Abstract
The present study aims to investigate the concentration and spatial distribution of trace metals in Tamirabarani river and estuary located in the southeast coast of India. Sediment samples collected from sixteen locations were analysed for Cu, Ni, Cr, Pb, Zn and Cd. The extent of pollution in these sediments was assessed using enrichment factor (EF), contamination factor (CF), geo-accumulation index (I geo). The EF shows enrichment of Ni in the northern part of the estuary and that of Cu in the south and it is mainly due to the process of weathering. The contamination factor indicates Cd is more highly contaminated than other metals. I geo index shows that Cd is moderately polluted and its spatial distributions clearly indicate that it is found in estuarine region. The comparison of metal concentration with other estuaries of India indicates that these metals are well below the permissible limit. The metals in the estuary of the study area indicate more of the predominance of natural process than other estuaries in India. It is evident that the samples of river and estuary area are dominantly due to the natural process rather than anthropogenic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of metal contamination in fluvial ecosystems is commonly due to urban and mining activities occurring in its watershed (Guasch et al. 2009; Ferreira da Silva et al. 2009; Sierra and Gomez 2010). Trace metals may produce toxic effects on aquatic organisms depending on metal speciation, which, in turn determines bioavailability, toxicity and metal accumulation in accordance with this environmental problem (Tessier and Turner 1995; Meylan et al. 2004).
Heavy metal pollution of an aquatic ecosystem has become a potential global problem; these heavy metals are among the most common environmental pollutants, and their occurrence in waters and sediments originated from natural or anthropogenic sources. A trace amount of heavy metals is always present in fresh waters from terrigenous sources such as weathering of rocks, which may be recycled through chemical and biological contaminates in sediments in these ecosystems (Muwanga 1997; Zvinowanda et al. 2009; Harikumar et al. 2009, 2010; Sekabira et al. 2010). Heavy metal contamination in sediments could affect the quality and bio-assimilation and bioaccumulation of metals in an aquatic ecosystem. Further, these metals are immobilised within the sediments and thus might be involved in absorption, co-precipitation and complex creation (Okafor and Opuene 2007; Mohiuddin et al. 2010; Sekabira et al. 2010). These elements accumulate in the sediments through heterogeneous physical and chemical adsorption mechanisms, depending upon the nature of the sediment matrix and elements, Fe–Mn hydroxides (Awofolu et al. 2005; Mwiganga and Kanisiime 2005; Rabee et al. 2011).
The effect of heavy metals on soil depends upon the series of physical and chemical characteristics, such as texture, organic matter, pH, redox potential, etc., and the amount of trace metals in sediments is usually low. A part from clay and colloidal materials is also found to be active and contain organic matter, and they can act as a shield in controlling the reflux of trace metals in sediments from an estuary towards the coastal region (Harikumar et al. 2010). The absorption of heavy metal into sediments can be a good sign of man-induced pollution rather than natural enrichment of the sediment by weathering. Human activities transformed the geochemical cycle of trace metals, which bring environmental contamination (Nriagu and Pacyna 1988).
Heavy metals may enter into the ecosystems from anthropogenic sources, such as industrial wastewater discharges, sewage wastewater, fossil fuel combustion and atmospheric deposition (Linnik and Zubenko 2000; Campbell 2001; Lwanga et al. 2003; El Diwani and El Rafie 2008; Idrees 2009; Sekabira et al. 2010). The elements like Pb and Cd, etc. show signs of extreme toxicity even at trace level (Nicolau et al. 2006; Harikumar et al. 2010). The continuous accumulation of heavy metals into the environment can cause a serious problem to society. Therefore, heavy metal concentration in sediment unravels the history and intensity of local and regional pollution (Nyangababo et al. 2005; Sekabira et al. 2010).
Rivers are dominant pathways for metals transport (Miller et al. 2003) and trace elements may become significant pollutants of many small riverine systems (Dassenakis et al. 1998). The behaviour of metals in natural waters is a function of the substrate sediment composition, the suspended sediment composition and the water physico-chemical properties. During transport, the trace elements undergo numerous changes due to dissolution, precipitation, sorption and complexation phenomena, which affect their behaviour and bioavailability (Dassenakis et al. 1998; Abdel-Ghani et al. 2007; Akcay et al. 2003; Nicolau et al. 2006). Verslycke et al. (2003) studied that salinity affects dissolved metal speciation and toxicity in surface waters and they reported a decreasing toxicity to the estuarine waters upon increasing the salinity from 5 to 25 ‰ and attributed this to lower activities of the free trace metal ions. The present work aims to understand the behaviour of certain heavy metals like Cu, Pb, Cr, Zn, Fe, Ni, and Cd in the surface sediment of the Tamirabarani river and estuary.
