Abstract
Glutamine metabolism is one of the hallmarks of cancers which is described as an essential role in serving as a major energy and building blocks supply to cell proliferation in cancer cells. Many malignant tumor cells always display glutamine addiction. The “kidney-type” glutaminase (GLS1) is a metabolism enzyme which plays a significant part in glutaminolysis. Interestingly, GLS1 is often overexpressed in highly proliferative cancer cells to fulfill enhanced glutamine demand. So far, GLS1 has been proved to be a significant target during the carcinogenesis process, and emerging evidence reveals that its inhibitors could provide a benefit strategy for cancer therapy. Herein, we summarize the prognostic value of GLS1 in multiple cancer type and its related regulatory factors which are associated with antitumor activity. Moreover, this review article highlights the remarkable reform of discovery and development for GLS1 inhibitors. On the basis of case studies, our perspectives for targeting GLS1 and development of GLS1 antagonist are discussed in the final part.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presently, metabolic reprograming is one of most important recognized cancer hallmarks. Previous research has shown that cancer cells could alter their own energy metabolism to grow rapidly in unfavorable environments. It is closely associated with their proliferation, metastasis and drug resistance [1,2,3]. Therefore, a comprehensive understanding of metabolic reprogramming will contribute to develop new anti-cancer drugs. With a deeper understanding of tumor metabolism, the glutamine metabolism pathway has attracted great attention in cancer research, which mainly produces adenosine triphosphate (ATP), nicotinamide adenine dinucleotide phosphate (NADPH) and supports the synthesis of nucleotides and lipids [4]. Moreover, it is found that these tumors are usually associated with specific genomes, including Myc upregulation, KRAS mutation, mTOR upregulation and NRF2 activation [5]. Therefore, the utilization of glutamine as an anti-cancer strategy is hopeful for the tumor types of glutamine addiction.
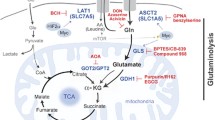
Mitochondrial glutaminase plays an important role in converting glutamine to glutamate which is regarded as a key enzyme in glutaminolysis [6]. And it takes effect in regulating cellular metabolism, maintaining redox balance and glutathione biosynthesis in cancer cells [6,7,8,9]. Interestingly, it was identified two different subtypes in mammals, the “kidney-type” glutaminase (GLS1) and “liver-type” glutaminase (GLS2). However, GlS1 played an important role in cancer progress instead of GLS2 [10, 11]. Compared to tumor adjacent tissue, the elevated expression of GLS1 has shown an abnormal expression in multiple cancer types, including colorectal cancer (CRC), prostate cancer and breast cancer [12,13,14,15]. In addition, it is identified that inhibiting the activity of GLS1 could reduce the rate of tumor growth and inhibit tumor progression [16, 17].
Overall, GLS1 may serve as a promising therapeutic agent in cancer treatment and attract more and more attention in the past ten years. However, as one of the potential therapeutic targets for cancer, researchers have investigated the role of GLS1 in various types of cancer. In this review, we summarize the prognosis and function of GLS1 in a variety of cancer types and introduce the potential mechanisms of GLS1 in pro-tumor effect. Then, we present the regulators and inhibitors of GLS1 and furtherly discuss the drug resistance mechanism of GLS1 inhibitors and the problem of non-specificity for patients to promote the development of clinical anti-tumor drugs.
Prognostic value of GLS1 in multiple cancer types
As shown in Fig. 1, we analyze the Cancer Cell Line Encyclopedia (CCLE) project and find that GLS1 presents different expressions in multiple tumor cell lines. Compared with adjacent tissues, GLS1 shows higher expression in tumor tissues through further TCGA database analyses in some solid tumors like, stomach adenocarcinoma (STAD), head and neck squamous cell carcinoma (HNSC), thymoma (THYM), testicular germ cell tumors (TGCT), liver hepatocellular carcinoma (LIHC), colon adenocarcinoma (COAD) and so on (Fig. 2). Besides, the patients’ plasma GLS1 levels showed dramatically expression in some solid tumors like, hepatocellular carcinoma, non-small cell lung cancer, colorectal cancer and breast cancer [14, 15, 18, 19]. Intriguingly, high expression of GLS1 is also related to tumor progression and poor prognosis in multiple cancer subtypes, particularly in gastric, ovarian, hepatoma carcinoma and the like via TCGA database and Kaplan–Meier plotter (KM) analysis (Fig. 3a–i). It means that there might be a certain correlation between the expression of GLS1 and the degree of tumor deterioration.
The expression of GLS1 in various cancer cell lines. The data are from the Cancer Cell Line Encyclopedia (CCLE) project (https://portals.broadinstitute.org/ccle/home)
GLS1 is significantly overexpressed in some solid tumors. According to the TCGA database, GLS1 expression is higher in tumor tissue (show in red) compared to normal tissue (show in black), including TCGA normal and GTEX data, in many cancer types, such as stomach adenocarcinoma (STAD, a), head and neck squamous cell carcinoma (HNSC, b), thymoma (THYM, c), testicular germ cell tumors (TGCT, d), liver hepatocellular carcinoma (LIHC, e) and colon adenocarcinoma (COAD, f). The data are analyzed from TCGA (http://cancergenome.nih.gov/)
Elevated GLS1 expression represents a poor prognostic signature in cancer patients. High GLS1 expression is accompanied by a short overall survival in gastric (a), ovarian (b) and liver (c) carcinoma based on the KM analysis. GLS1 is a unfavorable prognostic factor in various cancer types according to TCGA database, including colon adenocarcinoma (COAD, d), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC, e), ovarian serous cystadenocarcinoma (OV, f), stomach adenocarcinoma (STAD, g), thymoma (THYM, h), liver hepatocellular carcinoma (LIHC, i). The data are analyzed from TCGA (http://cancergenome.nih.gov/) and Kaplan–Meier Plotter (http://kmplot.com/analysis)
Hepatocellular carcinoma
In hepatocellular carcinoma (HCC), it is found that GLS1 is relevant to tumor aggressiveness and poor prognosis [15, 20, 21]. Compared with adjacent tissues, GLS1 expression was highly expressed in HCC tissues and was associated with clinical features, which could promote the tumor progression in HCC [22]. When GLS1 is knockdown or blocked, the growth of liver cancer cells slows down and the tumor progression is blocked [20,21,22,23,24,25]. Interestingly, further studies found that high expression of GLS1 had something to do with stemness phenotype and advances clinic pathological features in HCC [25]. In conclusion, GLS1 has a certain relationship with the malignant progression of liver cancer and it might serve as a new prognostic factor in HCC.
Colorectal cancer
In colorectal cancer, it was reported that GLS1 was drastically over-expressed in human CRC tissues compared to normal mucosae [12, 26, 27]. In addition, the inhibitors of GLS1 may suppress tumor growth and induce apoptosis in CRC cell lines, which indicated that GLS1 plays an important role in colon cancer (CRC) tumorigenesis [12, 28]. Subsequently, GLS1 inhibitors also provide therapeutic benefits in the treatment of CRC by regulating glutaminolysis [26]. Due to the functional importance of GLS1 in regulating glutamine metabolism, GLS1 has a critical association with glucose uptake and tumor progression [29]. Interestingly, costunolide as an inhibitor of GLS1 might activate p53 to blockade glutaminolysis and inhibit proliferation in CRC cells [82]. In a nutshell, as a marker for CRC, GLS1 makes a difference to tumor size, tumor grade and lymph node status [12, 27, 28]. Researchers also confirmed that GLS1 is an independent prognostic factor in overall survival. It is also showed that high expression of GLS1 is an independent predictor of poor clinical prognosis after surgical treatment of CRC patients and there is significant correlation between the expression of GLS1 and tumor budding in a patient population.
