Abstract
To identify polymorphism in interferon gamma (IFN-\(\upgamma \)) and interleukin-2 (IL-2) genes, blood samples were collected from 380 breeder hens of the Mazandaran native fowls breeding station. DNA extraction was performed through a modified salting-out method and fragments of 670 and 659 bp from the promoter regions of IFN-\(\upgamma \) and IL-2 genes were amplified by using specific primers, respectively. Following genotyping in the IFN-\(\upgamma \) gene using the Tsp509I restriction enzyme, two alleles of A and G with the frequencies of 0.55 and 0.45 and three genotypes of AA, AG and GG were observed with the frequencies of 0.32, 0.46 and 0.22, respectively. For the IL-2 gene, two alleles of A and G were also detected using the MnlI restriction enzyme with the frequencies of 0.58 and 0.42 and three genotypes of AA, AG and GG with the frequencies of 0.33, 0.50 and 0.17, respectively. Statistical analysis revealed significant associations between IL-2 gene single-nucleotide polymorphism and productive traits including the average egg weight (EW) at 345–375 days of age, egg number (EN) at 345–375 days of age and body weight (BW) at 8 weeks of age traits (\(P{<}0.05\)). Further, in a mean comparison analysis, there were also significant differences between different genotypes of the IL-2 gene in average EW at 28 and 30 weeks of age, in which AG genotypes showed higher performance. Additionally, for the IFN-\(\upgamma \) gene, a significant difference was found between the genotypes in average EW at 28 weeks of age trait. Therefore, it can be concluded that the above-mentioned polymorphisms could be considered as the pivotal genetic makers to improve Mazandaran native fowl breeding programmes to achieve the optimum performance in productive traits more efficiently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improving animal health is considered to be the basic goal of the animal breeding industry (Soller and Andersson 1998). The poultry industry, as one of the most important parts of the agriculture sector for providing the invaluable supply of proteins, fats, minerals and vitamins as well as its worthwhile economic outcomes, has expanded dramatically during the last decade (Vetrivel and Chandrakumarmangalam 2013). However, it is threatened by prevalent diseases progressively such as Newcastle, infectious bronchitis, avian influenza, Marek’s and mycoplasmas which can cause catastrophic losses (Roussan et al. 2008). To improve poultry health, reduce disease-associated losses and keep poultry health management at a reasonable cost, efforts need to continue to advance toward understanding of the genetic bases for resistance and immunity to avian diseases. In this context, it is critically important to focus efforts on identification and characterization of genes and their genetic variants affecting innate and adaptive immunity (Van der Poel and Parmentier 2003). Genetic improvement of the immune responses can also enhance vaccine efficacy and disease resistance in chickens (Lamont et al. 2002). Indeed, the reproductive system has a key role in ensuring the continuity of the species, while the immune system is essential for continued health and survival through internal protection. Chicken has been the primary species that have shed light on reproductive–immune interactions. In chicken, increase in \(\hbox {Ca}^{2+}\) mobilization and its effect on regulation of granulosa cell function (Soboloff et al. 1995), presence of lymphocytes and cells specific to B-cell (Zettergren and Cutlan 1992) and T-cell (Withanage et al. 2003) lineages in the gonads of birds, stimulation of T-cells in the hen ovary by oestrogen (Barua and Yoshimura 1999), increasing progesterone receptor (PR) expression during sexual maturation (Pasanen et al. 1998), modulation of growth and differentiation of granulosa cells by cytokines (Hales 2000), participating the immune cells in the ovulatory process (Hellberg et al. 1991) and the formation and demise of the corpus luteum (Benyo et al. 1991) have been noted. Cytokines have generally been identified and characterized as the main components of the peripheral immune system (Dhama et al. 2015). Although far from fully comprehended, the role of cytokines as the important immune modulators in the peripheral immune system is evident (Dhama et al. 2015). Interleukins are regarded as the cytokines that regulate the relationship between lymphocytes and other leukocytes. Interleukin-2 (IL-2) is known as a cytokine that plays an active role in enhancing innate and acquired immunity and acquired safety (Kogut et al. 2003), which is produced by type-I T cells and induces natural killer (NK) cells and lymphocyte-activated killer (LAK) cells, demonstrating strong B-cell growth factor activity and can stimulate monocytic lineage cells (Pintarič et al. 2008; Nagarajan et al. 2011). It also stimulates progesterone production in granulose cells (Sharma and Gandhi 2011), the proliferation and differentiation of T cells and the activation of monocytes and macrophages, thus playing a fundamental role in regulating the immune response in animals (Zhao et al. 2011). Additionally, it affects type B lymphocytes and enhances their growth and synthesizes a limited amount of immunoglobulins and also has an inductive role in the production of interferon gamma (IFN-\(\upgamma \)) and IL-15 (Zhou et al. 2001), as well as a direct effect on the activity of heterophils in poultry (Kogut et al. 2002). The chicken IL-2 gene includes four exons and three introns and are located on chromosome 4 from nucleotides 54073166 to 54076514 (NC_006091.4) (Kaiser and Mariani 1999; Ye et al. 2006). To date, significant associations were identified between SNPs of the IL-2 gene with the resistance against gastrointestinal infection by nematodes (Donadoni et al. 2011), the differentiation and homeostasis of the so-called natural Tregs, which are developed in the thymus of animals (Burchill et al. 2007), and mastitis disease in cattle (Alluwaimi et al. 2003). Researchers reported the presence of transcription factor-binding sites in the second intron of the IL-2 gene of dairy cows and stated that the SNPs in these sites may affect the binding of transcription factors resulting in different production patterns in genotypes (Lühken et al. 2005). It has been discovered that in chickens, host immune responses to Eimeria acervulina and Eimeria tenella infection are due to the high regulation of IL-2 secretion (Choi and Lillehoj 2000; Miyamoto et al. 2002), and IL-2 production after reinfection with E. tenella could be considered as an important factor causing genetic differences between SC chickens’ resistance (an inbred chicken strain which is resistance to coccidiosis) or TK chickens’ (an inbred chicken strain which is susceptible to coccidiosis) vulnerability to coccidiosis (Li et al. 2002). Chicken IL-2 has also been used as an adjuvant factor to improve vaccine responses to infectious bursal disease virus (Park et al. 2009), avian influenza virus (Hu et al. 2006), and E. tenella (Xu et al. 2008), indicating its functional importance in enhancing immune responses to vaccines (Zhang et al. 2011).