Materials and methods
Study area
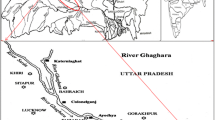
Tamirabarani river originates from western Ghat hills in the study area and confluences in the East coast of Bay of Bengal. The present estuarine region falls in the part of Tirunelveli and Thoothukudi districts, east coast of Tamil Nadu state, and is located between 8°25N and 9°10N latitudes and 77°10E and 78°15′E longitudes (Fig. 1). The study area is blessed with deltaic system with different active and inactive distributaries. The southwestern part is dominated by the river and the northern part by the sea. The tidal impact is also noted along the distributary channels (Magesh 2011).
Sediments sampling and analysis
In the study area, sixteen sediment samples were collected at the river mouth estuary and distributary channels (Fig. 2). Each sampling location was identified and recorded using a hand-held GPS (Magellan); surface sediment’s samples collected were packed in thick polyethylene bags. In the laboratory, the collected samples were frozen at −4 °C to avoid soil contamination. The freezing of the samples below −4 °C, prevents the growth of microbes or bacteria, which can result in the variation of metal in sediments. These samples were then dried in a hot-air oven at 40 °C and after homogenization using pestle and mortar; later they were sieved to less than 2 mm and stored in polyethylene bags for further analysis (Praveena et al. 1997; Shetye et al. 2009). The sediment’s samples were digested and extracted based on the procedure of Manasrah et al. (2010) subjected for the assessment of trace metals using AAS with specific flame and wavelength (Atomic Absorption Spectrometer, (Elico make)) using a series of solution over the range 2–10 mg/l. The concentration of the metals was normalised and inferred for the following parameter.
Determination of enrichment factor (EF)
Enrichment factor (EF) is the proportional abundance of the chemical elements that helps to assess the degree of contamination and to understand the distribution among the elements of anthropogenic origin from sites by individual elements in sediments. EF computed relative to the abundance of species in source material to that found in the Earth’s crust is considered as a better method for understanding the geochemical trends (Simex and Helz 1981; Praveena et al. 2007; Harikumar and Jisha 2010; Sekabira et al. 2010).
An element qualifies as a reference, if it is of low occurrence variability and is present throughout the environment in trace amounts (Loska et al. 2003; Sekabira et al. 2010). Different enrichment calculation methods and reference material have been reported by many researchers like Ogusola et al. (1994), Gaiero et al. (1997), and Sutherland (2000). Deely and Fergusson (1994) proposed Fe as an acceptable normalisation element to be used in the calculation of the EF, as they considered the Fe distribution was not related to other heavy metals. Fe usually has a high natural concentration; therefore, it is not substantially enriched from anthropogenic sources in estuarine sediments (Niencheski et al. 1994) (Table 1).
EF = (Cn/Fe) sample/(Cn/Fe) background, where, Cn is the concentration of element “n”. The continental crustal value of Fe was considered as background value (Turekian and Wedepohl 1961).
Determination of contamination factor
The levels of contamination of sediment by metals are frequently expressed in terms of a contamination factor (CF). Hakanson (1980) has suggested a CF and degree of contamination. Here CF is calculated as follows:
Crustal average values of specific metals were considered as background values (Turekian and Wedepohl 1961; Taylor and McLennan 1981). If CF < 1 refers to low contamination; 1 ≥ CF ≥ 3 means moderate contamination; 3 ≥ CF ≥ 6 indicates considerable contamination and CF > 6 indicates very high contamination (Table 2).
Geo-accumulation index (I geo)
Enrichment of metal absorption was calculated by adopting Muller (1969) methods, termed the geo-accumulation index (I geo). This method concludes the metal pollution in terms of seven (0 to 6) enrichment classes ranging from background concentration to very heavily polluted. It is calculated as follows:
where Cn = measured concentration of heavy metal in the Tamirabarani sediment. Bn = geochemical background value of average crustal value (Turekian and Wedepohl 1961) of the specific element (n). The factor 1.5 is used for the possible variations of the background data due to lithological variations. Seven different classes for geo-accumulation index provided by Muller (1969, 1981) have been used in the present study (Table 3).
In this I geo classification, 0 suggests the lack of contamination, while the I geo class 6 highlights upper limit of contaminations. Higher contamination shows the extreme enrichment of the metals relative to their background values (Harikumar et al. 2010; Sekabira et al. 2010; Riyadi et al. 2012).