Non-small cell lung cancer
In 2016, researchers found that the expression of GLS1 in non-small cell lung cancer (NSCLC) (N = 57) was markedly higher than the pneumocytes from normal lung tissues (N = 57), which is inversely proportional to the overall survival rate of NSCLC [30]. What is more, NSCLC cells rely on the cytoplasmic NADH to generate ATP through the NADH transport system malate-aspartate shuttle (MAS) [31]. This is consistent with previous reports that regardless of the rate of glycolysis, oxidative phosphorylation is the main source of ATP supply [32]. Oxidative phosphorylation is the main contribution of ATP generation in NSCLC through NADH transport. Hence, the inhibition of GLS1 or glutamine exhaustion in culture media could prominently reduce proliferation and ATP production in NSCLC cell lines [30]. Similarly, the treatment of BPTES, a specific inhibitor of GLS1, could induce cell death in NSCLC [30]. It is worth noting that the combination of GLS1 inhibitors and a variety of drugs will significantly enhance the effect of GLS1 inhibitors in NSCLC, such as heat shock protein 90 (HSP90) inhibitor THZ1 and thymidylate synthase inhibitor 5-fluorouracil (5-FU) [30, 33]. And we will introduce in detail the mechanism of the combination of GLS1 inhibitors in the following section.
Other cancers
It is found that GLS1 is associated with poor prognosis of other types of cancer, such as ovarian cancer, breast cancers and neck squamous cell carcinoma (HNSCC) based on TCGA database. Indeed, GLS1 has a certain impact on the poor prognosis of ovarian cancer and has gained some attention. The expression of GLS1 mRNA in tumor tissues is positively relevant to poor prognosis, while the decreased expression of GLS1 is associated with a high survival rate in ovarian cancer patients [25, 33]. Interestingly, the deletion of GLS1 could cause cell death in ovarian cancer [34]. At the same time, targeting glutamine addiction by inhibiting GLS1 also provides a viable novel treatment to overcome resistance to PI3K/Akt/mTOR inhibition. Further study demonstrated that inhibition of GLS1 and mTOR (CB839 and PP242) improved the efficacy of ovarian cancer therapy [25]. GLS1 has a certain influence on mTOR-related pathways, thus mTOR inhibitors PP242 could enhance antitumor effect of GLS1 inhibitors in ovarian cancer.
Also, significantly increased GLS1 expression was identified in tumors in comparison with normal tissues in breast cancer. GLS1 levels were significantly positively correlated with stage N, lymph node metastasis, stage grouping, and disease severity, while the higher expression of GLS1 observed in breast cancer patients were not associated with increased tumor tissue concentrations compared to the control group [35,36,37]. Furtherly, tissue microarray of 702 breast cancer patients and HER2 type showed the highest expression of GLS1 [37]. The up-regulated GLS1 expression levels have a certain relationship with glutamine metabolism and it might be the mechanism of ErbB2-induced paclitaxel resistance [35]. ErbB2 activation could promote the expression of GLS1 in breast cancer cells via the NF-κB pathway. It identifies another oncogenic signaling pathway that can stimulate GLS1 expression, thereby promoting the utilization of glutamine in breast cancer cells [36].
It is noted that the use of GLS1 inhibitor BPTES to prevent glutamine consumption could prevent head and neck squamous cell carcinoma (HNSCC) overgrowth, indicating that GLS1 seems to be a promising therapeutic target for the treatment of HNSCC [38]. Besides, when treated with BPTES, there is a significant increase in the number of dead cells in HNSCC cell. Previous study also showed that the combined use of BPTES and metformin has a cumulative inhibitory effect on the proliferation of HNSCC cells via induction of cell cycle arrest and apoptosis, but its mechanism is still unclear [39, 40]. Moreover, the study found that GLS1 is overexpressed in patients with gastric cancer, which has important clinical value and could be used as a biomarker for the diagnosis and treatment of early gastric cancer [39]. Considering all the above findings, it shows that in some cases, high expression of GLS1 in circulating blood might indicate a poor prognosis for cancer patients and the mechanism of GLS1 as a tumor biomarker in cancer needs to be further elucidated.
Function of GLS1 in cancer
As an important rate-limiting enzyme for glutamine metabolism, GLS1 plays a pivotal role in multiple cancers [38]. However, the function of GLS1 in cancer research has not been fully probed. Based on the Cancer Hallmarks Analytics Tool (http://chat.lionproject.net/) and the researches of GLS1, it is implicated in cellular energetics, redox homeostasis, sustaining proliferative signaling, induction of apoptosis, driving tumor progression, invasion and metastasis and conferring bad prognosis (Fig. 4).
Roles of GLS1 in cancer progression. Investigations of GLS1 in cancer research mainly focus on its crucial roles in genome instability and mutation, cellular energetics, sustain proliferative signaling and so on. The data are from Cancer Hallmarks Analytics Tool (http://chat.lionproject.net/)
Cellular energetics
As is known to all, GAC exists in mitochondria and KGA is found in the cytoplasm, which has an impact on cancer metabolism [39]. Generally, glutamine plays an important role in the growth and synthesis of cancer cells. When GLS1 is inhibited or knockdown, glutamine cannot be converted into glutamate and α-KG, thereby entering the tricarboxylic acid cycle. It leads to the obstruction of the energy metabolism and biosynthesis pathways of cancer cells, which induces cancer cell death [2, 5]. The inhibition or blockade of GLS1 would decrease the production of ATP, reduce oxygen consumption rate (OCR) and restrain TCA cycle, which could lead to cell death and induce apoptosis [40, 41].
Anti-proliferation activity
Glutamine not only provides energy for cancer cells, but also plays a certain biosynthetic role [39]. When glutamine is transported into the cell, it is metabolized by glutaminase and the biosynthesis of cancer cells is enhanced, thereby promoting tumor cells proliferation [41,42,43]. In addition to amino acids, glutamine is also an important raw material for nucleotides, proteins, and lipids [41, 44]. In addition, glutamine can also be used as a basis for biosynthesis, providing carbon and nitrogen sources for cancer cells. GLS1 is the key regulator of glutamine metabolism. Therefore, it is found that GLS1 could regulate the proliferation in many tumors. Recent research shows that the increase of GLS1 in the patient's cancer tissues helped to promote tumor cell proliferation [22, 24]. At the same time, the knockdown of GLS1 gene or GLS1 drug blockade could inhibit tumor growth in multiple mouse xenograft models which indicates that GLS1 mainly participates in the utilization of glutamine to promote tumor proliferation [27, 51, 52]. In other aspects, Kr-POK (POZ domain and Krüppel-like protein associated with kidney cancer) could inhibit tumor proliferation in vivo by inhibiting the expression of GLS1 in the xenograft model. Mechanistically, Kr-POK might activate GLS1 and increase glutamine uptake to promote rapid tumor growth in cancer cells [53]. In addition, GLS1 could also inhibit cancer cell proliferation by preventing pyrimidine synthesis and inducing DNA damage [30] which is different from previous studies.
