1 macrophages can change them to alternatively activated M2 macrophages. Based on this fact, investigators hypothesized that transfection of PDAC cells with M1 specific miRs (such as miR-155 and miR-125b) may skew macrophage polarization (Table 2). Both miR-155 and miR-125b were upregulated in macrophages in response to LPS and IFN-γ, respectively, and are known to play an important role in innate immunity [158, 159]. Plasmids encoding these miRNAs were transferred to Panc-1 cells by encapsulation in HA-PEI/HA-PEG nanoparticles, which transferred the DNA more efficiently than Lipofectamine, used as a positive control. J774A.1 macrophages are known to be polarized to M1 phenotype when incubated with LPS/IFN-γ or M2 phenotype when incubated with IL-4. The transfected Panc-1 cells were co-cultured with J774A.1 macrophages, which repolarized their phenotype from alternative activation to classical activation. The exosomes analyzed from Panc-1, and miR transfected Panc-1 cells had similar cargoes except for the increased load of miR-155 and miR125b in the exosomes isolated from the transfected cell line. When J774A.1 cells were directly incubated with these exosomes, M2 polarized macrophages were re-programmed toward an M1 phenotype as confirmed by an increase in the IL-1β/Arg-1 and iNOS/Arg-1 ratio [130].
Colorectal cancer-derived exosomes
Colorectal cancer (CRC) derived exosomes have the potential to inactivate macrophages when incubated with mixed M1 and M2 macrophages. This is also dependent on the duration of contact of the exosomes with macrophages [35]. EVs derived from the isogenic CRC cell lines SW480 and SW620 were reported to be internalized by human THP-1 monocytes. The uptake is mediated by dynamin-dependent endocytic pathway and resulted in the differentiation of THP-1 towards both M2 and M1 phenotypes hallmarked by the secretion of ROI, CXCL10, IL-6, IL-23, IL-12 or IL-10 and TGF- β [160, 161]. Interestingly, a study by Yingkuan Shao et al. (2018) suggests that exosomes from CRC cells specifically targeted liver tissue, which induced liver resident macrophages to polarize toward pro-inflammatory phenotype predominantly secreting IL-6 (Table 2). These exosomes were found to be rich in miR-21, which in turn created a pro-inflammatory environment in the liver and aided niche formation. Exosomes derived from SW480, and SW620 cells were incubated with RAW264.7 and THP-1 macrophage like cell lines for 24 h (long term incubation) revealed a significant increase in pro-inflammatory cytokines IL-6 and TNF-α while the levels of the anti-inflammatory Arg-1 and IL-10 remained similar to the control. The incubation of miR-21 analogs with macrophages resulted in increased expression of IL-6 and TNF-α, while incubation with miR-21 antagonists resulted in the opposite. Similarly, knocking out miR-21 by CRISPR/Cas9 resulted in decreased expression of IL-6 and TNF-α. Moreover, labelled miR-21 binds to TLR7 in RAW 264.7 macrophages. The knockdown of TLR7 in RAW 264.7 cells was shown to diminish activation of the miR-21-TLR7-IL-6 signaling axis. Additionally, the levels of miR-21 in the CRC derived exosomes and macrophage IL-6 expression were found to positively correlate with liver metastasis [35].
Exosome and ADCC
The immunological function of antibody-dependent cell-mediated cytotoxicity (ADCC) executed by immune effector cells like NK cells and macrophages are the major mechanism utilized as a therapeutic approach for cancer immunotherapy [162]. The macrophages mostly involve in antibody-dependent cellular phagocytosis (ADCP), are characterized to have Ptdlns 3-kinase/Akt pathway activation, which significantly increases its ability to mediate ADCC [163]. The ADCC/ADCP responses are mediated through the Fcγ receptor (FcγR) and in contrast to NK cells, macrophages are known to express all categories of these Fcγ receptors [164]. In cancer immunotherapies, tumour-targeting antibodies activate macrophages and NK cells mediated cytotoxicity via FcγRs, a key method of many antibodies therapies. The exosomes secreted from cancer cells are largely favour pro-cancer activities and help in immune suppression [165]. In the context of ADCC, the tumor-derived exosomes are the major resistance to antibody-based therapy. As discussed above, exoxomes bulging out from tumor cells carry various immunosuppressive payloads from the parent tumor cell. In addition to MHCs and co-stimulatory proteins, exosomes stimulate the immune response due to presence of tumor associated antigen. These tumor associated antigens with exosomes interfere with ADCC by sequestering the anti-tumor antibodies and significantly reducing the binding to the tumour cells. The HER2+ (human epidermal growth factor receptor 2) carrying exosomes derived from breast cancer cells sequester the anti-HER2+ antibody Trastuzumab (brand name Herceptin) and reduces the bioavailability to the target cell [162, 165]. Similarly and as discussed above, the monoclonal antibody therapy for B cell lymphoma using anti-CD20 antibody rituximab is intercepted by its derived exosomes and hence impairs the ADCC [166]. The sequestering of the anti-tumour Abs by tumour-derived exosomes strongly suggest the exosomes as a potential target which could be beneficial in unmasking the efficacy of ADCC.