Result and discussion
The total trace metal concentrations for each sampling site found in sediments of Tamirabarani river and estuary are depicted in Table 4. The metal content ranges as follows: Pb 12.930–4.480 ppm; Cd 4.920–1.410 ppm; Cr 145.500–58.300 ppm; Cu 17.820–2.200 ppm; Zn 39.740–9.300 ppm; Ni 15.200–7.830 ppm. The mean concentrations of these metals are 7.301, 2.610, 97.162, 7.758, 27.534, and 11.852 ppm for Cd, Cr, Cu, Zn and Ni, respectively. The ascending order of average concentration of metals is as follows: Cd < Pb < Cu < Ni < Zn < Cr. These are calculated from the enrichment factors of the elements in the sediment’s samples of Tamirabarani river. The difference in concentration is mainly attributed to the difference in the magnitude of input for each metal in the sediments or the difference in the removal rate of each metal from the sediment (Ghrefat and Yusuf 2006). The results specify that the Cd levels found in the sediments of study area are hazardous to the aquatic system and public health.
Sharma et al. (1999) used both Al and Fe to distinguish natural and anthropogenic sources in recent sediments from Texas estuaries. Abrahim and Parker (2008) have also used Fe as a normalising base in EF calculation to assess the contamination of marine sediments. Naturally, derived elements have an EF value of near identity, while elements of anthropogenic origin have EF values of several orders of magnitude (Kamau 2002; Valdes et al. 2005; Ghrefat and Yusuf 2006; Abrahim and Parker 2008; Akoto et al. 2008; Dragovic et al. 2008). Hence, Fe is used as the normalising base for the metals in sediments of the present study.
Based on Zhang and Liu’s (2002) categorization, if EF values between 0.5 and 1.5 indicate that these metals are entirely derived from crystalline materials or natural processes, while EF values greater than 1.5 suggest that the sources of these metals are of enriched relative to average continental crust, it could be of anthropogenic origin. All the heavy metals irrespective of locations have EF less than 1.5; they are comparable to continental crust, which indicates their source might be only from the natural weathering of exposed rocks at the river and the estuary without any additional input.
Few authors (Sekabira et al. 2010; Harikumar and Jisha 2010; Sutherland 2000) derived six categories as background concentration <1, depletion to minimal enrichment 1–2, moderate enrichment 2–5, significant enrichment 5–20, very high enrichment and 20–40 extremely high enrichment >40 (refer Table 1). It was found that all the samples fall below 1 and thus it is inferred that they represent the background concentration (Charkravarty and Patgiri 2009; Olubunmi and Olorunsola 2010; Mmolawa et al. 2011).
The spatial representation of the EF (Fig. 3) for analysed elements shows that comparatively higher enrichments of Ni and Cu are noted in the samples. It is also interesting to note that the EF of Ni is higher in the northern part of the estuary (10, 5 and 1) and that of Cu in the southern part and along the river course (12, 15, 14 and 16). Moreover, the samples with higher Ni concentration are found near the sea interface, which has high pH and salinity (Chidambaram et al. 2010); this also infers the fact that they are enriched in the finer size fractions than those samples in the southern part of the study area (Hema et al. 2002). Gambrell et al. (1991) also studied salinity effects during the oxidation of reduced metal-polluted brackish marshy sediments and Ni mobility. According to Millward and Liu (2003), the extent of metal desorption from sediments which were suspended in seawater followed the general order Ni > Cu, which is later adsorbed to surficial/bed sediments. Lores and Pennock (1998) concluded that salinity affects the binding of metals.
Statistical analysis is also used to understand the geochemical association between metals and to infer the hidden process (El-Hasan et al. 2006). The initial step in multivariate analysis is computation of correlation matrix, which gives interrelationship among the set of variables. After determining correlation matrix, correlation coefficient is measured of interrelationship for all pairs of constituents (Ashley and Lloyd 1978). The correlation coefficient expresses numerically the extent to which two variables are statistically associated. Correlation coefficients <0.5 are supposed to exhibit poor correlation. Correlation coefficient of 0.5 is termed as good correlation and >0.5 is termed to have excellent correlation. The correlation analysis of the metal concentration in sediments reveals that good correlation exists between Cu, Ni and Cr. Furthermore, between Pb and Cd, Pb and Cd have negative correlations with Cu, Ni and Cr, indicating a different source. Fe shows poor negative to poor positive correlation with other metals. This implies that metal concentration in sediments depends on release processes which are multiphasic, with the first set of processes possibly controlling early release and different sets controlling longer-term release (Forstner and Wittmann 1989; Caetano et al. 2003; Eggleton and Thomas 2004). For example, in the estuarine region of the study area bioturbation, microbial processes, and sediment oxidation can lead to significant fluctuations in metal content by changing their binding form (Simpson et al. 1998; Saulnier and Mucci 2000; Zoumis et al. 2001).