IFN-\(\upgamma \) is one of the most important cytokines that controls the primary immune response and cellular immunity (Savan et al. 2009), and is recognized as a macrophage-activating cytokine (Reemers et al. 2012; Vervelde et al. 2013), representing a substantial link between innate and adaptive immune responses. This cytokine enhances the activity of NK cells, increases the expression of classes I and II of the major histocompatibility complex (MHC) that modulate immune responses (Zhou et al. 2001), and plays a key role as an adjuvant factor in accelerating the immune response induced by vaccine antigens (Shah et al. 2010). The chicken IFN-\(\upgamma \) gene is located on chromosome 1 from nucleotides 35053221 to 35057368 (NC_006088.4) and composed of four exons and three introns. Polymorphisms of the promoter region of this gene have been identified in white Leghorn strains that were associated with resistance to Escherichia coli in lines \(6_{1}\) and \(7_{2}\) (Kaiser et al. 1998). Similar to mammalian IFN-\(\upgamma \), production of chicken IFN-\(\upgamma \) has been used to regulate cell-mediated immunity (CMI) from T cells upon recall antigen stimulation (Lambrecht et al. 2004), which plays a vital role in influenza A virus annihilation in chickens (Singh et al. 2014). Thus, evaluation of immune responses can be exploited as an indirect selection for improving genetic resistance and promoting vaccine efficacy in chickens (Kaiser et al. 2008). It was found that heterozygote genotypes of IFN-\(\upgamma \) gene in Leghorn and Fayoumi chicken breeds demonstrated the largest primary antibody response to the sheep red blood cell (SRBC) test (Zhou et al. 2003). Considering the importance of the disease resistance issue in the poultry industry and the role of IL-2 and IFN-\(\upgamma \) genes in the immune system, the present study was performed to identify allelic polymorphisms in the promoter region of these genes and their effects on some productive and reproductive traits in breeder hens of the Mazandaran native fowls breeding station.
Materials and methods
Experimental population
The Mazandaran native fowl breeding station was inaugurated in 1988 with the purpose of preserving the population of endangered native fowls. The research center has two major activities comprising reproduction and genetic improvement of this native population. In the present study, 380 native fowls were randomly selected that had been grown under the same conditions. The productive and reproductive traits included body weight (BW) at day 1, 8 and 12 weeks of age and puberty, age of sexual maturity (ASM), egg number (EN) at 120–270 and 345–375 days of age, laying intensity (LI), egg weight (EW) at puberty, average EW (AEW) at 28, 30 and 32 weeks of age and 345–375 days of age, percentage of fertility (PF) and percentage of hatchability (PH) were recorded and measured accurately by researchers.
Blood sampling and DNA extraction
A total of 380 blood samples were collected from the brachial wing vein of selected native breeder fowls and transferred into vacutainer tubes containing disodium ethylenediaminetetraacetic acid (EDTA) and then shipped to our laboratory in an insulated cooler with cold-packs and stored at \(-20^{\circ }\hbox {C}\) until the DNA extraction process. DNA was extracted through a modified salting-out method and then quantity and quality of the extracted DNA were evaluated by both spectrophotometry and agarose gel electrophoresis.