Conclusion
In conclusion, exosome reflects a great promise towards cellular therapy and disease diagnostic. Being involved in cell-to-cell communication, it regulates both normal and pathological processes to regulate body homeostasis. Extensive studies in the last decade show the association of exosomes with cancer metastasis and infectious diseases. These exosomes serve as biomarkers for early detection and diagnosis of diseases. For instance, urine exosomes are used to detect prostate cancer. Analysis of tumor exosomes content (RNA and protein) allows for delineation of tumor status, both for tumor genotype/phenotype and metastatic potential. Cancer cell-secreted exosomes provide immunosuppressive signals to local macrophages to promote tumor progression and metastasis to distant organs. Thus, targeting metastasis-associated exosomes opens up a new window for the development of active anti-tumor therapeutic agents. Additionally, in terms of therapeutics, the immune cells derived exosomes cargoes can be artificially modulated to provide pro-inflammatory, anti-tumorigenic microenvironment to suppress the tumor growth and metastasis. For instance, the growth factors released by macrophage favouring tumor growth, can be modified artificially to produce tumor-killing cytotoxic chemokines.
Apart from recruiting exosomes to precondition tumor microenvironment with cytotoxic chemokines to reduce metastasis, exosomes can be also be used as an effective drug delivery system. One of the major advantages of employing exosomes as a drug delivery system is that there is no unwanted homing of exosomes in the liver; it can easily avoid the first pass metabolic effect and can be effectively delivered to target sites. Encapsulating anti-cancer drugs with exosomes have shown potential delivery to the targeted tumor in the animal model. The functional exosomes with its natural ability to contain a therapeutic agent and its intracellular cargo would have significant potential to reduce cancer metastasis. To our best knowledge, this review provides compiled information on recent advancement in understanding the cancer progressions via cancer-derived exosomes providing immunosuppressive microenvironment. Hence they could be utilized to immunomodulate the tumor microenvironment through exosome targeted modifications for effectively promoting anti-tumor phenotype.
Targeting immunosuppressive elements of exosomes to destroy TAM-cancer cell-secreted exosomes provide immunosuppressive signals to local macrophages favoring progression and metastasis to distant organs. Thus, targeting metastasis-associated exosomes opens up a new window for the development of active anti-tumor therapeutic agents. Immune cells derived exosomes to produce a tumor cytotoxic effect-in terms of therapeutics, the immune cell derived exosome cargoes can be artificially modulated to provide pro-inflammatory, anti-tumorigenic microenvironment to suppress the tumor growth and metastasis. For instance, the growth factors released by macrophages favor tumor growth and can thus modified artificially to produce tumor-killing cytotoxic chemokines. One of the major advantages of employing exosome as drug delivery system is that there is no unwanted homing of exosomes in the liver as it can avoid the first pass metabolic effect and can be effectively delivered to target sites. Encasulation of exosomes with anti-cancer drugs have been shown to target tumor effectively in animal model. The functional exosomes with its natural ability to contain a therapeutic agent and its intracellular cargo would have significant potential to reduce cancer metastasis. Although modulating exosome cargo, targeting its secretion in a tumor microenvironment and loading of tumor-derived antigens/drugs onto exosomes remain the plausible therapeutic strategy; there remain several unopened questions about their role in tumorigenesis and normal state. For instance, targeting critical proteins likes GTPase Rab27a, Rab11 and Rab35 (via RNAi) which are involved in exosome release shows a reduced rate of tumor growth and development. In addition, one of the potential fields to explore is to develop techniques to yield high concentrations of exosomes with maximum purity and techniques to identify the accurate exosomal content that impacts tumorigenesis. Uptake of exosomes loaded with drug by host immune is one of the critical gaps to study. Rejection of injected exosomal cargo may elicit further immunological reactions in a host body, leading to hypersensitivity and adverse disease development. Thus, understanding the exosome-mediated signalling pathway and its machinery, remains an exciting area for further interrogation.