Further, it is obvious that the concentrations of Cr and Ni provide information on the provenance and shows enrichment when derived from a mafic source (Wronkiewicz and Condii 1989). Higher values of Cr and Ni, are also attributed to intense chemical weathering of the source rocks especially in tropical environments (Negender Nath et al. 2000). The correlation effect was strongest for Cd and Pb and attributed to their complexation with chloride ions. Hence, the adsorbed Ni, Cu, and Zn in sediments show that it is mainly governed by salinity of the water.
The comprehensive analysis of the contamination factor for the average values of the metals in the study is compared with the background and toxicological reference values of sediments. It appears that all the metals are low to moderately contaminated, except for Cd, which shows very high contamination. It is apparent that the average total concentration of Cadmium concentration in the sediment samples exceeded the geochemical background (average shale). It is mainly due to the fact that Cadmium uptake from water decreases with increasing salinity up to 10 ‰ (Du Laing et al. 2009).
This clearly illustrates that ascending salinity promotes Cd desorption from sediments, hence increases total Cd concentrations in the water column and Cd uptake by organisms. It is also substantiated by studies of Du Laing et al. (2002) that increasing Cd concentrations in ground-dwelling spiders, living on estuarine marshes with increasing salinities, indicates the prevelence of Cd in higher saline environments and the uptake by these organisms. It may also be understood that this less bioavailable Cd chloride complex may be adsorbed on the surficial sediment of estuarine. This further states that higher Cd bioavailability and toxicity with decreasing salinity is previously observed for living organisms in close contact with the water column, such as mussels (Fischer 1986; Stronkhorst 1993) and invertebrates (McLusky et al. 1986). The elemental contribution and enrichment of metals compared with the toxicological levels shows that Tamirabarani river and estuary sediments are moderately polluted. The spatial distribution (Fig. 4) of the contamination factor shows higher values for Cd are nearer the estuary region with varied salinity due to the tidal fluctuation, which is in agreement with the earlier interpretations.
The I geo method was used to calculate the metal contamination levels of the samples collected in the study area. The average I geo class <0 indicates unpolluted nature, but for that of Cd it shows moderately polluted to highly polluted levels. Details of the I geo values for individual elements in the 16 locations are presented in Table 3. The negative I geo values found in the table are the results of relatively low levels of contamination for all metals except Cd. This factor is not readily comparable to the other indices of metal enrichment due to the nature of the I geo calculation, which involves a log function. Based on the classification system proposed for I geo factors, all samples have moderately polluted to highly polluted index for Cd. This higher value is mainly due to the salinity factor as discussed earlier. The I geo “uncontaminated” designation is clearly inappropriate as part of an overall description of the heavy metal results for sediments from this estuary. Spatial representation of I geo (Fig. 5) shows that higher values of Cd are noted in the estuarine part of the study area and it decreases inland.
Earlier studies on this estuary by Magesh (2011) inferred that Cd concentration is due to pollution. The present study infers that it is due to natural source, similar to the present results that Lee et al. (2008) found that heavy metals concentration in the sediment varied with physico-chemical factors of seawater (i.e. dissolved oxygen, temperature and pH). Moreover, production of organic carbon in seawater due to phytoplankton growth and chelation of dissolved metals followed by their settlement increases the heavy metal concentration in the sediment (Lee et al. 2008). Hence, it is inferred that the variation of this metal is mainly due to the variation in physico-chemical factors in the estuary and not due to pollution.
Still, the velocity and duration of freshwater flow control the arrival of rainfall-entrained sediment to exposed intertidal creeks, and the focused flow is routed to the larger sub-tidal channels. In the sub-tidal zone, wider redistribution or export to the coastal ocean may occur with tidal circulation (Torres et al. 2004). Hence, the degree to which the intertidal creeks dissect the landscape reflects the sediment redistribution. In such a scenario, it is also inferred that the coarser sediments are more concentrated with Cu near the river mouth and the finer sediments are distributed along the estuary, which might have higher concentrations of Ni. Geomorphologically, it is witnessed that the coarser sediments are near the river mouth and the finer sediments are concentrated in the estuary as observed in the nearby Pichavaram Mangroves (Ramanathan et al. 1999). Hence, correlating the distribution of Cu and Ni shows that Cu is more concentrated near the river mouth, where the sediments are inferred to be coarser; and the Ni is concentrated in the fine-grained estuarine region. This relation is also well established in the correlation which indicates that Cu–Ni have good correlation indicating the same source but the distribution may be varying with respect to size of the particle. The fine-grained particles are found in the northern part of the study area near the river–ocean interface in northern part of estuary.
The comparison of the metal concentration with the other estuaries of India (Table 5) shows that they are well below the permissible limit and it is interesting to note that the estuary shows the predominance of natural process than other estuaries in India and toxicological reference of the world. Further, the study shows that Cd concentration is higher than the Crustal values (Table 6). The results specify that the Cd levels found in the sediments of the study area are hazardous to the aquatic system and public health.