Amplification of gene fragments and PCR reaction conditions
To amplify the targeted loci, PCR reaction solutions were prepared with the volume of \(25 \, \mu \hbox {L}\) as follows: \(2.5 \, \mu \hbox {L}\) of PCR buffer, \(1 \, \mu \hbox {L}\) of each primer (table 1), \( 1.5 \, \mu \hbox {L}\) of genomic DNA, \( 0.5 \, \mu \hbox {L}\) of dNTP, \(0.65 \, \mu \hbox {L}\) of \(\hbox {MgCl}_{{2}}\), \(0.2 \, \mu \hbox {L}\) (500 U) of Taq polymerase (cat. no. TA8109C; sinaclon) and \(17.65 \, \mu \hbox {L}\) of distilled water. Initial denaturation and final extension were performed at \(95^{\circ }\hbox {C}\) for 240 s and \(72^{\circ }\hbox {C}\) for 300 s, respectively.
Genotyping
To perform polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), \(5 \, \mu \hbox {L}\) of the PCR product of IL-2 gene was digested with \(0.3 \, \mu \hbox {L}\) of restriction enzymes at \(37^{\circ }\hbox {C}\) for 12 h. Digested products were segregated by agarose gel electrophoresis (2.5%). Then, the gel was stained with ethidium bromide and the fragments were observed using a UV transilluminator and Gel-doc machine. For the IFN-\(\upgamma \) gene, after digestion of PCR products by Tsp509I, the digested products were segregated using acrylamide gel (13%) for 3 h and 250 V, to observe the digested fragments and detect the alleles.
Statistical analysis
The chi-square test (\(\chi ^{2}\)) was used to verify the equilibrium of the genotypic frequencies with the Hardy–Weinberg equilibrium (HWE). Gene frequencies were also calculated using PopGene software. Statistical analyses were performed using SAS 9.1 software and the effects of IL-2 and IFN-\(\upgamma \) SNPs on the studied traits were evaluated with the GLM procedure.
Results
Amplification of the gene fragments
After DNA extraction, the fragments of promoter region of the IL-2 (659 bp) and the IFN-\(\upgamma \) (670 bp) genes were amplified using specific primers. All amplified fragments had a strong and clear single band, with no extra band or dimer.
Allelic and genotypic frequencies
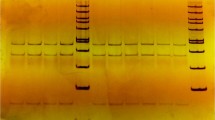
In this research, the RFLP technique was used to determine the genotypes of specific regions of IL-2 and IFN-\(\upgamma \) genes (table 2). The polymorphism of the IL-2 gene was detected by digestion of PCR products using the MnlI restriction enzyme (Zhou et al. 2001), and two alleles of A and G and three genotypes of AA (19, 115, 165 and 251 bp), GG (19, 112, 115, 139 and 165 bp) and AG (heterozygote of AG and GG) were observed (figure 1). The polymorphism of the IFN-\(\upgamma \) gene was also obtained by digestion of PCR products using the Tsp509I restriction enzyme (Zhou et al. 2001), and then, two alleles of A and G and three genotypes of AA (28, 53, 55, 56, 64, 88, 99, 104 and 123 bp), GG (28, 53, 55, 56, 88, 99, 123 and 168 bp) and AG (heterozygote of AG and GG) were detected (figure 1). The chi-square test was used to determine whether the subjects met the HWE, for IL-2 and IFN-\(\upgamma \) loci, the \(\chi ^{2}\) value was 0.86 and 0.15, respectively, both \(P{>}0.05\) (table 3). Also, observed and effective numbers of alleles of IL-2 and IFN-\(\upgamma \) loci are shown in table 4.
Association analysis
The association analysis between the genotypes of two genes with the studied traits in the Mazandaran native fowls population showed significant correlations between the IL-2 gene polymorphism and some of the studied traits. In the IL-2 locus, significant associations were found between the SNP and AEW at 375–345 days of age (\(P{<}0.05\)), EN at 345–375 days of age and BW at 8 weeks of age traits (almost achieved significance, \(P{\le } 0.058\)) (table 5). Further, mean comparison analysis indicated significant differences between different genotypes of the IL-2 gene in AEW at 28 and 30 weeks of age and also genotypes of the IFN-\(\upgamma \) gene only in AEW at 28 weeks of age traits (\(P{<}0.05\)). It was also determined that the AG genotypes, despite their lower BW at 8 weeks of age, had better performances in EN and AEW traits compared to the other two genotypes. Therefore, these genotypes could be used as useful markers for the next generations to improve performance in egg production traits.