Redox homeostasis
Generally, glutamine metabolism could produce reactive oxygen species (ROS) levels in cancer cells [45]. Therefore, the depletion of glutamine or knockdown of GLS1 could increase the level of ROS and promote endoplasmic reticulum stress in cancer cells [45]. The process of glutamine metabolism could protect cells from oxidative stress by reducing glutathione (GSH) levels and providing NADPH antioxidants [46]. When shRNA was used to knock down GLS1, the levels of GSH and oxidized glutathione (GSSG) in cancer cells were significantly decreased, which is consistent with previous research [47]. On the other hand, using GLS1 inhibitor BPTES or compound 968 in lung cancer cells and ovarian cancer, it was found that GLS1 inhibitors mainly increase ROS levels by inhibiting glutamine flow into the Kreb’s cycle and reducing oxidative phosphorylation of mitochondria [48]. Similarly, in ovarian and gastric cancer, various concentrations of compound 968 caused a decrease in GLS1 expression and increased ROS levels in a dose-dependent manner, which further proved that GLS1 may play an important role in ROS and participate in the progress of oxidative stress to driven tumor progression [49, 50].
Induction of apoptosis
When discussing the apoptotic effect of GLS1 on cancer cells, it is found that the apoptosis of GLS1 on cells is often accompanied by the production of ROS and many GLS1 inhibitors have a certain apoptosis effect on cancer cells [19, 38]. When the process of glutamine metabolism is blocked, the production of ROS was produced more, the expression of GSH is reduced, and the level of cell redox is promoted to induce cell apoptosis. Interestingly, GLS1 is a key enzyme in the process of glutamine metabolism. When it is knocked down or blocked, tumor cells will show high levels of apoptosis [19, 35, 39]. For example, after knocking down GLS1, the apoptosis rate of both THC8307 and THC8307 cell lines increased significantly [19]. Interestingly, GLS1 might induce apoptosis and enhance the drug sensitivity via affecting the mTOR pathway [19, 35]. Furthermore, the GLS1 inhibitor BPTES has a certain effect on the glutamine metabolism of cancer cells and causes the energy production of restrictions to induce apoptosis [38], indicating that GLS1 may serve as a role to induce cancer apoptosis and tumor progression. In conclusion, GLS1 has a close relationship with tumor cell apoptosis.
Others
Further investigations have also validated that GLS1 may show an anti-cell cycle action in NSCLC. When combining BPTES with 5-FU, it was found that the G1 cell cycle arrest of lung cancer cell A549 increased by approximately 20–30%, and significantly induced NSCLC cell cycle arrest, leading to cell death [30]. In addition, the research found that GLS1 has a certain relationship with the metastasis of cancer cells. When the GLS1 expression in CRC tissues increases, the tumor node metastasis of CRC cells increases significantly [30, 54, 55]. High expression of GLS1 could accelerate the metastatic process of CRC [57], but the mechanism still needs to be further investigated.
Regulators of GLS1 in cancer
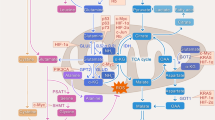
It is showed that the expression of GLS1 is regulated by various regulatory factors, thereby promoting glutamine metabolism in various cancer cell lines (Fig. 5). To be exact, there are many factors related to the induction of GLS1 during cancer progression, such as Myc [43], microRNA [58], phosphate (PI) [39], Retinoblastoma (Rb) protein [59] and nuclear transcription factor-κB (NF-κB) [60]. As a glutamine enzyme in glutamine metabolism, the regulators of GLS1 have been extensively studied in a variety of cancer types, but the molecular mechanism of GLS1 still needs to be further investigated.
Pathways regulating GLS1 expression in cancer research. The regulators of GLS1 can be mainly classified into Myc, Rb family members, MicroRNA, NF-κB and most of them are tumor-promoting factors. Rb: Retinoblastoma protein, NF-κB: nuclear transcription factor-κB, ASCT2: alanine-serine-cysteine transporter 2, GLN: glutamine, GCLC: Glutamate-cysteine ligase catalytic subunit, Cys: Cysteine, GSH: Glutathione, GDH: glutamate dehydrogenase, GLU: glutamate, α-KG: alpha-ketoglutarate, OOA: Oxaloacetate, Mal: Malic acid; OGDH: oxoglutarate dehydrogenase; NADPH: triphosphopyridine nucleotide; NAD+: nicotinamide adenine dinucleotide; IDH: isocitrate dehydrogenase; ME: malic enzyme, ROS: reactive oxygen species.
Myc
Myc is an oncogene and tumor suppressor which could cause “glutamine addiction” of a cancer cell and affecting tumor progression. In 2009, it is reported that Myc could regulate the expression of GLS, thereby regulating glutamine metabolism and preventing cancer progression [43]. Importantly, the Myc oncogene could produce Myc protein which directly or indirectly regulates the transcriptional expression of GLS1 [43] (Fig. 5). Researchers also identified that the dependence of growing cancer cells on glutamine and showed the depletion of glutamine potently induced apoptosis in cells with ectopically activated Myc [60]. In human PC3 prostate cancer cell lines, when Myc is knocked down, the expression of GLS1 is also reduced, which showed a certain correlation between Myc and GLS levels [61]. The levels of glutamine transporters and glutaminolysis decreased upon Myc deactivation (Myc-OFF) [62]. Further studies confirmed that GLS1 was shown to be positively regulated by Myc, and Myc could also affect glutamine metabolism via different pathways [43, 63, 64]. On the other hand, the c-Myc (or Myc) could regulate miRNAs [58, 65] and stimulate cell proliferation [66]. Therefore, it is found that Myc could adjust the transcriptional expression of GLS1 through miRNAs, and we will discuss in detail in the next section. In short, Myc has an important regulatory effect on glutamine metabolism. It could directly or indirectly affect the expression of GLS1. At the same time, it also has a certain effect on the uptake of glutamine which synergistically affect glutamine metabolism and cancer progression [43].
MicroRNA
MicroRNA (miRNA) is a type of non-coding RNA that regulates gene expression by blocking translation or promoting the degradation of mRNA. The study found that miRNA could regulate GLS1, and miRNA is directly regulated by Myc. In P493-6 cells, Myc inhibited miR-23a and miR-23b, thereby inhibiting the expression of GLS1 [58, 67]. Further research showed that miR-23 a/b targets and inhibits the activity of GLS via the 3′UTR. On the other hand, antisense miR-23 RNA could rescue GLS levels in PC3 cells [43]. It is also found that there is a negative correlation between the expression miR-145 and GLS1 in ovarian cancer. The up-regulation of miR-145 could inhibit the decomposition of glutamine by inhibiting the activity of GLS1 in ovarian cancer cells. Mechanistically, miR-145 might bind to the promoter region of GLS1, thereby promoting the activity of GLS1 [25]. Interestingly, miR-192 and miR-204 also showed a connection with GLS1 [23]. As shown above, microRNAs could regulate the expression of GLS1 and promote tumor progression. Nonetheless, the mechanisms of microRNA in glutamine metabolism and tumorigenesis still remain unknown.