Conclusion
A quantitative geochemical analysis of the sediments in Tamirabarani river and estuary sediments reveals dominance of metal concentration is as follows: Cu > Pb > Zn > Ni > Cr > Cd. The concentration of trace metals in the sediments is chiefly due to the influence of natural process. The spatial distribution of EF of different elements displays that Ni is higher in the northern part of the estuary and Cu in southern part along the river course. The contamination factor shows Cd with very high contamination and Cu, Ni, Cr, Pb, Zn are less contaminated. The study also adheres to the fact that the salinity and other physiochemical parameters of waters affect the binding property of metals. The contamination factor shows higher values for Cd near the estuary region and inferred as influenced by salinity. The Cd values in the estuary region are higher due to variation in salinity, which governs the formation of non-bio available Cd chloride complex. The higher Cd value in EF is found throughout the estuary sediment, and the I geo value reflects highly polluted nature and other elements are unpolluted. The study reflects the fact that the salinity and sediment size governs the EF. Further, the CF and I geo of sediments are governed by the physico-chemical parameters in the water, sediment interface.
References
Abdel-Ghani NT, Hefny M, El-Chaghaby GAF (2007) Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int J Environ Sci Technol 4(1):67–74
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Envron Monit Assess 136:227–238
Akcay H, Oguz A, Karapire C (2003) Study of heavy metal pollution and speciation in Buyak Menderes and Gediz river sediments. Water Res 37(4):813–822
Akoto O, Ephraim JH, Darko G (2008) Heavy metal pollution in surface soils in the vicinity of abundant railway servicing workshop in Kumasi, Ghana. Int J Environ Res 2(4):359–364
Ananthan G, Ganesan M, Sampathkumar P, Matheven Pillai M, Kannan L (1992) Distribution of trace metals in water, sediment and plankton of the Vellar estuary. Seaweed Res Utiln 15:69–75
Ashley RP, Lloyd JW (1978) An example of the use of factor analysis and cluster analysis in ground-water chemistry interpretation. J Hydrol 39:35–364
Awofolu OR, Mbolekwa Z, Mtshemla V, Fatoki OS (2005) Levels of trace metals in water and sediments from Tyume River and its effects on an irrigated farmland. Water SA 31(1):87–94
Biksham G, Subramanian V (1988) Elemental composition of Godavari sediments (Central and Southern Indian Subcontinent). Chem Geol 70:275–286
Caetano M, Madureira MJ, Vale C (2003) Metal remobilization during resuspension of anoxic contaminated sediment: short-term lab study. Water Air Soil Pollut 143:23–40
Campbell LM (2001) Mercury in Lake Victoria (East Africa): another emerging issue for a Beleaguered Lake. PhD dissertation, Waterloo, Ontario, Canada
Charkravarty M, Patgiri AD (2009) Metal pollution assessment in sediments of the Dikrong river, NE India. J Hum Ecol 27(1):63–67
Chidambaram S, Ramanathan AL, Prasanna MV, Karmegam U, Dheivanayagi V, Ramesh R, Johnsonbabu G, Premchander B, Manikandan S (2010) Study on the hydrogeochemical characteristics in groundwater, post-and pre-tsunami scenario, from Portnova to Pumpuhar, southeast coast of India. Environ Monit Assess 169(1):553–568
Dassenakis M, Scoullos M, Foufa E, Krasakopoulou E, Pavlidou A, Kloukiniotou M (1998) Effects of multiple source pollution on a small Mediterranean river. Appl Geochem 13(2):197–211
Deely JM, Fergusson JE (1994) Heavy metal and organic matter concentration and distribution in dated sediments of a small estuary adjacent to a small urban area. Sci Total Environ 153:97–111
Dragović S, Mihailović N, Gajić B (2008) Heavy metals in soils: distribution, relationship with soil characteristics and radionuclides and multivariate assessment of contamination sources. Chemosphere 74:491–495
Du Laing G, Bogeart N, Tack FMG, Verloo MG, Hendrickx F (2002) Heavy metal contents (Cd, Cu, Zn) in spiders (Pirata piraticus) living in intertidal sediments of the river Scheldt estuary (Belgium) as affected by substrate characteristics. Sci Total Environ 289:71–81
Du Laing G, Rinklebeb J, Vandecasteelec B, Meersa E, Tacka FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 466(30):973–980