Discussion
Genetic selection in the poultry industry was hitherto based on producing broilers with high growing rate and less feed conversion ratio (FCR). However, positive selection towards these traits can adversely impact on immune competence and lead to increase in the susceptibility of birds to various diseases (Janss and Bolder 2000; Cheema et al. 2003). Nowadays, in the poultry industry there is genetic selection for egg layers as well as for broilers. Poultry selection with an early innate immune response efficiently is an important task in the poultry industry because innate immune response directs the acquired response (Parish and O’neill 1997). Moreover, in broiler selection there is multi-environment selection to allow balanced breeding of production and health traits. Therefore, balanced genetic selection for both production and immune competence in contradictory environments is an efficient strategy to improve performance in productive traits. Numerous reports have already revealed a remarkable association between strong proinflammatory cytokine and chemokine profiles and increased resistance against diverse pathogens as well as various diseases (Heinrich et al. 2001; Sebastiani et al. 2002; Coussens et al. 2004; Swaggerty et al. 2004; Withanage et al. 2004). Swaggerty et al. (2004) indicated that the mRNA levels of proinflammatory cytokines and chemokines observed in the sires are influenced on the innate immune. Cytokines are identified as important players in different tissues and mostly have variety of functions (Goldsby et al. 2003). Moreover, former studies have indicated that cytokines act as intermediators for the two-way relationship between the neuro-endocrine and immune systems (Felten et al. 1997). In mammals, it has proven that cytokines play a key role in regression of different tissues such as the corpus luteum (Neuvians et al. 2004; Nishimura et al. 2004), follicles (Knight and Glister 2003), endometrium (Rahman et al. 2004) and mammary glands (Clarkson et al. 2003). Additionally, cytokines including TGF-\(\upbeta \), IFN-\(\upgamma \) and TNF-\(\upalpha \), IL-\(1\upalpha \), IL-\(1\upbeta \), IL-6, IL-8 and IL-10 are constitutively expressed in the oviduct and ovary of mammals (Kayisli et al. 2002; Dimitriadis et al. 2005). Also, increased cytokine mRNA expression was associated with follicular atresia and oviduct regression in chickens (Sundaresan et al. 2007). Although a number of former reports indicate that cytokines can have influence on autocrine and paracrine pathways in the ovary and oviduct, the exact mechanism of cytokine function and the normal cytokine profile in the reproductive system of chicken are not known (Sundaresan et al. 2007). Detection of the remarkable effect of the polymorphisms on traits of interest in chickens illustrates its potential for use in marker-assisted selection to improve their performance of birds such as immune function as well as reproduction and production efficiency. Considering the importance of these major genes and the role of cytokines in the immune system, reproduction system and even production, the present study was performed to identify different allelic forms in the flanking regions of IL-2 and IFN-\(\upgamma \) genes and their associations with some productive and reproductive traits in breeder hens of the Mazandaran native fowls breeding station. In the present study, in the promoter region of IL-2 and IFN-\(\upgamma \) genes, two alleles A and G, and three genotypes AA, AG and GG were identified. The results obtained from the association analysis of significant effects of each gene on different traits and the mean comparison analysis between genotypes with studied traits are comparable with those of the recent studies in this field.
IL-2
Regarding our findings, two alleles A and G and three genotypes AA, AG and GG were detected with different frequencies in the promoter region of the IL-2 gene in the Mazandaran native hen population, demonstrating the polymorphic pattern of this gene site in this native population. Also, significant correlations were discovered between the IL-2 gene polymorphisms in the promoter region with EN (\(P{<}0.015\)) and AEW at 345–375 days of age traits (almost achieved significance, \(P {\le } 0.058\)), while the AG and GG genotypes had higher performance in both traits compared with the AA genotypes, which could indicate the positive and additive effects of the G allele in this population. In addition, mean comparison analysis identified significant differences between the genotypes for AEW at 28 and 30 weeks of age (\(P {\le } 0.05\)), except that the overall effects of SNP on these traits were found to be non-significant. In recent studies, significant correlations have been found between the SNPs of IL-2 gene with BW at 7 days of age (\(P{<}0.01\)), BW at 40 days of age and food conversion ratio (\(P{<}0.05\)). In addition, investigation of the polymorphism in intron 2 of IL-2 gene in chickens has revealed 15 new haplotypes in 66 breeds of native chickens and commercial lines (Zhang et al. 2011). In a study, genetic polymorphism of IL-2 gene with egg production traits in a native turkey population was detected using the PCR-single-stranded conformation polymorphism (PCR-SSCP) method. Two alleles A (52.65) and B (47.35) and three genotypes AA, AB and BB were observed with the frequency of 13.83, 77.66 and 8.51, respectively. The allelic and genotypic frequencies had a similar pattern to the obtained results from the polymorphism of the IL-2 gene promoter in the Mazandaran native fowls population. These researchers found significant differences between the genotypes in terms of EN and weight of egg mass production, while the AA genotypes showed better performances than the other genotypes (\(P {\le } 0.01\)). However, no significant association was revealed between this polymorphism and the AEW trait (Erfaniasl et al. 2015).