Availability of data and materials
Not applicable, all information in this review can be found in the reference list.
References
Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11.
Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2013:2.
Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol. 2019;2019:1–8.
Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes. 2013;4:152–70.
Bae S, Brumbaugh J, Bonavida B. Exosomes derived from cancerous and non-cancerous cells regulate the anti-tumor response in the tumor microenvironment. Genes Cancer. 2018;9:87–100.
Rajagopal C, Harikumar KB. The origin and functions of exosomes in cancer. Front Oncol. 2018;8:66.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491.
Nonnenmacher Y, Hiller K. Biochemistry of proinflammatory macrophage activation. Cell Mol Life Sci. 2018;75:2093–109.
Cohen HB, Mosser DM. Extrinsic and intrinsic control of macrophage inflammatory responses. J Leukoc Biol. 2013;94:913–9.
Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23.
Lichtnekert J, Kawakami T, Parks WC, Duffield JS. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–64.
Liu B, Zhang M, Zhao J, Zheng M, Yang H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Therapeut Med. 2018;16:5009–144.
Saqib U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R. Phytochemicals as modulators of M1–M2 macrophages in inflammation. Oncotarget. 2018;9:17937.
Zhou X, Li W, Wang S, Zhang P, Wang Q, Xiao J, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and Gut microbial homeostasis. Cell Rep. 2019;27(1176–1189):e5.
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–62.
Brown M, O'Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin Exp Immunol. 2019;195:310–21.
Laurent P, Sisirak V, Lazaro E, Richez C, Duffau P, Blanco P, et al. Innate immunity in systemic sclerosis fibrosis: recent advances. Front Immunol. 2018;9:1702.
Mori S, Chang JT, Andrechek ER, Matsumura N, Baba T, Yao G, et al. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28:2796–805.
Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–8.
Chen Z, Yang L, Cui Y, Zhou Y, Yin X, Guo J, et al. Cytoskeleton-centric protein transportation by exosomes transforms tumor-favorable macrophages. Oncotarget. 2016;7:67387–402.
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–73.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–90.
Ireland LV, Mielgo A. Macrophages and fibroblasts, key players in cancer chemoresistance. Front Cell Dev Biol. 2018;6:131.
Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75.
Owen JL, Mohamadzadeh M. Macrophages and chemokines as mediators of angiogenesis. Front Physiol. 2013;4:159.
Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;2018:145.
Bhattacharjee O, Ayyangar U, Kurbet AS, Ashok D, Raghavan S. Unraveling the ECM-immune cell crosstalk in skin diseases. Front Cell Dev Biol. 2019;7:68.
Hwang I. Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Mol Cells. 2013;36:105–11.
Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–35.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72:659–71.
Lobb RJ, Lima LG, Moller A. Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol. 2017;67:3–10.
Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421.
Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39:1368–79.
Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS, et al. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep. 2018;8:3853.
Piao YJ, Kim HS, Hwang EH, Woo J, Zhang M, Moon WK. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. 2018;9:7398–410.
Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, et al. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front Immunol. 2018;9:871.
Kanda T, Ogasawara S, Chiba T, Haga Y, Omata M, Yokosuka O. Current management of patients with hepatocellular carcinoma. World J Hepatol. 2015;7:1913–20.
Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Ooka Y, et al. Real-world experiences with the combination treatment of ledipasvir plus sofosbuvir for 12 weeks in HCV genotype 1-infected Japanese Patients: achievement of a sustained virological response in previous users of peginterferon plus ribavirin with HCV NS3/4A inhibitors. Int J Mol Sci. 2017;2017:18.
Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41.
Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014;2014:3.
Li X, Lei Y, Wu M, Li N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA TUC339. Int J Mol Sci. 2018;2018:19.
Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172172.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30.
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–42.
Hsu YL, Hung JY, Chang WA, Jian SF, Lin YS, Pan YC, et al. Hypoxic Lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol Therapy. 2018;26:568–81.