El Diwani G, El Rafie S (2008) Modification of thermal and oxidative properties of biodiesel produced from vegetable oils. Int J Environ Sci Technol 5(3):391–400
El-Hasan T, Batarseh M, Al-Omari H, Ziadat Anf, El- Alali A, Al-Naser F, Berdanier BW, Jiries A (2006) The distribution of heavy metal in urban street dust of Karak City Jordan. Soil Sediment Contam 15:357–365
Environment Canada (2002) Canadian sediment quality guidelines for the protection of aquatic life: summary table. http://www.doeal.gov/SWEIS/OtherDocuments/328%20envi%20canada%202002.pdf
Ferreira da Silva E, Almeida SFP, Nunes ML, Luis AT, Borg F, Hedlund M, Mar-ques de Sá C, Patinha C, Teixeira P (2009) Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci Total Environ 407:5620–5636
Fischer (1986) Influence of temperature, salinity, and oxygen on the cadmium balance of mussels Mytilus edulis. Mar Ecol Prog Ser 32(1986):265–278
Forstner U, Wittmann GTW (1989) Metal pollution in aquatic environment. Springer, New York
Gaiero DM, Ross GR, Depetris PJ, Kempe S (1997) Spatial and temporal variability of total non-residual heavy metals content in stream sediments from the Suquia river system, Cordoba, Argentina. Water Air Soil Pollut 93:303–319
Gambrell RP, Wiesepape JB, Patrick WH Jr, Duff MC (1991) The effects of pH, redox, and salinity on metal release from contaminated sediment: water. Air Soil Pollut 57–58:359–367
Gamo T (ed) (2007) Environmental geochemistry. Baihu-kan, Japan, pp 118–119
Ghrefat H, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65:2114–2121
Govindasamy C, Kannan L (1991) Rotifers of the Pichavaram mangroves hydrobiological approach. Mahasagar Bull Nat Inst Oceanogr 24(1):39–45
Guasch H, Leira M, Montuelle B, Geiszinger A, Roulier JL, Tornés E, Serra A (2009) Use of multivariate analyses to investigate the contribution of metal pollution to diatom species composition: search for the most appropriate cases and explanatory variables. Hydrobiologia 627:143–158
Hakanson L (1980) Ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(5):975–1001
Harikumar PS, Jisha TS (2010) Distribution pattern of trace metal pollutants in the sediments of an urban wetland in the southwest coast of India. Int J Eng Sci Technol 2(5):840–850
Harikumar PS, Nasir UP, Mujeebu Rahman MP (2009) Distribution of heavy metals in the core sediments of a tropical wetland system. Int J Environ Sci Technol 6(2):23–225
Harikumar PS, Nasir UP, Mujeebu Rahman MP (2010) Distribution of heavy metals in the core sediments of a tropical wetland system. Int J Environ Sci Technol 6(2):23–225
Hema A, Richardmohan D, Srinivasalu, Selvaraj K (2002) Trace metals in the sediment cores of estuary and tidal zones between Chennai and Pondicherry, along the east coast of India. Indian J Mar Sci 31:141–149
Idrees FA (2009) Assessment of trace metal distribution and contamination in surface soils of Amman, Jordan. J Chem 4(1):77–87
Jones DS, Sutter W, Hull RN (1997) Toxicological benchmarks for screening contaminants of potential concern for effects on sediment-associated biota: 1997 Revision, ES/ ER/TM-95/R4. Oak Ridge National Laboratory, US Department of Energy. http://www.ornl.gov/webworks/cpr/rpt/68667.pdf
Kamau JN (2002) Heavy metal distribution and enrichment at Port-Reitz creek, Mombasa. West Indian Ocean J Mar Sci 1(1):65–70
Lee M, Bae W, Chung J, Jung H, Shimd H (2008) Seasonal and spatial characteristics of seawater and sediment at Youngil bay, Southeast Coast of Korea. Mar Pollut Bull 225:467–474
Linnik PM, Zubenko IB (2000) Role of bottom sediments in the secondary pollution of aquatic environments by heavy metal compounds, lakes and reservoirs. Res Manage 5(1):11–21
Lores EM, Pennock JR (1998) The effect of salinity on binding of Cd, Cr, Cu and Zn to dissolved organic matter. Chemosphere 37:861–874
Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A (2003) Assessment of arsenic enrichment of cultivated soils in Southern Poland. Pol J Environ Stud 12(2):187–192
Lwanga MS, Kansiime F, Denny P, Scullion J (2003) Heavy metals in Lake George, Uganda with relation to metal concentrations in tissues of common fish specie. Hydrobiologia 499(1–3):83–93
Magesh NS (2011) Spatial analysis of trace element contamination in sediments of Tamirabarani estuary, southeast coast of India Estuarine. Coast Shelf Sci 92:618–628