IFN-\({\varvec{\upgamma }}\)
Here, we found two alleles A and G and three genotypes AA, AG and GG with different frequencies in the promoter region of IFN-\(\upgamma \) gene in the Mazandaran native hen population and significant difference was observed between the genotypes of IFN-\(\upgamma \) gene in AEW at 28 weeks of age trait and the effect of IL-2 gene on BW at 8 weeks of age trait was shown to be significant (\(P {\le } 0.05\)). The findings of recent research have indicated significant relationships between the polymorphisms in the promoter region of the IFN-\(\upgamma \) gene even among other species such as total milk yield, lactation length and daily milk yield in the Indian crossbreed cattle (\(P{<}0.05\)) (Prakash et al. 2010), and rectal temperature as well as resistance to heat stress in the Nigerian goat breeds (\(P{<}0.05\)) (Yakubu et al. 2016). But, in chickens, significant associations of the polymorphism in the promoter region of IFN-\(\upgamma \) gene were observed with food conversion ratio (\(P{<}0.01\)) in broiler chicken lines (Ye et al. 2006), EN and weight of egg mass production in the Iranian indigenous Turkey breeds (\(P {\le } 0.01\)) (Erfaniasl et al. 2015) and Salmonella enteritidis burden in the cecum and spleen of the Malaysian indigenous chickens (\(P{<}0.05\)) (Tohidi et al. 2012). Also, in previous research, the polymorphism analysis of the IFN-\(\upgamma \) gene has shown to be significantly associated with the initial antibody response to SRBC, Brucella abortus (BA) and BW at 12 weeks of age in two-layer lines (\(P{<}0.05\)) (Ahmed 2010), the log transformed number of Ascaridia galli in the brown-layer line (\(P{<}0.05\)) (Lühken et al. 2011), IFN-\(\upgamma \) protein expression after both primary and secondary immunizations in chickens (\(P{<}0.05\)) (Zhou et al. 2002) and the secondary humoral immune response to the HPAI vaccine in a red jungle fowl population (\(P{<}0.01\)) (Ji et al. 2015). By studying the SNP and expression of IFN-\(\upgamma \) gene and its role against Haemonchus contortus in two Indian indigenous sheep breeds, in addition to one SNP detected at exon 3 of the IFN-\(\upgamma \) gene in all resistant sheep groups, researchers also reported that the level of mRNA in the susceptible groups was significantly higher (\(P {\le } 0.05\)) in comparison with the resistant groups (Patra et al. 2016). In a previous survey, researchers analysed the polymorphism of the promoter regions of IFN-\(\upgamma \) and IL-2 candidate genes with the primary and secondary antibody responses to BA and SRBC antigens in Leghorn (G-B1) and Fayoumi (M15.2 and M5) chicken lines (Zhou et al. 2001). The fragments obtained through enzyme digestion, alleles and genotypes discovered for these two genes were identical to the alleles and genotypes observed in the Mazandaran native fowls population, namely two alleles of A and G and three genotypes of AA, AG and GG were demonstrated. The results of this researcher’s study showed that there was a significant relationship between the IFN-\(\upgamma \) gene promoter region polymorphism with primary and secondary antibody responses to both BA and SRBC antigens (Zhou et al. 2001). In another study, besides the confirmation of the results mentioned above related to the immune response to antibodies, there were also significant differences between genotypes in the M5.1 line for BW at 6 and 12 weeks of age traits, and in the M15.2 line for BW at 2 and 6 weeks of age traits, respectively (\(P{<}0.05\)) (Ahmed 2010).
In conclusion, the association between SNP in the IL-2 and IFN-\(\upgamma \) genes with some productive and reproductive traits in the Mazandaran native fowls population was investigated. Significant effects were detected from the IL-2 gene polymorphisms in the promoter region on BW at 8 weeks of age, EN at 345–375 days of age (almost achieved significance, \(P {\le } 0.058\)) and AEW at 345–375 days of age (\(P{<}0.01\)). Also, in the mean comparison analysis, in addition to the above-mentioned traits, there were significant differences between the genotypes of the IL-2 gene in AEW at 28 and 30 weeks of age traits, and for the IFN-\(\upgamma \) gene only in AEW at 28 weeks of age trait. At the IL-2 gene locus, besides their higher frequency, the AG genotypes had better productive performance than AA and GG genotypes, representing the fact that the AG genotypes could be considered as the competent parents for the next generation due to their higher indexes in EN and AEW traits. In fact, this higher performance of AG genotypes could be related to the association between their lower BW at 8 weeks of age and higher records in EN and AEW traits. Therefore, it can be concluded that the immune system genes were positively affective on some economically important productive traits in this native population. Consequently, the polymorphisms and associations found at these gene loci can be exploited as the pivotal genetic makers to improve Mazandaran native fowls breeding programs to achieve increased livability by increased resistance against pathogens as well as the optimum performance in reproductive and productive traits more efficiently.
References
Ahmed A. 2010 Associations of polymorphisms in four immune-related genes with antibody kinetics and body weight in chickens. Asian-Australas. J. Anim. Sci. 23, 1089–1095.
Alluwaimi A., Leutenegger C., Farver T., Rossitto P., Smith W. and Cullor J. 2003 The cytokine markers in Staphylococcus aureus mastitis of bovine mammary gland. J. Vet. Med. B Infect. Dis. Vet. Public Health 50, 105–111.
Barua A. and Yoshimura Y. 1999 Effects of aging and sex steroids on the localization of T cell subsets in the ovary of chicken, Gallus domesticus. Gen. Comp. Endocrinol. 114, 28–35.
Benyo D., Haibel G., Laufman H. P. and Pate J. L. 1991 Expression of major histocompatibility complex antigens on the bovine corpus luteum during the estrous cycle, luteolysis and early pregnancy. Biol. Reprod. 45, 229–234.
Burchill M. A., Yang J., Vang K. B. and Farrar M. A. 2007 Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 114, 1–8.