PI
GLS1 is also known as "phosphate-dependent glutaminase", and generally requires a high concentration of phosphate as a necessary cofactor for activating the enzyme [68]. Inorganic phosphate levels could be used as a sensor for GAC, and were first discovered and found overexpressed in human breast cancer cell lines. Early research on kidney-type isoenzymes showed that mitochondrial glutaminase is an inactive dimer in organelles and could tetramerize and activate enzymes in the presence of phosphate [69, 70]. It has been found that glutamate is competitive with phosphate. Under hypoxic conditions, F1Fo ATPase activity that uses PI to produce ATP is significantly reduced, leading to cancer progression. However, its mechanism has not been elucidated. It is speculated that PI may affect the activity of GA, thereby changing the metabolic level of cancer cells, thus affecting the cancer process [71,72,73,74,75,76]. In conclusion, PI could play an important role in activating GLS1 to promote glutamine metabolism and act as an antagonist of glutamine [39].
Rb
Retinoblastoma (Rb) protein is a tumor suppressor and is abnormally expressed in multiple cancer types. Rb plays an anti-tumor role mainly by suppressing the cell cycle-promoting transcription factor E2F family to inhibit cell cycle progression. The deletion of the Rb genome leads to increased activity of glutamine transporters ASCT2 and GLS1, thereby enhancing glutamine metabolism. It promotes the glutamine-dependent cell proliferation and survival. On the other hand, isotope experiments show that the deletion of overall Rb function leads to a significant increase in 13C-glutamine uptake, and promotes the upregulation of glutamine transporter ASCT2 expression and the activity of GLS1, thereby flowing glutamate to TCA cycle [59]. Interestingly, recombination of Rb-1 in Rb dysfunction cells could reduce the uptake of glutamine by inhibiting ASCT2 expression via E2F-3, thereby inhibiting cancer progression [70] (Fig. 5).
NF-κB
It is found that elevated GAC activity is mainly related to Rho GTPases and nuclear transcription factor-κB (NF-κB) expression in transformed fibroblasts and breast cancer cells [60]. At the same time, the transformed fibroblasts and breast cancer cells showed glutamine addiction and high glutaminase activity, which revealed a previously unrecognized link between NF-κB activation and cell metabolism [60]. In addition, the oncomine microarray data set indicates that NF-κB and GLS1 are co-overexpressed in human liver cancer samples. It have demonstrated that glutaminase activity might mainly rely on the activation of GLS1 and P53, which provides a mechanisms for elevating glutamine metabolism needs [60]. Importantly, in breast cancer cells, ErbB2 could activate NF-κB and P53 to up-regulate the expression of GLS1 [24]. In conclusion, GLS1 is controlled by NF-κB, thus affecting glutamine metabolism (Fig. 5).
Others
In addition to the above regulatory factors, there is also a certain correlation between the expression of HIF-1α/2α and GLS1. Previous studies have shown that HIF-1α stabilization could stimulate glutaminase-mediated glutathione synthesis and maintain redox homeostasis. Under the circumstances of hypoxia, HIF activity will usually increase. It could also regulate oxygen and glutamine metabolism to maintain cell growth and redox balance [76, 77]. On the other hand, the HIF-1α signal might increase glycogen storage and prevent energy deficiency during nutrition or oxygen deprivation [78]. Importantly, HIF-1 could bind to HRE in the GLS1 gene, thereby increasing the expression of GLS1 [26, 56]. All of these studies indicated that HIF-1α/2α may regulate glutamine metabolism and glutaminase activity. Further experiments showed that GLS1 inhibitors might have a certain effect on the de novo pathway of pyrimidine biosynthesis of PPP (pentose phosphate pathway) by regulating HIF-1α/2α in RCC cells [56]. Therefore, when combining GLS1 inhibitor BPETES with thymidylate synthase inhibitor 5-fluorouracil (5-FU), the anticancer effect would be potentiated in tumor cells.
Interestingly, HGF (hepatocyte growth factor) and its receptor tyrosine kinase MET/HGFR were firstly identified in the 1980s and they could hyper-activate in multiple HCC types [6,7,8,9]. In addition, the HGF-MET signaling pathway could promote the Warburg effect, glutamine and biosynthesis by activating GLS Interestingly, drugs targeting HGF-MET kinase could only inhibit the Warburg effect and glutamine decomposition without destroying biogenesis, providing a hint to drugs targeting HGF-MET kinase. In addition, it is speculated that HGF-MET may exert its inhibitory effect on glutamine decomposition via GLS1 [79]. In fact, previous researches demonstrated that KARS mutations are also related to the regulation of GLS1 in pancreatic cancer [80] and NSCLC [81]. In addition, it is also found that P53 mutations is related to the activation of GLS1 in CRC cells [82]. Glutamine metabolism serves as a vital role in cancer cells, and there are multiple pathways for the regulation mechanism of GLS1 (Fig. 5). When designing drugs for the GLS1 target, there is no necessary correlation between the therapeutic effect of the drug and the expression of GLS1 and its inhibitors often occur drug tolerance [30, 33]. Therefore, the research on the mechanism of GLS1 will of great value for the development and clinical use of GLS1 inhibitors.
Inhibitors
Based on the widely proven effects on cellular energy, tumor progression, and redox homeostasis over the past few decades, GLS1 has become a potential therapeutic target for certain cancers. A series of inhibitors have been developed to treat glutamine-dependent cancer patients. And early therapeutic treatment for glutamine metabolism in cancers mainly focused on the use of glutamine removal. Clinically, L-ornithine and phenylacetate were used to reduce the toxicity level of ammonia in cancer [109]. Recently, it reveals a clinical situation that most treatments regulate glutamine metabolism by inhibiting the initial stage of glutamine decomposition. And the GlS1 inhibitors, such as DON, BPTES, 968, CB-839, UPGL00004 and ebselen (Table 1), significantly inhibit GLS1 by different mechanism and inhibit tumor progression [14, 49, 83,84,85]. It is worth noting that CB-839 is currently the only small molecule inhibitor targeting GLS1 in the clinical trials.
DON
6-diazo-5-oxo-L-norleucine (DON) as a substrate glutamine analog could be used as a competitive inhibitor of GLS. It mainly covalently binds to the active site of GLS and modifies the catalytic serine (S291), thereby regulating the biological function of the enzyme [86]. DON showed the potential antitumor activity in several animal tumor models, including the LI210 leukemia model (with a dose range of 2.5 to 40 mg/kg, life expectancy (ILS) of more than 50%, and fatal at 80 mg/kg)) and a colon cancer model of 26 (70% ILS at a dose of 12.5 mg/kg). In addition, in the xenograft experiment of LX1 lung cancer, DON might cause significant tumor regression [7]. Nonetheless, compared with other GLS inhibitors, the toxicity and weaker binding selectivity of DON hinder its clinical research [87]. Given the anti-tumor potential of DON, it can not only inhibit the growth of tumors, but also regulate the tumor microenvironment (TME) to enhance immunotherapy. Recently, researchers have developed a lot of DON prodrugs. These prodrugs are designed to circulate in the body and have a certain degree of inertness. They will be activated in TME after enzymatic cleavage of proteins, thereby reducing the toxicity of glutamine antagonists to susceptible tissues such as the intestine [88, 89]. Among them, JHU083, the best prodrug of DON, could inhibit the oxidation and glycolysis of cancer cells, resulting in hypoxia, acidosis, and reduced nutrient consumption in MC38 tumor-bearing mice; at the same time, the drug can upregulate effector T cells oxidative metabolism, enhance immune function. Of note, this drug provides a new direction for the clinical treatment of DON and reveals the metabolic plasticity between cancer cells and effector T cells [90].