Manasrah W, Hilat I, El-Hasan T (2010) Heavy metal and anionic contamination in the water and sediments in Al-Mujib Reservoir, central Jordan. Environ Earth Sci 60(3):613–621
McLusky DS, Bryant V, Campbell R (1986) The effects of temperature and salinity on the toxicity of heavy metals to marine and estuarine invertebrates. Oceanogr Mar Biol Ann Rev 24:481–520
Meylan S, Behra R, Sigg L (2004) Influence of metal speciation in natural fresh-water on accumulation of copper and zinc in periphyton: a microcosm study. Environ Sci Technol 38:3104–3111
Miller CV, Foster GD, Majedi BF (2003) Baseflow and stormflow metal fluxes from two small agricultural catchments in the coastal plain of Chesapeake Bay Basin, United States. Appl Geochem 18(4):483–501
Millward GE, Liu YP (2003) Modelling desorption kinetics in estuaries. Sci Total Environ 314–316:613–623
Mmolawa KB, Likuku AS, aboutloeloe GK (2011) Assessment of heavy metal pollution in soils along major roadside areas in Botswana. Afr J Environ Sci Technol 5(3):186–196
MOE-Japan (Ministry of Environment, Japan) (2004) Environmental quality standards for water pollution. Tokyo, Japan
Mohiuddin KM, Zakir HM, Otomo K, Sharmin S, Shikazono N (2010) Geochemical distribution of trace metal pollutants in water and sediments of downstream of an urban river. Int J Environ Sci Technol 7(1):17–28
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geo J 2(3):108–118
Muller G (1981) The heavy metal pollution of the sediments of Neckars and its tributary: a stocktaking. Chem Zeit 105:157–164
Muwanga A (1997) Environmental impacts of copper mining at Kilembe, Uganda: a geochemical investigation of heavy metal pollution of drainage waters, stream, sediments and soils in the Kilembe valley in relation to mine waste disposal. PhD dissertation. Universitat Braunschweig, Germany
Mwiganga M, Kanisiime F (2005) Impact of Mpererwe Landfill in Kampala Uganda, on the surrounding environment. Phys Chem Earth 30(11–16):744–750
Negender Nath B, Kunzendorf H, Pluger WL (2000) Influence of provenance, weathering and sedimentary processes on the elemental ratio of the fine grained fraction of the bed load sediments from the Vembanad lake and the adjoining continental shelf, southwest coast of India. J Sed Res 70:1081–1094
Nicolau R, Galera-Cunha A, Lucas Y (2006) Transfer of nutrients and labile metals from the continent to the sea by a small Mediterranean river. Chemosphere 63(3):469–476
Niencheski LF, Windom HL, Smith R (1994) Distribution of particular trace metal in patos lagoon Estuary (Brazil). Mar Pollut Bull 28:96–102
Nriagu JO, Pacyna J (1988) Quantitative assessment of worldwide contamination of air. Water Soil Trace Metals Nat 333:134–139
Nyangababo JT, Henry E, Omutange E (2005) Lead, cadmium, copper, manganese and zinc in wetland waters of Victoria Lake Basin, East Africa. Bull Environ Contam Toxicol 74(5):1003–1010
Ogusola OJ, Oluwole AF, Asubiojo OI, Olaniyi HB, Akeredolu FA, Akanle OA, Spyrou NM, Ward NI, Ruck W (1994) Traffic pollution: preliminary elemental characterization of roadside dust in Lagos, Nigeria. Sci Total Environ 146(147):175–184
Okafor ECH, Opuene K (2007) Preliminary assessment of trace metals and polycyclic aromatic hydrocarbons in the sediments. Int J Environ Sci Technol 4(2):233–240
Olubunmi FE, Olorunsola OE (2010) Evaluation of the status of heavy metal pollution of sediment of Agbabu bitumen deposits area, Nigeria. Eur J Sci Res 41(3):373–382
OMOE (Ontario Ministry of Environment) (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Ontario Ministry of Environment and Energy, Queen’s Printerfor Ontario
Praveena SM, Ahmed A, Radojevic M, Abdullah MH, Aris AZ (1997) Factor-cluster analysis and enrichment study of mangrove sediments-an example from Mengkabong, Sabah. Malays J Anal Sci 11(2):421–430
Praveena SM, Radojevic M, Abdullah MH (2007) The assessment of mangrove sediment quality in mengkabong lagoon: an index analysis approach. Int J Environ Sci Educ 2(3):60–68
Rabee AM, Al-Fatlawy YF, Abd own A-A-HN, Nameer M (2011) Using pollution load index (PLI) and geoaccumulation index (I-Geo) for the assessment of heavy metals pollution in tigris river sediment in Baghdad region. J Al-Nahrain Univ 14:108–114
