Abstract
This study analyses the heavy metal contamination in freshly deposited sediment of 210 km stretch of River Ganga between Kanpur and Prayagraj, Uttar Pradesh (U.P.), India. As per APHA (2012)30 digested samples were prepared after collecting from 10 sampling sites. Then the samples were analyzed for 8 heavy metals namely Cadmium (Cd), Chromium (Cr), Copper (Cu), Iron (Fe), Manganese (Mn), Nickel (Ni), Lead (Pb), and Zinc (Zn) by Atomic Absorption Spectroscopy (AAS) (Shimadzu AA-6300). Risk assessment was done by employing four pollution indices. The average values of contamination factor (CF) indicate that the level of contamination by Pb is moderate (1 < CF < 3) and significant by Cd (3 < CF < 6). Whereas, the average value of Geo-accumulation index (Igeo) indicates that the sediments are uncontaminated or in a moderate level of contamination by Pb (0 < Igeo < 1) and in a moderate level of contamination by Cd (1 < Igeo < 2), which may have harmful effects on Ganga River ecology. The values of Pollution load index (PLI) indicate that the Ganga River sediment has a low level of contamination. Based on various pollution indicators, it has been observed that concentrations of Cd and Pb in the river sediment is a matter of concern and may lead to further deterioration of sediment quality in near future due to increasing anthropogenic activities in the river basin, hence proper management strategies should be taken to control the direct dumping of both domestic and industrial wastewater in the river.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal contamination in rivers is a serious threat to aquatic life as they are highly toxic, long-term persistent in the environment, and get accumulated in water bodies [1, 2]. Causes of heavy metal contamination in water bodies include atmospheric deposition [3] erosion of soil [4], disposal of effluent (treated or untreated) [5], use of fertilizers and pesticides which contain heavy metals [6], surface run-off [7] and various chemicals from agricultural, urban and industrial activities [8, 9]. Some studies have also suggested that the quality of the sediments is an indication of pollution levels in the river and can also help to detect the history of pollution from the past to the present [7]. The presence of elevated levels of toxic heavy metals in sediments can pose a potential risk to the health of humans as they may transfer into aquatic species and then enter the food chain [10, 11].
In India, Ganga is the largest river with a stretch of 2525 km originating from Gangotri Glacier and ending in the Bay of Bengal, catering to around 400 million people of India. River water quality is degraded in the last few decades [12, 13]. Discharge from industries is a primary source of pollution in the Ganga River [14, 15]. Only 3500 MLD of sewage is treated out of 8250 MLD of total sewage which is generated from more than 220 cities in the Ganga River basin. 2460 MLD is dumped directly into the Ganga River, 4510 MLD is discharged into its tributaries, and the remaining is dumped on land or low-lying areas that leach downstream [15]. Since 1985, the government has taken many steps but still, the quantity of untreated sewage being discharged into the Ganga River has increased [12]. In addition to sewage from urban areas, approximately 2500 MLD sewage load of industrial units which include textiles, sugar mills, electroplating, power plants, distilleries, paper and pulp, etc. discharge their waste directly into the Ganga River [16]. The Middle section of the Ganga River i.e. from Kannauj to Varanasi receives most of the industrial discharge to the river majorly from tannery industries [17].
Heavy metal studies have been done on various rivers including the Ganga River, India [18], Indus river, Pakistan [19], Gomti River, India [20], Hindon River, India [21] and Nile River, Egypt [22].
The main objectives of this study are (i) to find the heavy metal concentrations in the Ganga River sediments of the selected stretch, (ii) to identify their source i.e., naturally enriched or anthropogenic using various pollution indices like enrichment ratio (ER) and geo-accumulation index (Igeo), (iii) to study spatial distribution pattern of heavy metal concentrations in the selected stretch and (iv) to evaluate the risk of heavy metals on the environment in the selected area by comparing the current metal concentrations values with standard quality guidelines. This study is also conducted to access the pollution level in the Ganga River and provides a base for the environmental health risk assessment of the consumers and also to manage the problems related to Ganga River pollution in the studied area.