BPTES
The GLS1 selective inhibitor bis-2-(5-phenylacetamido-1, 3, 4-thiadiazol-2-yl) ethyl sulfide (BPTES) introduced by Robinson is known to inhibit GAC through an allosteric mechanism by stabilizing inactive tetramers [38, 90]. BPTES has been shown to inhibit the growth of cancer cells in various tumor models, including NSCLC [30, 38], RCC [62], breast cancer [36], glioblastoma [61] and B cell lymphoma [91]. For example, when treated with BPTES in NSCLC, it could induce cell cycle arrest through GLS1 and cause glutamate reduction to decrease ATP levels, which ultimately leads to cancer cell death [30]. Otherwise, in the xenograft experiment of A549 mice, when combining treatment with BPTES and thymidylate synthase (TYMS) inhibitor 5-FU could significantly inhibit tumor growth. The combination of GLS1 inhibitor BPETES and thymidylate synthase inhibitor 5-fluorouracil (5-FU) could potentiate the anticancer effect in NSLCL. Then, it is reported that the activity of TYMS could affect the function of glutamine metabolism via two directions. First, the knockdown or inhibition of GLS1 could induce significant ATP reduction. ATP plays an important role in nucleotide synthesis and is regarded as a major components of building material. The second, there is ATP-dependent cell cycle checkpoints at G2/M transition and the decrease of ATP production could delay the cell cycle progression in G2/M transition [30]. Interestingly, combination of BPTES and metformin could also prevent HNSCC cell proliferation [14]. Notably, Metformin could inhibit glycolysis and glycogen synthesis which is a drug used for the treatment of diabetes [92]. Metabolomics analyses revealed that surviving tumor cells following glutaminase inhibition mainly relied on glycolysis and glycogen synthesis. Therefore, when combining BPTES with metformin, tumor volume is much more greatly reduced compared with either drug alone. This review has already explained that MYC can regulate the expression of GLS1.Therefore, in Myc-off renal cell line E28, BPTES could prevent the growth of Myc-induced RCC tumor progression via the inhibition of GLS1 in vivo and vitro, confirming that Myc-induced renal adenocarcinoma has a certain dependence on glutamine metabolism [56, 62]. Similarly, BPTES could also significantly prolong the survival time of animals with Myc-induced HCC [52]. Nonetheless, BPTES has poor water solubility and pharmacokinetic characteristics, which seriously hinders its clinical application, so there is still a long way for BPTES to carry out clinical trials [94]. It is worth noting that the researchers have made great progress in polyethylene glycol nanoparticles containing BPTES. This nano-capsule can be safely administered and is currently being tested in a preclinical mouse model [94].
Compound 968
Compound 968 which is also called 5- [3-Bromo-4- (dimethylamino) phenyl] -2, 3, 5, 6-tetrahydro-2-dimethyl-benzo [a] phenanth-ridin-4 (1H) -one is known to be a non-competitive allosteric inhibitor of GAC (IC50≈2.5 µM), by interfering with the interaction of two GAC monomers to form a GAC dimer. Recent studies showed that compound 968 is a unique small molecule inhibitor that regulates intracellular glutamine metabolism by inhibiting GLS1 [18, 95, 96]. When the breast cancer cells showed elevated GLS1 activity, the sensitivity of GLS1 to small molecule inhibitor 968 increased [97]. Moreover, compound 968 also predicted a certain effect on the cell cycle of breast cancer cells, but research on other cancers needs to be furtherly investigated.
CB-839
CB-839 has been more extensively studied for its anti-tumor activity than the other GLS1 inhibitors discussed above and is the only small molecule GLS inhibitor being studied in the clinical trials. CB-839, as a selective inhibitor of GLS1, is currently undergoing multiple clinical studies including TNBC, lung, RCC and so on [18, 98]. It is shown that the sensitivity of GLS1 inhibitors has a certain relationship with the genetic phenotype of tumors and the expression of GLS1 levels, but the expression of this enzyme does not play a decisive role [5, 14, 98]. Given the potential of GLS1 for targeting cancer and the lack of reliable biomarkers to predict pharmacodynamics, there is an urgent need for a biomarker that could predict the response of GLS1 inhibitors between various indications, which is consistent with ASCT2. Recently, researchers have discovered that this drug exhibits anti-proliferative activity on TNBC cells in vitro [14, 101]. Of note, CB-839 showed anti-proliferative activity in TNBC cells HCC-1806 and MDA-MB-231, while the anti-proliferation was not observed in estrogen receptor-positive/HER2-negative (ER+/HER2−) T47Dcells [101]. On the other hand, CB-839 is currently in phase 2 clinical trials for advanced TNBC in combination with paclitaxel (NCT03057600). At the 2017 Keystone Symposium, Emberly reported that CB-839 had anti-tumor activity as a treatment for RCC [102]. In addition to solid tumors, CB-839 has been broadly studied for its activity in blood cancers and hematologic malignancies, such as MDS, AML [103], multiple myeloma (MM) [104], and lymphoma [105]. Interestingly, the combination of heat shock protein 90 (HSP90) inhibitor THZ1 and GLS1 inhibitor CB-839 had a synergistic effect on reducing tumor proliferation and cell cycle progression in NSCLC cells. In terms of mechanism, THZ1 could block glucose metabolism and affect the expression of GLS1. Thus, a combination treatment with heat shock protein 90 (HSP90) inhibitor THZ1 and GLS1 inhibitor CB-839 in NSCLC cells could enhance the efficiency of NSCLC treatment. Furtherly, a combination treatment could activate mTORC1 and protein toxicity, inhibition of GLS1 and HSP90 could increase the sensitivity of cell death by increasing oxidative stress [33].
As discussed above, researchers are currently working on the development of multiple clinical effects of CB-839. It is the main drug candidate of the human glutaminase inhibitor program, which could be used for the treatment of various cancers. However, the sensitivity GLS1 inhibitor is not completely relevant to the expression of this enzyme, we still need to research for biomarkers of GLS1 inhibitors to achieve precise treatment.