Riyadi AS Itail T, Isobe T, Ilyas M, Sudaryanto A, Setiawan IE, takahashi S, Tanabe S (2012) Spatial profile of trace elements in marine sediments from Jakarta Bay, Indonesia. In: Kawaguchi M, Misaki K, Sato H, Yokokawa T, Itai T, Nguyen M, Ono J, Tanabe S (eds) Interdisciplinary studies on environmental chemistry—environmental pollution and ecotoxicology, pp 141–150
Ramanathan AL, Subramaniam V, Ramesh R, Chidambaram S, James A (1999) Environmental geochemistry of the Pichavaram mangrove ecosystem (Tropical), southeast coast of India. Environ Geol 37:223–233
Saulnier I, Mucci A (2000) Trace metal remobilization following the resuspension of estuarine sediments: Saguenay Fjord, Canada. Appl Geochem 15:191–210
Sekabira K, Origa HO, Basamba TA, Mutumba G, Kakulidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Sci Technol 7(3):435–446
Sharma VK, Rhudy KB, Koenig R, Baggett AT, Hollyfield S, Vazquez FG (1999) Metals in sediments of Texas estuaries, USA. J Environ Sci Health 34:2061–2073
Shetye SS, Sundhakar M, Mohan R, Tyagi (2009) Implications of organic carbon, trace elemental and CaCO3 variation in a sediment core from the Arabian sea. Indian J Mar Sci 38:432–438
Sierra MV, Gomez N (2010) Assessing the disturbance caused by an industrial discharge using field transfer of epipelic biofilm. Sci Total Environ 408:2696–2705
Simex SA, Helz GR (1981) Regional geochemistry of trace elements in Chesapeake Bay. Environ Geol 3:315–323
Simpson SL, Apte SC, Batley GE (1998) Effect of short-term resuspension events on trace metal speciation in polluted anoxic sediments. Environ Sci Technol 32:620–625
Stronkhorst J (1993) The environmental risks of pollution in the Scheldt estuary. Neth J Aquat Toxicol 27:383–393
Sutherland RA (2000) Bed sediment associated trace metals in an urban stream Oahu, Hawaii. Environ Geol 39(6):611–627
Taylor SR (1964) Abundances of chemical elements in the continental crust: a new table. Geochimica et Cosmochimica Acta 28:1273–1285
Taylor SR, McLennan M (1981) The composition and evolution of the continental crust: rare earth element evidence from sedimentary rocks. Phil Trans R Soc Lond A 301:381–399
Tessier A, Turner DR (1995) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester
Torres R, Goni MA, Voulgaris G, Lovell CR, Morris JT (2004) Effects of low tide rainfall on intertidal zone material cycling, pp 93–114
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–182
US EPA (U.S. Environmental Protection Agency) (1999) Screening level ecological risk assessment protocol for hazardous waste combustion facilities, vol 3. Appendix E: Toxicity reference values, EPA530- D99-001C
Valdes J, Vargas G, Sifeddine A, Orttlieb L, Guinez M (2005) Distribution and enrichment evaluation of heavy metals in Mejillones bay (23°S), Northern Chile: geochemical and statistical approach. Mar Pollut Bull 50:1558–1568
Verslycke T, Vangheluwe M, Heijerick D, De Schamphelaere K, Van Sprang P, Janssen CR (2003) The toxicity of metal mixtures to the estuarine mysid Neomysis integer (Crustacea: Mysidacea) under changing salinity. Aquat Toxicol 64:307–315
Wronkiewicz DJ, Condie Kent C (1989) Geochemistry and provenance of sediments from the pengola supergroup South Africa: evidence for a 3.0 Ga—old continental craton. Geochem Cosmochim Leta 53(7):1537–1549
Zhang J, Liu CL (2002) Estuar Coast Shelf Sci 54:1051
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266:195–202
Zvinowanda CM, Okonkwo JO, Shabalala PN, Agyei NM (2009) A novel adsorbent for heavy metal remediation in aqueous environments. Int J Environ Sci Technol 6(3):425–434
Acknowledgments
The authors wish to express their sincere thanks to University authorities to undertake this project work. We are also thankful to the Ministry of Earth Sciences (MOES/MRDF-11/1/25/P/09-PC-III) for the financial grant given for this work. Thanks are as well due to the technical staffs of CIRT laboratory and geochemical laboratory for analysis work undertaken. We express our sincere gratitude to manuscript reviewers for the suggestion to reconstruct and refine our work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrasekaran, A., Mukesh, M.V., Chidambaram, S. et al. Assessment of heavy metal distribution pattern in the sediments of Tamirabarani river and estuary, east coast of Tamil Nadu, India. Environ Earth Sci 73, 2441–2452 (2015). https://doi.org/10.1007/s12665-014-3593-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3593-y