Though in the past, studies were conducted to assess heavy metal contamination in the Ganga River water [17, 23] and Ganga River bed sediment [24, 25] but very limited data is available for the Jajmau location of Ganga River which is in the downstream of Kanpur city. Jajmau is a hub of tannery industry involving heavy metals in their manufacturing process and other industries like paints, electroplating, leather, etc. which lead to a huge untreated sewage load in the Ganga River which could be a possible source of heavy metal contamination in the Ganga River [26]. Further, this can also lead to contamination to downstream of the Ganga River in the holy city of Prayagraj where lakhs of people take holy baths daily and do many rituals and spiritual activities. This gave us the motivation to select this particular stretch of Ganga River between Kanpur and Prayagraj city for this study. In addition, the entire selected region is used for agricultural purposes using various pesticides and fertilizers which can contaminate River Ganga with heavy metals. Besides, chromium tannery effluent contain some other heavy metal like lead, cadmium and copper which are toxic in excess amount. Cu, Fe, Mn, Ni, and Zn metals are required as nutrients in trace amount for life processes in plants and microorganisms but become toxic at higher concentrations wheras Pb, Cr, and Cd metals have no biological function and are toxic elements. Therefore, in this study, the deposition of these eight heavy metals namely Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn was analyzed to evaluate its spatial distribution in the Ganga River sediments.
2 Methodology
2.1 Study area, sampling sites and collection of sediment samples
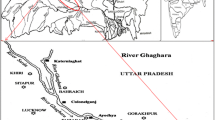
The Ganga River flows through Kanpur and joins the Yamuna river in the Triveni Sangam in Prayagraj city, a sacred confluence in Hinduism. Between Kanpur and Praygraj, the Ganga River flows 230 km through these 10 selected sampling sites: Jajmau (S1), Maharajpur (S2), Sarsaul (S3), Dalmau—Fatehpur Bridge (S4), Unchahar (S5), Nawabganj (S6), Manikpur (S7), Shringverpur (S8), Phaphamau (S9) and Sangam (S10) (table 1; figure 1).
Sediment sampling was done in the summer (March–June) between 7:00 AM and 9:00 AM in the year 2019. From each sampling location, 3 sediment samples were collected from a depth of 10–15 cm from the top surface of the river bank of the running river. In total 30 samples were collected from 10 selected sites using standard procedure. Each sediment sample of approximately 500 g was collected with the help of a grab sampler and then packed in impermeable polythene bags [27]. All sediment samples were then brought to the laboratory and were air dried for 72 h before further analysis.
2.2 Sample preparation and analytical aspect
According to APHA (2012), collected samples of sediment were digested and analyzed to find the concentrations of heavy metals. Sediment samples were dried in the oven at 100 °C for 1–2 h. to get the constant mass and then crumbled and ground to get < 63 µm fractions. Sediment samples each weighing 1 g were digested by adding 10 mL 1:2 solution of perchloric acid and nitric acid initially placed on a hot plate for 60 min at low temperature and then at 120 °C temperature until fumes started arising and the solution became clear and transparent. Then digested samples were filtered using Whatman filter paper 42. Then 0.1 N HNO3 is added to dilute the filtrate till the final volume of 50 mL is reached. Finally, the concentrations of heavy metals namely Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn were measured in the digested samples using atomic absorption spectroscopy (AAS) (Shimadzu AA-6300).
2.3 Calculation of enrichment ratio, geo-accumulation index, contamination factor and pollution load index
Enrichment ratio (ER) helps to know the level of environmental pollution and to know whether the source of pollution is natural or anthropogenic, based on the standards of heterogeneous sediments [7, 28]. Fe is used as reference metal and heavy metal concentrations were found with respect to it. ER of metals in the sediment samples for all sites was calculated using equation (1), [11].
where [(Xx)/(XFe)]s denotes the ratio of heavy metal X concentration and Fe concentrations in the sample and [(Xx)/(XFe)]b denotes the ratio of metal X concentration and Fe concentration taken from the background values. The background values of concentrations of Fe, Ni, Cd, Cu, Cr, Mn, Pb and Zn were taken from geometric mean values given by Singh et al [24] mentioned in table 3. The Enrichment levels were determined based on ER values using table 2.