Other inhibitors
The newly discovered inhibitors ebselen, celandine and apomorphine have an affinity of 10 to 1500 times higher than DON and BPTES, and the efficiency of inhibiting GLS1 has increased by more than 100 times. Although they are non-selective, it is still worth noting that the affinity of ebselen for glutaminase is more effective than any other activity that has not been described. Ebselen, celandine, and apomorphine seem to be more effective than DON or BPTES, but due to the poor selectivity and high toxicity of such compounds, they have not yet entered clinical trials [85]. In addition, UPGL00004 is an effective GAC inhibitor and binds to GAC significantly. Moreover, UPGL00004 could effectively inhibit the proliferation of highly aggressive TNBC cell lines. When combining treatment with the monoclonal antibody bevacizumab, this compound could effectively inhibit tumor growth in a patient-derived xenograft model for breast cancer [84].
Discussion and prospect
On the basis of the observations above, there are still some challenges to target GLS1 in cancer research. Due to these vital roles of GLS1 in tumor-promoting and glutamine metabolism, GLS1 has gained extensive attention. First, When GLS1 is inhibited or knockdown, it will cause the blockade of glutamine metabolism, which will cause the change of mechanisms and phenomena that glutamine is blocked in tumor, such as the proliferation pathway related to mTORC, autophagy caused by NH4+ accumulation, and oxidative stress caused by glutathione reduce [33, 79, 83] (Fig. 5). Second, it has figured out that glutamine provides important energy and material basis for cancer cells [5]. When GLS1 is inhibited or knockdown, glutamine cannot be converted into glutamate and α-KG, thereby entering the tricarboxylic acid cycle. It leads to the obstruction of the energy metabolism and biosynthesis pathways of cancer cells, which induces cancer cell death and produces a series of biological effects [2, 5] (Fig. 5). Notably, some cancer cells might not rely on the glutamine metabolic pathway, and there is no inevitable relation between the expression and function of GLS1, such as non-triple-negative breast cancer cells [101]. When inhibiting glutamine metabolism, cancer cells will have a certain compensatory effect. Such problems will affect the clinical progress of targets which regulate glutamine metabolism, but there is currently no research report about this problem.
On the other hand, the therapeutic responses of GLS1 inhibitors in cancer research is still not clear. Although GLS1 inhibitors have definite curative effects on a variety of cancers, they still face many challenges. First, due to the subtype of cancer and metabolic heterogeneity, they do not have targets specificity. For example, CB-839 showed anti-proliferative activity in TNBC cells, such as HCC-1806 and MDA-MB-231, while the anti-proliferation was not observed in estrogen receptor-positive/HER2-negative (ER+/HER2−) T47D cells [101]. It is found that different breast tumor subtypes showed systematic variation in glutamine dependence, which is related to mammary differentiation; basal breast cancer cells showed more glutamine dependence than luminal breast cancer cells [109]. Therefore, if the tumor cells showed high expression of GLS1 but are not glutamine-dependent, GLS1 inhibitors might not have a good antitumor effect. However, whether it is associated with the subtypes of cancer or metabolic heterogeneity still need to be further investigated. To be precise, we need find the relevant biomarkers of GLS1 inhibitors, so as to guiding the clinical application of these inhibitors. The second, since GLS1 could produce certain anti-tumor effects on cancer cells via multiple metabolic pathways (Fig. 5), the efficacy of GLS1 inhibitors will be greatly increased when combining with inhibitors of related pathways, such as shock protein 90 (HSP90) inhibitor THZ1, thymidylate synthase inhibitor 5-fluorouracil (5-FU), Metformin [30, 33, 92]. GLS1 inhibitors mainly enhance the efficacy of combination drugs by influencing nucleotide synthesis, glycolysis and glycogen synthesis and other pathways. Therefore, the detail research on the mechanism of GLS1 would be valuable for the development of combined applications of GLS1 inhibitors. Finally, some GLS1 inhibitors like DON and BPTES, showed high cell toxicity, weaker binding selectivity or a poor water solubility, which seriously hinder its clinical research. Therefore, the researchers synthesized a series of prodrugs or designed nanocapsule to overcome this problem [90, 94].
Overall, GLS1 acts as a poor prognostic target and has an impact on the pro-tumor activity of a variety of cancers, especially CRC, breast cancer, HCC and ovarian cancer [19, 49, 106, 107]. Glutaminase plays a crucial role in new therapies targeting glutamine metabolism. In conclusion, it is showed that the target GLS1 is relevant in antagonizing tumor cell growth and homeostasis. Many characteristic GLS1 inhibitors have been discovered, but at present, the discovery of selective and effective small molecule GLS1 inhibitors and glutamine metabolism is limited. In addition, although the compensation mechanism for inhibiting GLS1 and knocking down cells has been determined, its mode of action in tumorigenesis still needs to be further elucidated. In conclusion, we have summarized our understanding of the functions, modulators, structure and inhibitors of GLS1 in cancer, and hope to have a comprehensive understanding of GLS1. To continue to study the tissue- and cell-specific expressions of GLS1, more detailed information on regulatory mechanisms and structure–activity relationships can still be used to update GLS1 inhibitors and their mechanisms of action involving anti-tumor effects.
Data availability statement
The raw date supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
References
Koppenol WH, Bounds PL, Dang CV, et al. Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–37.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–30.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Geck RC, Toker A. Nonessential amino acid metabolism in breast cancer. Adv Biol Regul. 2016;62:11–7.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–34.
Curthoys NP, Watford M, et al. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–59.
DeBerardinis RJ, Cheng T. The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–24.
Aledo JC, Gomez-Fabre PM, Olalla L, et al. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm Genome. 2000;11(12):1107–10.
Curthoys NP, Kuhlenschmidt T, Godfrey SS, et al. Phosphate-dependent glutaminase from rat kidney. Cause of increased activity in response to acidosis and identity with glutaminase from other tissues. Arch Biochem Biophys. 1976;172(1):162–7.
Kristin GA, David SD, Ayellet VS, et al. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60.
Matés J, Segura J, Martín-Rufián M, Campos-Sandoval J, Alonso F, Márquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr Mol Med. 2013;13(4):514–34.
Huang F, Zhang Q, Ma H, et al. Expression of glutaminase is upregulated in colorectal cancer and of clinical significance. Int J Clin Exp Pathol. 2014;7(3):1093–100.
Pan T, Gao L, Wu G, et al. Elevated expression of glutaminase confers glucose utilization via glutaminolysis in prostate cancer. Biochem Biophys Res Commun. 2015;456(1):452–8.
Gross MI, Demo SD, Dennison JB, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Canc Therapeut. 2014;13(4):890–901.
Yu DC, Shi XB, Meng G, et al. Kidney-type glutaminase (GLS1) is a biomarker for pathologic diagnosis and prognosis of hepatocellular carcinoma. Oncotarget. 2015;6(10):7619–31.
Mohamed A, Deng X, Khuri FR, et al. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 2014;15(1):7–15.
Katt WP, Cerione RA. Glutaminase regulation in cancer cells: a drugable chain of events. Drug Discov. 2014;19(4):450–7.
Wang ES, Frankfurt O, Orford KW. Phase 1 study of CB-839, a first-in-class, orally administered small molecule inhibitor of glutaminase in patients with relapsed/refractory leukemia. Blood. 2015;126:2566.
Lu WQ, Hu YY, Lin XP, et al. Knockdown of PKM2 and GLS1 expression can significantly reverse oxaliplatin-resistance in colorectal cancer cells. Oncotarget. 2017;8(27):44171–85.