Deep G, Panigrahi GK (2015) Hypoxia-induced signaling promotes prostate cancer progression: exosomes role as messenger of hypoxic response in tumor microenvironment. Critical Reviews™ in Oncogenesis, p 20
Ragnum HB, Vlatkovic L, Lie AK, Axcrona K, Julin CH, Frikstad KM, et al. The tumour hypoxia marker pimonidazole reflects a transcriptional programme associated with aggressive prostate cancer. Br J Cancer. 2015;112:382–90.
Stewart GD, Gray K, Pennington CJ, Edwards DR, Riddick AC, Ross JA, et al. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol Rep. 2008;20:1561–7.
Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys. 2012;82:e433–e439439.
Vergis R, Corbishley CM, Norman AR, Bartlett J, Jhavar S, Borre M, et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–51.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–122.
Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Can Res. 2001;61:1100–6.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin. 2011;61:69–90.
Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital. 2007;27:113–7.
Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–62.
Cai J, Qiao B, Gao N, Lin N, He W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am J Physiol Cell Physiol. 2019;316:C731–C740740.
Lu L, Xue X, Lan J, Gao Y, Xiong Z, Zhang H, et al. MicroRNA-29a upregulates MMP2 in oral squamous cell carcinoma to promote cancer invasion and anti-apoptosis. Biomed Pharmacother. 2014;68:13–9.
Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90.
Momen-Heravi F, Bala S. Extracellular vesicles in oral squamous carcinoma carry oncogenic miRNA profile and reprogram monocytes via NF-kappaB pathway. Oncotarget. 2018;9:34838–54.
Xiao M, Zhang J, Chen W, Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37:143.
Wang JY, Zhang Q, Wang DD, Yan W, Sha HH, Zhao JH, et al. MiR-29a: a potential therapeutic target and promising biomarker in tumors. Biosci Rep. 2018;2018:38.
Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Can Res. 2016;76:1770–80.
King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421.
Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteom. 2010;9:1085–99.
Yasuda M, Schmid T, Rubsamen D, Colburn NH, Irie K, Murakami A. Downregulation of programmed cell death 4 by inflammatory conditions contributes to the generation of the tumor promoting microenvironment. Mol Carcinog. 2010;49:837–48.
Hsu DS, Wang HJ, Tai SK, Chou CH, Hsieh CH, Chiu PH, et al. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell. 2014;26:534–48.
Hsieh CH, Tai SK, Yang MH. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia. 2018;20:775–88.
Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36.
Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, et al. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci USA. 1999;96:14037–42.
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–33.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58.
Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–7.
Yang CH, Yue J, Pfeffer SR, Fan M, Paulus E, Hosni-Ahmed A, et al. MicroRNA-21 promotes glioblastoma tumorigenesis by down-regulating insulin-like growth factor-binding protein-3 (IGFBP3). J Biol Chem. 2014;289:25079–87.
Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120–9.
Sahin E, Haubenwallner S, Kuttke M, Kollmann I, Halfmann A, Dohnal AM, et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193:1717–27.
Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6:19.
Chang LC, Huang CF, Lai MS, Shen LJ, Wu FL, Cheng WF. Prognostic factors in epithelial ovarian cancer: a population-based study. PLoS One. 2018;13:e0194993.
Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137.
Rodriguez GM, Galpin KJC, McCloskey CW, Vanderhyden BC. The tumor microenvironment of epithelial ovarian cancer and its influence on response to immunotherapy. Cancers (Basel). 2018;2018:10.
Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32–42.
Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38:522–8.
Rashed MH, Kanlikilicer P, Rodriguez-Aguayo C, Pichler M, Bayraktar R, Bayraktar E, et al. Exosomal miR-940 maintains SRC-mediated oncogenic activity in cancer cells: a possible role for exosomal disposal of tumor suppressor miRNAs. Oncotarget. 2017;8:20145–64.
Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91.
Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–87.
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3.
Tamimi AF, Juweid M (2017) Epidemiology and Outcome of Glioblastoma. In: De Vleeschouwer S (ed) Glioblastoma. Brisbane (AU)
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–16.
Bodmer S, Strommer K, Frei K, Siepl C, de Tribolet N, Heid I, et al. Immunosuppression and transforming growth factor-beta in glioblastoma Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222–9.
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Can Res. 2006;66:3294–302.
Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, et al. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother. 2015;64:419–27.