For the determined heavy metal concentrations, the geo-accumulation index (Igeo) was calculated by using equation (2) given below [30] This index is used to assess the anthropogenic impact.
where Cn denotes the concentration of metal in (ppm) determined in the sediment sample, Bn denotes the geochemical background concentration value in ppm (table 3) of that metal in the background sample and factor 1.5 is used to reduce the errors in the background values which may be at due to lithogenic effects. Igeo values were used to report the Igeo class and pollution levels as given in table 2.
Pollution load index (PLI) is an indicator used to conclude the overall heavy metal pollution load at each site and is calculated by using equation (3) [31]. PLI = 0 indicates a perfect state of pollution; PLI = 1 points indicate only baseline levels of pollutants present and PLI above 1 would indicate progressive deterioration of sites.
where CFn is the contamination factor (CF) of the heavy metal ‘n’ in the sediment sample calculated using equation (4). CF is a quantification of the degree of contamination relative to either average crustal composition of a respective metal or to the measured background values from geologically similar and uncontaminated area.
CF less than 1 indicates a low degree of contamination; CF between 1 and 3 indicates moderate contamination, CF between 3 and 6 indicates a considerable degree of contamination, and CF above 6 indicates very high contamination.
2.4 Comparison of data using sediment quality guidelines (SQGs)
The concentrations of heavy metals in the Ganga River sediments are determined and compared with the corresponding SQGs values [32] in table 3. SQGs help in assessing the extent to which concentrations of heavy metals in sediments may adversely affect aquatic plants and animals and are designed to interpret the quality of sediment [33]. Two types of SQGs are developed for the ecosystem in freshwater [32]: probable effect concentration (PEC) and threshold effect concentration (TEC). PEC represents the concentration above which adverse effects are expected to occur and the TEC represents the concentration below which adverse effects are unlikely to occur.
3 Results and discussions
3.1 Concentrations and spatial distribution of heavy metals in the sediments of the selected sites
Heavy metal concentrations in the sediment samples collected from the selected sites are reported in table 3. On average, the heavy metal mean concentrations followed a decreasing order of i.e. Fe > Mn > Cr > Zn > Ni > Pb > Cu > Cd where Fe is in the highest concentrations and Cd is in the lowest concentration in the collected sediment samples.
3.1.1 Lead (Pb)
Lead is a toxic and unessential heavy metal. It is generated from both natural and anthropogenic sources. The main sources of lead concentrations are airborne particles, effluents of leather factories, forest fires, emissions from vehicles, volcanoes, waste incineration, paints, and pesticides. Earth's crust contains 15–20 ppm of Pb concentration [36]. Pb concentrations in this study ranged between 29.5 and 39.6 ppm with a mean value of 33.68 ppm. Pb concentration in the sites follows this order S3 < S9 < S8 < S2 < S10 < S5 < S1 < S4 < S6 < S7 with highest value at site S7 and lowest at site S3. Values of Pb concentrations at all sites exceeded the world surface rock average of 16 ppm indicating an increase in anthropogenic activities. Higher levels of Pb is a threat to fisheries resources, whereas, in plants, it initially results in enhanced growth, but from a concentration of 5 ppm onwards, this is counteracted by severe growth retardation, discoloration, and morphological abnormalities (figure 2).
3.1.2 Cadmium (Cd)
Cadmium is an unessential element and adversely affects the growth of plants. Cd is a by-product of Zn and Pb mining and smelting and is more mobile in aquatic environments than most of the other metals. Cd is released into the environment by metallurgical industries, atmospheric deposits, power plants, fertilizers, natural atmospheric processes, municipal solid waste (MSW) incineration, and discharge of toxic effluents from industries and wastewater treatment units [37]. It is extremely important to study this contaminant because it is highly toxic and water soluble. Earth's crust contains 0.1–0.5 ppm of Cd [38]. The Cd concentration in this study ranged between 0.95 and 3.45 ppm with a mean value of 1.94 ppm. Cd concentration in the sites followed this order S9 < S1 < S6 < S10 < S2 < S3 < S5 < S7 < S8 < S4 with highest at S4 and lowest at S9. Values of Cd concentrations at all sites exceeded the World Surface Rock Average of 0.2 ppm indicating an increase in anthropogenic activities. This can cause toxicity to some aquatic species in the vicinity (figure 3).