Liu RQ, Li YJ, Tian LT. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in Human Hepatocellular Carcinoma. Cancer Lett. 2019;28(443):34–46.
Mariia O, Yuneva WM, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metabolism. 2012;15(2):157–70.
Xia JB, Sun YC, Zhang MT, et al. GLS1 promotes proliferation in hepatocellular carcinoma cells via AKT/GSK3β/CyclinD1 pathway. Exp Cell Res. 2019;381(1):1–9.
Ge YX, Yan XD, Jin YG, et al. FMiRNA-192 and miRNA-204 directly Suppress lncRNA HOTTIP and Interrupt GLS1 Mediated Glutaminolysis in Hepatocellular Carcinoma. PLoSGenet. 2015;12(1):e1005825.
Dong M, Miao L, Zhang FM, et al. Nuclear factor-κB p65 regulates glutaminase 1 expression in human hepatocellular carcinoma. Onco Targets Ther. 2018;11:3721–9.
Li BH, Cao YJ, Gang M, et al. Targeting glutaminase 1 attenuates stemenss properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. 2019;39:239–54.
Xiang LS, Mou J, Shao B, et al. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. 2019;10(2):40.
Xu XY, Li JY, Sun X, et al. Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression. Oncotarget. 2015;6(28):26161–76.
Huang F, Zhang Q, Ma H, et al. Expression of glutaminase is upregulated in colorectal cancer and of clinical significance. Int J Clin Exp Pathol. 2014;7(3):1093–100.
Li J, Song P, Jiang T, et al. Heat shock factor 1 epigenetically stimulates glutaminase-1-dependent mTOR activation to promote colorectal carcinogenesis. Mol Ther. 2018;26(7):1828–39.
Lee JS, Kang JH, Lee SH, et al. Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC. Cell Death Dis. 2016;7(12):e2511.
Kang JH, Lee SH, Lee JS, et al. Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion. Oncotarget. 2016;7(31):49397–410.
Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313(3):459–65.
Li J, Csibi A, Yang S, et al. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proc Natl Acad Sci. 2015;112(1):E21–9.
Guo L, Zhou B, Liu Z, et al. Blockage of glutaminolysis enhances the sensitivity of ovarian cancer cells to PI3K/mTOR inhibition involvement of STAT3 signaling. Tumour Biol. 2016;37(8):11007–15.
Fu AQ, Yu Z, Song YB, et al. Silencing of glutaminase 1 resensitizes Taxol-resistant breast cancer cells to Taxol. Mol Med Rep. 2015;11(6):4727–33.
Qie S, Clarissa C, Li WH, et al. ErbB2 activation upregulates glutaminase 1 expression which promotes breast cancer cell proliferation. J Cell Biochem. 2014;115(3):498–509.
Kim S, Kim DH, Jung WH, et al. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer. 2013;20(3):339–48.
Proteomics C, Guo YQ, Wonkyu S, et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci Rep. 2019;9(1):4995.
Cassago A, Ferreira AP, Ferreira IM, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl. 2012;109(4):1092–7.
Willems L, Jacque N, Jacquel A, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122(20):3521–32.
Anne MR, Cl’ement L, Godelieve M, et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood. 2015;126(11):1346–56.
Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–34.
Gao P, Tchernyshyov I, Chang TC, et al. C-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–5.
Qu X, Sun J, Zhang YM, et al. C-Myc-driven glycolysis via TXNIP suppression is dependent on glutaminase-MondoA axis in prostrate cancer. Biochem Biophys Res Commun. 2018;504(2):415–21.
Yuan L, Sheng X, Willson AK, et al. Glutamine promotes ovarian cancer cell proliferation through the mTOR/S6 pathway. Endocr Relat Cancer. 2015;22(4):577–91.
Shanware NP, Mullen AR, DeBerardinis RJ, et al. Glutamine: pleiotropic roles in tumor growth and stress resistance. J Mol Med. 2011; 89(3):229–36.
Martin RM, Nascimento GR, Higuero A, et al. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J Mol Med (Berl). 2014;92(3):277–90.
Ulanet DB, Couto K, Jha A, et al. Mesenchymal phenotype predisposes lung cancer cells to impaired proliferation and redox stress in response to glutaminase inhibition. PLoS ONE. 2014;9(12):e115144.
Yuan L, Sheng X, Clark LH, et al. Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer. Am J Transl Res. 2016;8(10):4265–77.
Zhen J, Zhang CH, Gan L, et al. ITRAQ-based quantitative proteomics approach identifies novel diagnostic biomarkers that were essential for glutamine metabolism and redox homeostasis for gastric cancer. Proteomics Clin Appl. 2019;13(4):e1800038.
Cetindis M, Biegner T, Munz AT, et al. Glutaminolysis and carcinogenesis of oral squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273(2):495–503.
Xiang Y, Stine ZE, Xia J, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125(6):2293–306.
Hura MW, Yoona JH, Kima MY, et al. Kr-POK (ZBTB7c) regulates cancer cell proliferation through glutamine metabolism. BBA-Gene Regul Mech. 2017;1860(8):829–38.
Song Z, Wei B, Lu C, et al. Glutaminase sustains cell survival via the regulation of glycolysis and glutaminolysis in colorectal cancer. Oncol Lett. 2017;14(3):3117–23.
Zhao J, Zhou R, Hui K, et al. Selenite inhibits glutamine metabolism and induces apoptosis by regulating GLS1 protein degradation via APC/C-CDH1 pathway in colorectal cancer cells. Oncotarget. 2017;8(12):18832–47.
Okazaki A. Glutaminase and poly (ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J Clin Investig. 2017;127(5):1631–45.
Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18(9):534–43.
Chang TC. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40(1):43–50.
Miriam RR, Andrew NL, Brian R, et al. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33(5):556–66.
Yuneva M, Zamboni N, Oefner P, et al. Deficiency in glutamine but not glucose induces Myc-dependent apoptosis in human cells. J Cell Biol. 2007;178(1):93–105.
Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–7.
Shroff EH, Eberlin LS, Dang VM, et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc Natl Acad. 2015;112(21):6539–44.
Wise DR, DeBerardinis RJ, Mancuso AS, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad. 2008;105(48):18782–7.
Wang JB, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2011;18(3):207–19.
O’Donnell KA, Wentzel EA, Zeller KI, et al. C-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–43.
Lombardi L, Newcomb EW, Dalla-Favera R, et al. Pathogenesis of Burkitt lymphoma: expression of an activated c-Myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987;49(2):161–70.
Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610.
Kristin F, Wilson JW, Erickson MA, et al. Rho GTPases and their roles in cancer metabolism. Trends Mol Med. 2013;19(2):74–82.
Godfrey S, Kuhlenschmidt T, Curthoys N, et al. Correlation between activation and dimer formation of rat renal phosphate dependent glutaminase. J Biol Chem. 1977;252(6):1927–31.
Morehouse RF, Curthoys NP. Properties of rat renal phosphate-dependent glutaminase coupled to Sepharose. Evidence that dimerization is essential for activation. Biochem J. 1981;193(3):709–16.
Sayre FW, Roberts E. Preparation and some properties of a phosphateactivated glutaminase from kidneys. J Biol Chem. 1958;233(5):1128–34.