Manda SV, Kataria Y, Tatireddy BR, Ramakrishnan B, Ratnam BG, Lath R, et al. Exosomes as a biomarker platform for detecting epidermal growth factor receptor–positive high-grade gliomas. J Neurosurg. 2018;128:1091–101.
Chae M, Peterson TE, Balgeman A, Chen S, Zhang L, Renner DN, et al. Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model. Neuro-oncology. 2015;17:978–91.
Fries G, Perneczky A, Kempski O. Glioblastoma-associated circulating monocytes and the release of epidermal growth factor. J Neurosurg. 1996;85:642–7.
Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+ HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-oncology. 2010;12:631–44.
Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-oncology. 2010;12:351–65.
Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016;2016:1.
Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-oncology. 2010;12:1113–25.
Gabrusiewicz K, Li X, Wei J, Hashimoto Y, Marisetty AL, Ott M, et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7:e1412909.
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nature Rev Gastroenterol Hepatol. 2019;16:361–75.
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nature Rev Dis Primers. 2015;1:15065.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108.
Wu J, Li H, Xie H, Wu X, Lan P. The malignant role of exosomes in the communication among colorectal cancer cell, macrophage and microbiome. Carcinogenesis. 2019;40:601–10.
Pardieck IN, Jawahier PA, Swets M, van de Velde CJ, Kuppen PJ. Novel avenues in immunotherapies for colorectal cancer. Expert Rev Gastroenterol Hepatol. 2016;10:465–80.
Braster R, Bogels M, Beelen RH, van Egmond M. The delicate balance of macrophages in colorectal cancer; their role in tumour development and therapeutic potential. Immunobiology. 2017;222:21–30.
Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013;47:197–205.
Lian G, Chen S, Ouyang M, Li F, Chen L, Yang J. Colon cancer cell secretes EGF to promote M2 polarization of TAM through EGFR/PI3K/AKT/mTOR pathway. Technol Cancer Res Treatment. 2019;18:1533033819849068.
Takano Y, Masuda T, Iinuma H, Yamaguchi R, Sato K, Tobo T, et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8:78598–613.
Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, et al. Regulated polarization of tumor-associated macrophages by miR-145 via colorectal cancer-derived extracellular vesicles. J Immunol. 2017;199:1505–15.
Lin L, Hou J, Ma F, Wang P, Liu X, Li N, et al. Type I IFN inhibits innate IL-10 production in macrophages through histone deacetylase 11 by downregulating microRNA-145. J Immunol. 2013;191:3896–904.
Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100.
Hu H, Hang JJ, Han T, Zhuo M, Jiao F, Wang LW. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016;37:8657–64.
Linton SS, Abraham T, Liao J, Clawson GA, Butler PJ, Fox T, et al. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLoS One. 2018;13:e0206759.
Yang M, McKay D, Pollard JW, Lewis CE. Diverse functions of macrophages in different tumor microenvironments. Can Res. 2018;78:5492–503.
Boyd AW, Wawryk SO, Burns GF, Fecondo JV. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci. 1988;85:3095–9.
Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55.
Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–21111.
Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12.
Hiraide T, Ikegami K, Sakaguchi T, Morita Y, Hayasaka T, Masaki N, et al. Accumulation of arachidonic acid-containing phosphatidylinositol at the outer edge of colorectal cancer. Sci Rep. 2016;6:29935.
Kim HY, Lee KM, Kim SH, Kwon YJ, Chun YJ, Choi HK. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget. 2016;7:67111–28.
De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727.
Borges FT, Reis LA, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46:824–30.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;2019:8.
Crenshaw BJ, Sims B, Matthews QL. Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. Nanomedicines: IntechOpen. 2018. https://doi.org/10.5772/intechopen.80225.
Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol. 2019;10:648.
Su MJ, Aldawsari H, Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of microRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep. 2016;6:30110.
Poh AR, Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 2018;8:49.
Zheng X, Turkowski K, Mora J, Brune B, Seeger W, Weigert A, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;8:48436–52.
Cianciaruso C, Beltraminelli T, Duval F, Nassiri S, Hamelin R, Mozes A, et al. Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep. 2019;27(3062–3080):e11.
Van Dalen FJ, Van Stevendaal MHME, Fennemann FL, Verdoes M, Ilina O. Molecular repolarisation of tumour-associated macrophages. Molecules. 2019;24:9.