3.1.3 Copper (Cu)
Copper is an essential nutrient in freshwater and river sediments for the growth of aquatic life, but its higher concentration is toxic. It is induced in the environment from natural sources such as decaying vegetation, volcanic eruptions, sea spray, forest fires, and also from anthropogenic activities like wastewater from industries and municipal corporations [39]. After several natural processes, the dissolved Cu finally gets adsorbed in the sediment. In this study, Cu concentration ranged from 3.8 to 17.1ppm with a mean value of 8.17 ppm. Cu concentration in the sites followed this order S10 < S3 < S4 < S1 < S9 < S8 < S2 < S5 < S7 < S6 with highest at S6 and lowest at S10. Values of Cu concentrations at all sites are well below the World Surface Rock Average of 32 ppm and IRS average value of 28 ppm indicating Cu concentrantions in river sediment are safe for aquatic life (figure 4).
3.1.4 Zinc (Zn)
Sediment may contain up to 100 ppm of zinc in its natural state. Human activities and natural processes are the sources of Zn. The use of fertilizers for agricultural purposes in the catchment of the Ganga River leads to increase in Zn concentrations in river sediments. In this study, concentrations of Zinc ranged from 27.5 to 58.1ppm with a mean value of 38.33 ppm. Cu concentration in the sites followed this order S10 < S3 < S9 < S4 < S1 < S8 < S5 < S2 < S6 < S7 with highest at S7 and lowest at S10. Values of Zn concentrations at all sites are less than the World Surface Rock Average of 127 ppm; however, it exceeded the IRS average value of 16 ppm [34] which may cause adverse effect on aquatic biota (figure 5).
3.1.5 Iron (Fe)
Iron (Fe) concentrations were found to be highest among all elements in Ganga River sediment due to many natural reasons like weathering, erosion, etc., and human activities such as urban discharge, industrial effluent, construction and demolition wastes, municipal solid waste, and agricultural activities. In this study, Fe concentration ranged between 15,110 and 30,017 ppm with a mean value of 21072.2 ppm (table 3). Fe concentration in the sites followed this order S3 < 10 < S9 < S2 < S1 < S8 < S5 < S4 < S7 < S6 being highest at S6 and lowest at S3. Values of Fe concentrations at all sites are less than the world surface rock average of 35900 ppm (figure 6).
3.1.6 Manganese (Mn)
Mn concentration in the earth’s crust ranges between 40 and 900 ppm [40]. Sewage sludge, municipal wastewater, mining waste, waste from various metal processing units, alloy production unit emissions, and fossil fuel combustion is the source of manganese in sediments. In this study, Mn concentration lies between 349 and 679 ppm with a mean value of 491.1 ppm. Mn concentration in the sites followed this order S9 < S8 < S3 < S10 < S7 < S1 < S2 < S5 < S4 < S6 being highest at S6 and lowest at S9. Mn concentrations at sites S4 and S6 exceeded the IRS average value of 605 ppm [34] whereas concentrations at all sites are less than the world surface rock average of 750 ppm (figure 7).
3.1.7 Nickel (Ni)
Ni is used in alloys with chromium, iron, copper, and zinc. It is widely used in the industry such as fuel production, household appliances, food production, electroplating, pigments, ceramics, jewelry manufacturing, heat exchangers, magnets, coins, medical prostheses, batteries, etc. In this study, Ni concentration remained below its baseline (46 ppm) ranging between 32.2 and 42.6 ppm with a mean value of 35.16 ppm, indicating a less contaminated condition by this metal. Ni concentration in the sites followed this order S3 < S4 < S1 < S2 < S9 < S10 < S5 < S8 < S6 < S7 with lowest at S3 and highest at S7. Ni concentrations at sites S6 and S7 exceeded the IRS average value of 37 ppm [34] whereas concentrations at all sites are less than the world surface rock average 49 ppm. This indicates the low level of the contamination of the river sediment (figure 8).