Cheema M., Qureshi M. and Havenstein G. 2003 A comparison of the immune response of a 2001 commercial broiler with a 1957 random bred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82, 1519–1529.
Choi K. D. and Lillehoj H. S. 2000 Role of chicken IL-2 on \(\upgamma \delta \) T-cells and Eimeria acervulina-induced changes in intestinal IL-2 mRNA expression and \(\upgamma \delta \) T-cells. Vet. Immunol. Immunopathol. 73, 309–321.
Clarkson R. W., Wayland M. T., Lee J., Freeman T. and Watson C. J. 2003 Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 6, R92.
Coussens P. M., Verman N., Coussens M. A., Elftman M. D. and McNulty A. M. 2004 Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 72, 1409–1422.
Dhama K., Saminathan M., Jacob S. S., Singh M., Karthik K., Tiwari R. et al. 2015 Effect of immunomodulation and immunomodulatory agents on health with some bioactive principles, modes of action and potent biomedical applications. Int. J. Pharmacol. 11, 253–290.
Dimitriadis E., White C., Jones R. and Salamonsen L. 2005 Cytokines, chemokines and growth factors in endometrium related to implantation. Hum. Reprod. Update 11, 613–630.
Donadoni F., Tizioto P., Meirelles S., Malago Junior W., Giglioti R., Ibelli A. et al. 2011 Identification of a SNP in the gene IL2 and its association with resistance against gastrointestinal infection by nematodes in goat. J. Anim. Sci. 89(e-suppl.); J. Dairy Sci. 94(e-suppl. 1), 280, 2011. Abstract T45. Abstracts of ADSA-ASAS Joint Annual Meeting, July 2011, New Orleans.
Erfaniasl Z., Hashemi A., Zarringhabaie G. E. and Farhadian M. 2015 Association of the interleukin-2 gene polymorphism with egg performance in a native Turkey population. Genetika 47, 417–424.
Felten S., Madden K., Bellinger D., Kruszewska B., Moynihan J. and Felten D. 1997 The role of the sympathetic nervous system in the modulation of immune responses. Adv. Pharmacol. 42, 583–587.
Goldsby R., Kindt T., Osborne B. and Kuby J. 2003 Immunology, 5th edition. Freeman, New York.
Hales D. B. 2000 Cytokines and testicular function. Cytokines Hum. Reprod. 17–42, ISBN 0-471-35242-X.
Heinrich J.-M., Bernheiden M., Minigo G., Yang K. K., Schütt C., Männel D. N. et al. 2001 The essential role of lipopolysaccharide-binding protein in protection of mice against a peritoneal Salmonella infection involves the rapid induction of an inflammatory response. J. Immunol. 167, 1624–1628.
Hellberg P., Thomsen P., Olof Janson P. and Brännström M. 1991 Leukocyte supplementation increases the luteinizing hormone-induced ovulation rate in the in vitro-perfused rat ovary. Biol. Reprod. 44, 791–797.
Hu H., Wang H., Yuan B., Zhou S., Li X., Huang Y. et al. 2006 Immunoenhancement of eukaryotic expression plasmids with chicken IL-2 or IL-15 genes on H5 subtype avian influenza vaccine. Vet. Sci. China 10, 805–810.
Janss L. and Bolder N. 2000 Heritabilities of and genetic relationships between salmonella resistance traits in broilers. J. Anim. Sci. 78, 2287–2291.
Ji B., Sun T., Ma Z., Lu Q., Hu W., Jian Z. et al. 2015 Possible association of IFN-g Gene -316A/G SNP with humoral immune response to killed H5N1 HPAI vaccine in a red jungle fowl population. J. Interferon Cytokine Res. 11, 844–849.
Kaiser P. and Mariani P. 1999 Promoter sequence, exon: intron structure, and synteny of genetic location show that a chicken cytokine with T-cell proliferative activity is IL2 and not IL15. Immunogenetics 49, 26–35.
Kaiser P., Wain H. M. and Rothwell L. 1998 Structure of the chicken interferon-\(\upgamma \) gene, and comparison to mammalian homologues. Gene 207, 25–32.
Kaiser P., Howell J., Fife M., Sadeyen J., Salmon N., Rothwell L. et al. 2008 Integrated immunogenomics in the chicken: deciphering the immune response to identify disease resistance genes. Dev. Biol. 132, 57–66.
Kayisli U. A., Mahutte N. G. and Arici A. 2002 Uterine chemokines in reproductive physiology and pathology. Am. J. Reprod. Immunol. 47, 213–221.
Knight P. G. and Glister C. 2003 Local roles of TGF-\(\upbeta \) superfamily members in the control of ovarian follicle development. Anim. Reprod. Sci. 78, 165–183.
Kogut M., Rothwell L. and Kaiser P. 2002 Differential effects of age on chicken heterophile functional activation by recombinant chicken interleukin-2. Dev. Comp. Immunol. 26, 817–830.