Yang L, Wang F, Wang L, Huang L, Wang J, Zhang B, et al. CD163+ tumor-associated macrophage is a prognostic biomarker and is associated with therapeutic effect on malignant pleural effusion of lung cancer patients. Oncotarget. 2015;6:10592.
Wang Y, Lin YX, Qiao SL, An HW, Ma Y, Qiao ZY, et al. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–63.
Na YR, Yoon YN, Son D, Jung D, Gu GJ, Seok SH. Consistent inhibition of cyclooxygenase drives macrophages towards the inflammatory phenotype. PLoS One. 2015;10:e0118203.
Batista IA, Melo SA. Exosomes and the future of immunotherapy in pancreatic cancer. Int J Mol Sci. 2019;2019:20.
Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015–28.
Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9:1714–27.
Saintigny P, Zhang L, Fan YH, El-Naggar AK, Papadimitrakopoulou VA, Feng L, et al. Gene expression profiling predicts the development of oral cancer. Cancer Prev Res. 2011;4:218–29.
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Can Res. 2004;64:7022–9.
Go A, Ryu Y-K, Lee J-W, Moon E-Y. Cell motility is decreased in macrophages activated by cancer cell-conditioned medium. Biomol Ther. 2013;21:481.
Narod SA, Iqbal J, Miller AB. Why have breast cancer mortality rates declined? J Cancer Policy. 2015;5:8–17.
Belizario JE, Loggulo AF. Insights into breast cancer phenotying through molecular omics approaches and therapy response. Cancer Drug Resist. 2019;2:527–38.
American Cancer Society. Breast Cancer Facts & Figures 2013-2014. Atlanta: American Cancer Society, Inc. 2013. p. 1–37.
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-κB. J Exp Med. 2008;205:1261–8.
Kindler HL. A glimmer of hope for pancreatic cancer. N Engl J Med. 2018;379:2463–4.
McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61.
Deshwar AB, Sugar E, Torto D, De Jesus-Acosta A, Weiss MJ, Wolfgang CL, et al. Diagnostic intervals and pancreatic ductal adenocarcinoma (PDAC) resectability: a single-center retrospective analysis. Ann Pancreatic Cancer. 2018;2018:1.
Winter JM, Brennan MF, Tang LH, D'Angelica MI, Dematteo RP, Fong Y, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–75.
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–23.
Yu Z, Zhao S, Ren L, Wang L, Chen Z, Hoffman RM, et al. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget. 2017;8:63461–83.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta General Subj. 2012;1820:940–8.
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Rel. 2015;219:278–94.
Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochem Biophys Acta. 2012;1826:103–11.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69.
Banzhaf-Strathmann J, Benito E, May S, Arzberger T, Tahirovic S, Kretzschmar H, et al. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer's disease. EMBO J. 2014;33:1667–800.
Popena I, Abols A, Saulite L, Pleiko K, Zandberga E, Jekabsons K, et al. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun Signal. 2018;16:17.
Baj-Krzyworzeka M, Mytar B, Szatanek R, Surmiak M, Weglarczyk K, Baran J, et al. Colorectal cancer-derived microvesicles modulate differentiation of human monocytes to macrophages. J Transl Med. 2016;14:36.
Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Investig. 2016;126:1216–23.
Joshi T, Ganesan LP, Cheney C, Ostrowski MC, Muthusamy N, Byrd JC, et al. The PtdIns 3-kinase/Akt pathway regulates macrophage-mediated ADCC against B cell lymphoma. PLoS One. 2009;4:e4208.
Weiskopf K, Weissman IL (2015) Macrophages are critical effectors of antibody therapies for cancer. mAbs 7:303–10.
Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60:639–48.
Marleau AM, Chen C-S, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134.
Acknowledgements
The authors are thankful to the Department of Biotechnology (DBT) for the Government of India sponsored Ramalingaswami Fellowship to MSB (BT/RLF/Re-entry/26/2013). The authors are thankful to the Department of Science and Technology (DST), Government of India for providing financial support under Early Career Research (ECR) Award (ECR/2016/00852) to MSB and American Heart Association support to KW. The authors also gratefully acknowledge the Indian Institute of Technology Indore (IITI) (MHRD) for providing facilities and other support.
Funding
The authors declare no funding support was received for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
No ethics approval was required for this review that did not involve patients or patient data.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baig, M.S., Roy, A., Rajpoot, S. et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 69, 435–451 (2020). https://doi.org/10.1007/s00011-020-01318-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01318-0