3.1.8 Chromium (Cr)
Chromium has an average concentration of 100 ppm in the earth's crust [41]. Chromium compounds are used in dyes, paints factories, and leather industries, these compounds get transported in the soil and groundwater of industrial sites. Paints base containing chromium is used for automotive refinishing applications that lead to chromium pollution in the Ganga River. In this study, Cr ranged from 31.4 to 100.2 ppm with a mean of 61.13 ppm. Cr concentration in the sites followed this order S1 < S9 < S8 < S3 < S10 < S5 < S4 < S6 < S7 < S2 with lowest at S1 and highest at S2. Cr concentrations at sites S2, S6, and S7 exceeded the both IRS average value and world surface rock average of 87 ppm and 71 ppm respectively whereas concentrations at all other sites are less than the IRS average value and the world surface rock average. Increase Cr concentration at some sites is alarming can cause lethality to some aquatic species in the river system (figure 9).
3.2 Distribution of heavy metals in Ganga River sediments
Following pattern based on the decreasing order of concentration of heavy metals at various sites was observed (see table 3): S7 > S6 > S2 > S5 > S4 > S8 > S1 > S3 > S10 > S9. Among these sites, the Manikpur (S7) site is the most contaminated site receiving the most industrial discharges from the surrounding upstream Kanpur city. All sampling sites receive toxic waste from the upstream flow and the catchment agricultural areas. Among the heavy metals examined, Cd and Pb concentrations exceeded the bottom limit of 0.2 ppm and 16 ppm by up to 15 and 2.4 times, respectively. Industrial cities like Kanpur and Prayagraj lead to industrial and municipal waste in the water of the Ganga River. At some sites, the Cd concentration exceeded the permissible reference point due to low water flow, which also leads to the deposition of different heavy metals in the sediments of the Ganga River at these sites.
3.3 Estimation of pollutant indices
3.3.1 Enrichment ratio (ER) and geo-accumulation index (Igeo)
ER depicts the contamination levels of heavy metals and their source of contamination in a specific environment [42, 43]. ER < 2 indicates the crustal source of the elements in the sediment, whereas ER > 2 reflects the high levels of anthropogenic pollution. The ER values of studied heavy metals in this study are presented in table 4.
The ER values range for Mn (0.36–0.64), Cr (0.44–1.42), Zn (0.56–0.78), Pb (2.50–3.40), Ni (1.17–1.83), Cu (0.15–0.42) and Cd (2.67–10.59). Enrichment levels are identified using table 2 based on calculated values of ER. The ER values for all heavy metals at all sites except for Cd and Pb are less than 2 which indicates the enrichment is at mineral depletion level by the other metals. For most sites, the ER values of Cd show significant contamination (i.e 5 < ER < 20) of which, site S3 and S4 were the most significant sites with ER value of 10.59 and 10.42 respectively indicating the pollution due to anthropogenic activities. Cd has high geochemical activity in the environment and can be transported for a long distance as it is mobile and soluble in water compared to other heavy metals. The values of ER for Pb are between 2 and 5 for all sites which indicates moderate contamination. The difference in ER values for different heavy metals from different locations was probably because of variation in metal input or the variation in the removal rate of each heavy metal from the sediments [44].
The Igeo class was determined with reference to Abrahim and Parker Classification [45], reported in table 2. This index is used to assess the anthropogenic impact. The Igeo values for the studied 8 heavy metals for each sampling location are shown in table 5. The Igeo values for Cr, Cu, Fe, Mn, Ni, and Zn were less than zero for all sites which lie in class 0, indicating that the sediments of Ganga River at these sampling sites are unpolluted by these metals and the Average value of Igeo for Pb is calculated to be as 0.02 which lies in class 1 (i.e. Igeo value lies between 0 and 1) indicate unpolluted stage to moderately pollution stage. Igeo value of Cd at most sites falls in class 2 (i.e. Igeo value lies between 1 and 2) with a mean value of 1.03 which indicates moderate pollution by this metal.