Kogut M. H., Rothwell L. and Kaiser P. 2003 Priming by recombinant chicken interleukin-2 induces selective expression of IL-8 and IL-18 mRNA in chicken heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enterica serovar enteritidis. Mol. Immunol. 40, 603–610.
Lambrecht B., Gonze M., Meulemans G. and van denBerg T. P. 2004 Assessment of the cell-mediated immune response in chickens by detection of chicken interferon-\(\upgamma \) in response to mitogen and recall Newcastle disease viral antigen stimulation. Avian Pathol. 33, 343–350.
Lamont S., Kaiser M. and Liu W. 2002 Candidate genes for resistance to Salmonella enteritidis colonization in chickens as detected in a novel genetic cross. Vet. Immunol. Immunopathol. 87, 423–428.
Li G., Lillehoj E. P. and Lillehoj H. S. 2002 Interleukin-2 production in SC and TK chickens infected with Eimeria tenella. Avian Dis. 46, 2–9.
Lühken G., Stamm I., Menge C. and Erhardt G. 2005 Functional analysis of a single nucleotide polymorphism in a potential binding site for GATA transcription factors in the ovine interleukin 2 gene. Vet. Immunol. Immunopathol. 107, 51–56.
Lühken G., Gauly M., Kaufmann F. and Erhardt G. 2011 Association study in naturally infected helminth layers shows evidence for influence of interferon-gamma gene variants on Ascaridia galli worm burden. Vet. Res. 42, 84.
Miyamoto T., Min W. and Lillehoj H. S. 2002 Kinetics of interleukin-2 production in chickens infected with Eimeria tenella. Comp. Immunol. Microbiol. Infect. Dis. 25, 149–158.
Nagarajan U. M., Sikes J., Prantner D., Andrews C. W., Frazer L., Goodwin A. et al. 2011 MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect. Immun. 79, 486–498.
Neuvians T., Schams D., Berisha B. and Pfaffl M. 2004 Involvement of pro-inflammatory cytokines, mediators of inflammation, and basic fibroblast growth factor in prostaglandin F2\(\upalpha \)-induced luteolysis in bovine corpus luteum. Biol. Reprod. 70, 473–480.
Nishimura R., Bowolaksono A., Acosta T. J., Murakami S., Piotrowska K., Skarzynski D. J. et al. 2004 Possible role of interleukin-1 in the regulation of bovine corpus luteum throughout the luteal phase. Biol. Reprod. 71, 1688–1693.
Parish C. R. and O’neill E. R. 1997 Dependence of the adaptive immune response on innate immunity: some questions answered but new paradoxes emerge. Immunol. Cell Biol. 75, 543–552.
Park J. H., Sung H. W., Yoon B. I. and Kwon H. M. 2009 Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-\(\upgamma \). J. Vet. Sci. 10, 131–139.
Pasanen S., Ylikomi T., Palojoki E., Syvälä H., Pelto-Huikko M. and Tuohimaa P. 1998 Progesterone receptor in chicken bursa of Fabricius and thymus: evidence for expression in B-lymphocytes. Mol. Cell. Endocrinol. 141, 119–128.
Patra G., Jas R., Ghosh J., Borthakur S. K. and Paul A. 2016 Single nucleotide polymorphism and expression studies of the interferon gamma gene and its role against Haemonchus contortus in Garole and Sahabadi sheep. Asian Pac. J. Trop. Dis. 6, 106–112.
Pintarič M., Gerner W. and Saalmüller A. 2008 Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-\(\upgamma \) production of porcine natural killer cells. Vet. Immunol. Immunopathol. 121, 68–82.
Prakash V., Bhattacharya T. K. and Pandey O. 2010 Genetic polymorphism study of promoter region of interleukin-2 gene and its association with certain milk associated traits in Indian crossbred cattle. J. Mol. Genet. 2, 15–19.
Rahman A., Snibson K. J., Lee C. S. and Meeusen E. N. 2004 Effects of implantation and early pregnancy on the expression of cytokines and vascular surface molecules in the sheep endometrium. J. Reprod. Immunol. 64, 45–58.
Reemers S. S., Van Haarlem D. A., Sijts A. J., Vervelde L. and Jansen C. A. 2012 Identification of novel avian influenza virus derived CD8+ T-cell epitopes. PLoS One 7, e31953.
Roussan D., Haddad R. and Khawaldeh G. 2008 Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 87, 444–448.
Savan R., Ravichandran S., Collins J. R., Sakai M. and Young H. A. 2009 Structural conservation of interferon gamma among vertebrates. Cytokine Growth Factor Rev. 20, 115–124.
Sebastiani G., Blais V., Sancho V., Vogel S. N., Stevenson M. M., Gros P. et al. 2002 Host immune response to Salmonella enterica serovar Typhimurium infection in mice derived from wild strains. Infect. Immun. 70, 1997–2009.
Shah M. A. A., Song X., Xu L., Yan R., Song H., Ruirui Z. et al. 2010 The DNA-induced protective immunity with chicken interferon gamma against poultry coccidiosis. Parasitol. Res. 107, 747–750.