Campos-Sandoval JA. Expression of functional human glutaminase in baculovirus system: affinity purification, kinetic and molecular characterization. Int J Biochem Cell Biol. 2007;39(4):765–73.
Kenny J. Bacterial expression, purification and characterization of rat kidney-type mitochondrial glutaminase. Protein Expres Purif. 2003;31(1):140–8.
Gorman MW, He MX, Hal CS, et al. Inorganic phosphate as regulator of adenosine formation in isolated guinea pig hearts. Am J Physiol. 1997;272(2 Pt 2):H913–20.
Nguyen T, Mourad O, Johnson JA. Delta protein kinase C interacts with the dsubunit of the F1F0 ATPase in neonatal cardiac myocytes exposed to hypoxia or phorbol ester. Implications for F1F0 ATPase regulation. J Biol Chem. 2008;283(44):29831–40.
Aragones J, Fraisl P, Bases M, et al. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9(1):11–22.
Deberardinis RJ, Lum JJ, Hatzivassiliou G, et al. The biology of cancer. Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008; 7(1):11–20.
SteveStegen, Nickvan G, GuyEelen, et al. HIF-1α Promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab 2016;23(2):265–79.
Huang X, Gan GM, Wang XX, et al. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy. 2019;15(7):1258–79.
Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–5.
Bruneli L, Caiola E, Marabese M, et al. Capturing the metabolomics diversity of KRAS mutants in non-small-cell lung cancer cells. Oncotarget. 2014;5(13):4722–31.
Hua M, Liu LS, Yao WR, et al. Activation of p53 by costunolide blocks glutaminolysis and inhibits proliferation in human colorectal cancer cells. Gene. 2018;678:261–9.
Rahman A, Smith FP, Luc PT, et al. Phase I study and clinical pharmacology of 6-diazo-5-oxo-L-Norleucine (DON). Invest New Drugs. 1985;3(4):369–74.
Huang Q, Stalnecker C, Zhang C, et al. Characterization of the interactions of protent allosteric inhibitors with glutaminase C, a key enzyme in cancer cell glutamin metabolism. J Biol Chem. 2018;293(10):3535–45.
Thomas AG, Rojas C, Tanega C, et al. Kinetic characterization of ebselen, chelerythrine and apomorphine as glutaminase inhibitors. Biochem Biophys Res Commun. 2013;438(2):243–8.
Thangavelu K, Chong QY, Low BC, et al. Structural basis for the active site inhibition mechanism of human kidneytype glutaminase (KGA). Sci Rep. 2014;4:3827.
Ortlund E, Lacount MW, Lewinski K, et al. Reactions of Pseudomonas 7A glutaminase-asparaginase with diazo analogues of glutamine and asparagine result in unexpected covalent inhibitions and suggests an unusual catalytic triad Thr-Tyr-Glu. Biochemistry. 2000;39(6):1199–204.
Kathryn M, Lemberg. We’re Not “DON” Yet: Optimal Dosing and Prodrug Delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther. 2018;17(9):1824–32.
Rana R, Andrej J, Lukáš T, et al. Discovery of 6-Diazo-5-oxo-l-norleucine (DON) prodrugs with enhanced csf delivery in monkeys: a potential treatment for glioblastoma. J Med Chem. 2016;59(18):8621–33.
Robinson MM, McBryant SJ, Tsukamoto T, et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2 (5-phenylacetamido-1, 2, 4-thiadiazol-2-yl) ethyl sulfide (BPTES). Biochem J. 2007;406(3):407–14.
Lee A, Lane AN, Hamaker M, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15(1):110–21.
Goodwin PJ, Wendy RP, Karen AG, et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. 2015;107(3):006.
Elgogary A, Xu QG, Brad P, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci USA. 2016;113(36):E5328–36.
Shukla K, Ferraris DV, Thomas AG, et al. Design, synthesis and pharmacological evaluation of bis-2 (5-phenylacetamido-1, 2, 4-thiadiazol-2-yl) ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55(23):10551–63.
Stalnecker CA, Ulrich SM, Li Y, et al. Mechanism by which a recently discovered allosteric inhibitor blocks glutamine metabolism in transformed cells. Proc Natl Acad. 2015;112(2):394–9.
Natalie E, Simpson VP, Tryndyak FA, et al. An in vitro investigation of metabolically sensitive biomarkers in breast cancer progression. Breast Cancer Res Treat. 2012;133(3):959–68.
Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–82.
Michaud J, Simpson KM, Escher R, et al. Integrative analysis of RUNX1 downstream pathways and target genes. BBMCGenomics. 2008;9:363.
Van den Heuvel AP, Wooster RF, Bachman KE, et al. Analysis of glutamine dependency in non-small cell lung cancer. Cancer Biol Ther. 2012;13(12):1185–94.
Parlati F, Gross M, Janes J, et al. Glutaminase inhibitor CB-839 synergizes with pomalidomide in preclinical multiple myeloma models. Blood. 2014;124:4720.
DeMichele A, Harding JJ, Telli ML, et al. Phase 1 study of CB-839, a small molecule inhibitor of glutaminase (GLS) in combination with paclitaxel (Pac) in patients (pts) with triple negative breast cancer (TNBC). J Clin Oncol. 2016;34:1011.
Song M, Kim SH, Chun YJ, et al. Recent development of small molecule Glutaminase inhibitors. Curr Top Med Chem. 2018;18:1–12.
Matre P, Shariati M, Velez J, et al. Efficacy of novel glutaminase inhibitor CB-839 in acute myeloid leukemia. Blood. 2014;18(11):1937–46.
Das DS, Ravillah D, Ray A, et al. Anti-myeloma activity of a novel glutaminase inhibitor CB-839. Blood. 2014;124:3439.
Parlati F, Bromley-Dulfano S, Demo S, et al. Antitumor activity of the glutaminase inhibitor CB-839 in hematological malignances. Blood. 2013;77(23):6746–58.
Jalan R, Wright G, Davies NA, et al. L-Ornithine phenylacetate (OP): a novel treatment for hyperammonemia and hepatic encephalopathy. Med Hypotheses. 2007;69(5):1064–9.
Simpson NE, Tryndyak VP, Beland FA, et al. An in vitro investigation of metabolically sensitive biomarkers in breast cancer progression. Breast Cancer Res Treat. 2012;133:959–68.
Manabu K, Kiyotaka O, Hideyuki S, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nature communication. 2020;11(1):1320.
Kung HN, Marks JR, Chi JT, et al. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 2011;7(8):e1002229.
Jin N, Bi AW, Lan XJ, et al. Identification of metabolic vulnerabilities of receptor tyrosine kinases-driven cancer. Nat Commun. 2019;10(1):2701.
Funding
This study is supported by the National Key Research and Development Program of China (Grant No. 2017YFA0205200), the National Natural Science Foundation of China (Grant Nos. 81771957, 81801811).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest associated with submission of this manuscript for all the authors listed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, W., Yang, X., Zhang, Q. et al. Targeting GLS1 to cancer therapy through glutamine metabolism. Clin Transl Oncol 23, 2253–2268 (2021). https://doi.org/10.1007/s12094-021-02645-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02645-2