3.3.2 Contamination factor (CF), pollution load index (PLI) and comparison with sediment quality guidelines (SQGs)
CF values are shown in table 6, CF values for Fe, Mn, Cr, Zn, Ni and Cu in all sediment samples were found to be < 1 with an average of 0.52, 0.28, 0.42, 0.37, 0.75 and 0.15 respectively which shows that the Ganga River sediment is not polluted by these metals. CF values for Pb at all locations lie between 1 and 3 with an average of 1.53 which indicates moderate contamination by Pb at all sites. The maximum CF value of for Pb was measured as 1.80 for site S7 (Manikpur) while the minimum value measured was 1.34 for site S3 (Sarsaul) and S9 (Phaphamau). The average value of CF for Cd is calculated as 3.34 which shows river sediments are contaminated by Cd in considerable amount. CF values of Cd for sites S1, S6, S9 and S9 lie between 1 and 3 which indicate moderate contamination by Cd at these sites whereas CF value for Cd at sites S2, S3, S4, S5, S7 and S8 lies between 3 and 6 which indicate a considerable amount of contamination by Cd at these sites. The maximum CF value of 5.95 for Cd was computed for site S4 while the minimum value of 1.64 was recorded at site S9. The general outcome from calculated CF values demonstrates that the river sediment is not in disturbing condition for metals Fe, Mn, Cr, Zn, Ni and Cu at present. However, it has moderate contamination by Pb and considerable contamination by Cd which may increase in the future and may be alarming to the river ecosystem with the rapidly increasing population in the river basin.
Pollution load index (PLI) indicates the overall heavy metal pollution at any site. PLI values calculated for the selected sites for the investigated heavy metals are shown in table 6. It indicates the highest pollution at Manikpur site (0.964) followed by Nawabganj (0.66), Maharajpur (0.187), Unchahar (0.154), Dalmau-Fatehpur Bridge (0.143), Shringverpur (0.035), Jajmau (0.018), Sarsaul (0.016) Sangam (0.012), whereas the lowest polluted was Phaphamau site (0.007). PLI value for site S7 (Manikpur) is close to 1 indicating close to baseline level of pollutionn and thus require quick attention to control the further deterioration of river sediment.
The reference values TEC and PEC for river sediments are shown in table 3. The concentrations of Zn and Cu were found below the reference TEC values in all sampling locations, indicating rare chances of harmful effects to the aquatic bio-system whereas the concentrations of Fe, Mn, Cr, Pb, Ni, and Cd exceeded the TEC level by 48.6, 44.7, 128, 8.93, 63, and 200% for some samples, indicating harmful effects on the aquatic plants and animals.
4 Conclusions
This study focused to assess the risk level, spatial distribution, and geo-accumulation of 8 heavy metals namely Cadmium (Cd), Chromium (Cr), Copper (Cu), Iron (Fe), Manganese (Mn), Nickel (Ni), Lead (Pb), and Zinc (Zn) in sediments of the Ganga River between Kanpur and Prayagraj stretch, U.P (India). The distribution of these metals in the sediments is not uniform throughout the studied stretch of the Ganga River and the change in metal concentration is due to the discharge of these metals from various anthropogenic sources like untreated wastewater from nearby industries of battery manufacturing and electroplating, pesticides, fertilizers, paint, tannery, etc (figure 10).
The concentrations of Cadmium (Cd) and lead (Pb) were found above the recommended SQG values of 0.99 and 35.8, indicating that the Ganga River is contaminated by Cd and Pb which could result in an adverse effect on the ecosystem of Ganga River. The values of Igeo and ER for the analyzed sediment samples revealed that they were moderately contaminated by Cd and Pb and unpolluted by other heavy metals. PLI values reveal that Manikpur is the most polluted site and Phaphamau is the least polluted site.