Sharma R. K. and Gandhi E. 2011 Effect of interleukin-2 on steroidogenesis of goat ovary. J. Immunol. Immunopathol. 13, 20–24.
Singh S., Pei J. and Collisson E. 2014 Viral specific CD8 + T lymphocyte immunity provides protection against heterotypic stains of avian influenza viruses and avian coronaviruses. Int. Trends. Immun. 2, 36–46.
Soboloff J., Desilets M. and Tsang K. 1995 Influence of tumor necrosis factor alpha on intracellular \(\text{ Ca }^{2+}\) in hen granulosa cells in vitro during follicular development. Biol. Reprod. 53, 546–552.
Soller M. and Andersson L. 1998 Genomic approaches to the improvement of disease resistance in farm animals. Rev. Sci. Tech. 17, 329–345.
Sundaresan N., Anish D., Sastry K., Saxena V., Mohan J. and Ahmed K. 2007 Cytokines in reproductive remodeling of molting White Leghorn hens. J. Reprod. Immunol. 73, 39–50.
Swaggerty C. L., Kogut M. H., Ferro P. J., Rothwell L., Pevzner I. Y. and Kaiser P. 2004 Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and-susceptible chickens. Immunology 113, 139–148.
Tohidi R., Idris I. B., Panandam J. M. and Bejo M. H. 2012 The effects of polymorphisms in IL-2, IFN-\(\upgamma \), TGF-\(\upbeta \)2, IgL, TLR-4, MD-2, and iNOS genes on resistance to Salmonella Enteritidis in indigenous chickens. Avian Pathol. 41, 605–612.
Van der Poel J. and Parmentier H. 2003 Genetic bases for resistance and immunity to Avian Disease. S. l.: S. n. (Annual Report, contributing project to NE-1016) - 4 p, (https://www.nimss.org/projects/view/SAES/3474).
Vervelde L., Matthijs M., Van Haarlem D., de Wit J. and Jansen C. 2013 Rapid NK-cell activation in chicken after infection with infectious bronchitis virus M41. Vet. Immunol. Immunopathol. 151, 337–341.
Vetrivel S. and Chandrakumarmangalam S. 2013 The role of poultry industry in Indian Economy. Rev. Bras. Cienc. Avic. 15, 287–293.
Withanage G., Sasai K., Fukata T., Miyamoto T., Lillehoj H. and Baba E. 2003 Increased lymphocyte subpopulations and macrophages in the ovaries and oviducts of laying hens infected with Salmonella enterica serovar Enteritidis. Avian Pathol. 32, 583–590.
Withanage G., Kaiser P., Wigley P., Powers C., Mastroeni P., Brooks H. et al. 2004 Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72, 2152–2159.
Xu Q., Song X., Xu L., Yan R., Shah M. A. A. and Li X. 2008 Vaccination of chickens with a chimeric DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 induces protective immunity against coccidiosis. Vet. Parasitol. 156, 319–323.
Yakubu A., Salako A., Donato M., Takeet M., Peters S., Wheto M. et al. 2016 Interleukin-2 (IL-2) gene polymorphism and association with heat tolerance in Nigerian goats. Small Rumin. Res. 141, 127–134.
Ye X., Avendano S., Dekkers J. and Lamont S. 2006 Association of twelve immune-related genes with performance of three broiler lines in two different hygiene environments. Poult. Sci. 85, 1555–1569.
Zettergren L. D. and Cutlan R. T. 1992 Immunoglobulin-containing cells in chick embryo urogenital tissues: a new site for early B lineage cells in endothermic vertebrates. J. Exp. Zool. Part A 262, 458–461.
Zhang D., Zhao H., Luo Y. and Han J. 2011 Characterization of haplotype diversity defined by discontinuous insertions/deletions within the intron 2 of interleukin 2 in different domestic chicken populations. J. Biol. Sci. 11, 261–267.
Zhao K., He W., Gao W., Lu H., Han T., Li J. et al. 2011 Orf virus DNA vaccines expressing ORFV 011 and ORFV 059 chimeric protein enhances immunogenicity. J. Virol. 8, 562.
Zhou H., Buitenhuis A., Weigend S. and Lamont S. 2001 Candidate gene promoter polymorphisms and antibody response kinetics in chickens: interferon-\(\upgamma \), interleukin-2, and immunoglobulin light chain. Poult. Sci. 80, 1679–1689.
Zhou H., Lillehoj H. S. and Lamont S. J. 2002 Associations of interferon-\(\upgamma \) genotype and protein level with antibody response kinetics in chickens. Avian Dis. 46, 869–876.
Zhou H., Li H. and Lamont S. 2003 Genetic markers associated with antibody response kinetics in adult chickens. Poult. Sci. 82, 699–708.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Silvia Garagna
Rights and permissions
About this article
Cite this article
Kazemi, H., Najafi, M., Ghasemian, E. et al. Polymorphism detection of promoter region of IFN-\(\gamma \) and IL-2 genes and their association with productive traits in Mazandaran native breeder fowls. J Genet 97, 843–851 (2018). https://doi.org/10.1007/s12041-018-0981-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-018-0981-1