Heavy metal concentration levels and distribution were found higher at locations that are downstream of industrial and urban areas. This also shows that increase in industries and urban cities near the studied area were the likely causing metal contamination. Industrial units should ensure proper treatment of toxic effluent wastewater before disposing into the Ganga River. Government agencies like Central Pollution Control Borard (CPCB) and State Pollutions Control Board (SPCB) must ensure strict compliance with environmental standards of municipal and industrial effluents to save the Ganga River from further pollution by these metals. Remediation of technology such as In-situ physical/chemical treatments like capping, solidification/stabilization, Ex-situ physical/chemical treatments like soil washing, solidification/stabilization, and chelation, or Ex-situ thermal treatment like vitrification can be used for the majorly of polluted sites.
References
Qadir A and Malik R N 2011 Heavy metals in eight edible fish species from two polluted tributaries (Aik and Palkhu) of the River Chenab, Pakistan. Biol. Trace Elem. Res. 143(3): 1524–1540
Fu J, Zhao C and Luo Y 2014 Heavy metals in surface sediments of the Jialu River, China: their relations to environmental factors. J. Hazard. Mater. 270: 102–109
Demirak A, Yilmaz F and Tuna A L 2006 Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere. 63: 1451–1458
Kaushik A, Kansal A and Kumari S 2009 Heavy metal contamination of river Yamuna, Haryana, India: assessment by metal enrichment factor of the sediments. J. Hazard Mater. 164: 265–270
Zheng N A, Wang Q and Liang Z 2008 Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China. Environ. Pollut. 154: 135–142
Iqbal J and Shah M H 2014 Occurrence, risk assessment, and source apportionment of heavy metals in surface sediments from Khanpur Lake, Pakistan. J. Anal. Sci. Technol. 5: 1–12
Zahra A, Hashmi M Z and Malik R N 2014 Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah—feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 470: 925–933
Park J and Presley B J 1997 Trace metal contamination of sediments and organisms from the Swan Lake area of Galveston Bay. Environ. Pollut. 98: 209–221
Xiao R, Bai J and Huang L 2013 Distribution and pollution, toxicity and risk assessment of heavy metals in sediments from urban and rural rivers of the Pearl River delta in southern China. Ecotoxicology. 22: 1564–1575
Varol M and Sen B 2012 Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena 92: 1–10
Salati S and Moore F 2010 Assessment of heavy metal concentration in the Khoshk River water and sediment, Shiraz, Southwest Iran. Environ. Monit. Assess. 164: 677–689
Dwivedi S, Mishra S and Tripathi R D 2018 Ganga water pollution: a potential health threat to inhabitants of Ganga basin. Environ. Int. 117: 327–338
Pandey J, Pandey U and Singh A V 2014 Impact of changing atmospheric deposition chemistry on carbon and nutrient loading to Ganga River: integrating land-atmosphere-water components to uncover cross-domain carbon linkages. Biogeochemistry 119: 179–198
Yadav A and Pandey J 2017 Contribution of point sources and non-point sources to nutrient and carbon loads and their influence on the trophic status of the Ganga River at Varanasi, India. Environ Monit. Assess. 189: 1–19
CPCB 2013 Pollution assessment: River Ganga
Trivedi R C 2010 Water quality of the Ganga River-an overview. Aquat. Ecosyst. Heal & Manag. 13: 347–351
Pandey J, Shubhashish K and Pandey R 2010 Heavy metal contamination of Ganga river at Varanasi in relation to atmospheric deposition. Trop. Ecol. 51: 365–373
Pandey J and Singh R 2017 Heavy metals in sediments of Ganga River: up-and downstream urban influences. Appl. Water Sci. 7: 1669–1678
Tariq J, Ashraf M and Jaffar M 1996 Pollution status of the Indus River, Pakistan, through heavy metal and macronutrient contents of fish, sediment and water. Water Res. 30: 1337–1344
Singh V K, Singh K and Mohan D 2005 Status of heavy metals in water and bed sediments of river Gomti—a tributary of the Ganga river, India. Environ. Monit. Assess. 105: 43–67
Suthar S, Nema A K and Chabukdhara M 2009 Assessment of metals in water and sediments of Hindon River, India: impact of industrial and urban discharges. J. Hazard. Mater. 171: 1088–1095
Rifaat A E 2005 Major controls of some metals distribution in sediments off the Nile Delta, Egypt. Egyptian Journal of Aquatic Research 31(2): 16–28
Aktar W, Paramasivam M, Ganguly M, Purkait S and Sengupta D 2010 Assessment and occurrence of various heavy metals in surface water of Ganga river around Kolkata: a study for toxicity and ecological impact. Environ. Monit. Assess. 160: 207–213
Singh M, Müller G and Singh I B 2003 Geogenic distribution and baseline concentration of heavy metals in sediments of the Ganges River, India. J. Geochemical Explor. 80: 1–17
Singh H, Yadav S, Singh B K, Dubey B, Tripathi K, Srivastava V and Shukla D N 2013 Assessment of geochemical environment from study of river sediments in the middle stretch of River Ganga at Ghazipur, Buxar and Ballia area. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 83(3): 371–384
Ansari A A, Singh I B and Tobschall H J 2000 Importance of geomorphology and sedimentation processes for metal dispersion in sediments and soils of the Ganga Plain: identification of geochemical domains. Chem. Geol. 162: 245–266
USEPA 2001 Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses: technical manual. EPA 823-B-01-002 US
Zhang L, Ye X, Feng H, Jing Y, Ouyang T, Yu X, Liang R, Gao C and Chen W 2007 Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Mar. Pollut. Bull. 54: 974–982
Hakanson L 1980 An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 14: 975–1001
Muller G 1969 Index of geoaccumulation in sediments of the Rhine River. GeoJournal. 2: 108–118
Usero J, Gonzalez-Regalado E and Gracia I 1997 Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ. Int. 23: 291–298
MacDonald D D, Ingersoll C G and Berger T A 2000 Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 39: 20–31
Wenning R J 2005 Use of sediment quality guidelines and related tools for the assessment of contaminated sediments. SETAC
Subramanian V, Van’t Dack L and Van Grieken R 1985 Chemical composition of river sediments from the Indian sub-continent. Chem. Geol. 48: 271–279
Martin J M and Meybeck M 1979 Elemental mass-balance of material carried by major world rivers. Mar. Chem. 7: 173–206
Abadin H, Ashizawa A, Llados F and Stevens Y W 2007 Toxicological profile for lead. US Dep. Heal Hum. Serv
WHO 2010 Exposure to cadmium: a major public health concern. Prev. Dis. through Heal Environ. 3–6
Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L and Rudisill C 2013 Toxicological profile for cadmium. US Dep. Heal Hum. Serv. US
Dorsey A and Ingerman L 2004 Toxicological profile for copper. US Dep Heal Hum. Serv. US
Williams M, Todd G D, Roney N, Crawford J, Coles C, McClure P R, Garey J D, Zaccaria K and Citra M 2013 Toxicological profile for manganese. US Dep. Heal Hum. Serv. US
Abadin H, Fay M, Ingerman L, Tencza B, Yu D and Wilbur S B 2012 Toxicological profile for chromium. Agency for toxic substances and disease registry. Public Heal Serv US Dep. Heal Hum. Serv. US
Feng H, Han X, Zhang W and Yu L 2004 A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar. Pollut. Bull. 49: 910–915
Chen C W, Kao C M, Chen C F and Dong C D 2007 Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66: 1431–1440
Ghrefat H A, Abu-Rukah Y and Rosen M A 2011 Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environ. Monit. Assess. 178: 95–109
Abrahim G M S and Parker R J 2008 Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Envion. Monit. Assess. 136: 227–238
Funding
The authors declare that no funds, grants, or other support were received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Sample collection and preparation, testing and analysis were performed by Mohit Aggarwal. The first draft of the manuscript was written by Mohit Aggarwal. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
AGGARWAL, M., ANBUKUMAR, S. & VIJAYA KUMAR, T. Heavy metals concentrations and risk assessment in the sediment of Ganga River between Kanpur and Prayagraj, U.P., India. Sādhanā 47, 195 (2022). https://doi.org/10.1007/s12046-022-01972-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12046-022-01972-6