Abstract
A new water-soluble copper(II) complex bearing 4-bromobenzoate/2,2’-dipyridylamine ligands was successfully synthesized and characterized by using single crystal X-ray diffraction and spectroscopic techniques. The catalytic activity of the compound was investigated as a homogeneous catalyst in the oxidation of several alcohols (benzyl alcohol, cinnamyl alcohol, 1-phenylethanol, cyclohexanol, 1-heptanol) and alkenes (styrene, ethylbenzene, cyclohexene) in aqueous medium. The copper(II) catalyst was found to be active for the studied alcohols and alkenes. \(\hbox {H}_{2}\hbox {O}_{2}\) was used as an active oxidant for alcohol oxidation, while t-BuOOH (TBHP) was used for alkenes. The compound exhibited high selectivity toward benzaldehyde (88%) in cinnamyl alcohol oxidation under mild conditions (70 \({^{\circ }}\hbox {C}\)) after 4 h. Particularly, remarkable results were obtained for the oxidation of styrene and cyclohexene; transformation via allylic oxidation to 2-cyclohexene-1-one as one product in 2 h \((\hbox {TOF} = 50\,\hbox {h}^{-1})\) and benzaldehyde in 1 h (100% conversion, TOF = 86 \(\hbox {h}^{-1}\), 100% selectivity). The Cu(II)/TBHP (or \(\hbox {H}_{2}\hbox {O}_{2})/\hbox {H}_{2}\hbox {O}\) system proved to be an alternative for catalytic oxidations in the green chemistry concept.
Graphical Abstract
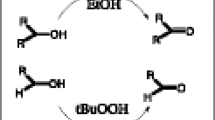
New Copper(II)-complex bearing 4-bromobenzoate/2,2’-dipyridylamine ligands was synthesized and characterized by single crystal diffraction technique. The complex exhibited good catalytic oxidation activity on alcohols and alkenes in water, a green solvent.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chemical reaction in green solvent systems is one of the major investigation areas of green synthesis. The number of published environmentally friendly catalytic systems in the literature is increasing over time. There are many examples of catalytic reactions performed in supercritical fluids, [1] ionic liquids, [2] fluorous solvents, [3] or solvent-free systems.[4, 5] Reactions especially applied in water solvent systems are extremely important because they have many advantages, such as non-toxicity, absence of hazards, low wastage in production, economical method, and so on. Water has been used as a solvent in many types of transition metal-catalyzed reactions, i.e., carbonylation, [6] arylation, [7] hydroformylation, [8] and hydrogenation. [9] In synthetic chemical production processes, such as those in the dye, pigment, medicine, and perfume industries, selective alcohol and alkene oxidations are important reactions for obtaining numerous starting or intermediate chemicals, aldehydes, ketones, diols, and carboxylic acids. Expensive transition metal catalysts, i.e., as palladium, [10] platinum, [11] and ruthenium [12] have been reported to be efficient catalysts for alcohol and alkene oxidations in water. Compared to the aforementioned high-cost metal compounds, relatively low-cost metal catalysts, such as copper, [13] iron, [14] and manganese [15] compounds are a lot scarce for homogeneous catalytic oxidations in water. In this respect, the catalytic oxidation of alcohol and alkenes in water to the corresponding products has always attracted interest in the past decade.[13, 16,17,18,19,20,21,22]
Many copper complexes have been found to catalyze for peroxidative oxidation in homogeneous and heterogeneous catalytic systems.[23,24,25,26,27] Some Cu–Schiff base complexes in a heterogeneous system can lead to a higher yield (\(\sim \)90%, TOF = 18.2 \(\hbox {h}^{-1})\) of products in cyclohexene oxidation. [28] Mizar et al., published a study of an effective catalyst for benzyl alcohol for benzaldehyde transformation with copper(II)-2-N-arylpyrrolecarbaldimine catalysts under atmospheric oxygen pressure at 80 \({^{\circ }}\hbox {C}\) within 2 h in water (yield : 68–99%). [29] Hu et al., synthesized and used some copper complexes with tetradentate pyridyl-imine terminated Schiff base ligands to investigate their aerobic alcohol oxidation activities in aqueous media. [30]
To the best of our knowledge, water-soluble carboxylate-Cu(II)-catalyzed reactions of alkenes to ketones and also the oxidation of alcohols in water are very scarce. Recently, we published a successful application of a new copper(II)/triphenylacetic acid/bipyridyl complex for oxidation of primary and secondary alcohols with \(\hbox {H}_{2}\hbox {O}_{2}\) under mild conditions in pure water. [31] Water-soluble copper(II) complexes with sulfonic-functionalized arylhydrazone of \(\upbeta \)-diketone are used as catalyst precursors for the selective peroxidative (with TBHP) allylic oxidation of cyclohexene to cyclohex-2-enol (Cy-ol) and cyclohex-2-enone (Cy-one) with a total yield of \(\sim \)70% and TONs of up to 350, [29] but that is lower than those (>90%) we observed in our Cu(II)-complex. Copper(II) catalytic systems with mixed-ligand aminoalcohol-dicarboxylate coordination polymers act as homogeneous catalysts in aqueous acetonitrile under mild conditions (50–60 \({^{\circ }}\hbox {C}\)): the oxidation of cyclohexene through \(\hbox {H}_{2}\hbox {O}_{2}\) and in the presence of a trifluoroacetic acid promoter results in total product yields (\(\sim \)15–18% based on the substrate).[32,33,34,35,36] Cu-MOF, [Cu(bpy)\((\hbox {H}_{2}\hbox {O})_{2}(\hbox {BF}_{4})_{2}\)(bpy)] (bpy: 4,4’-bipyridine), exhibited catalytic activity and high selectivity in the allylic oxidation of cyclohexene with molecular oxygen under solvent-free conditions (TON = 13–37). [25] However, these catalysts show lower selectivity than our systems. Therefore, the title Cu(II)-complex is the best catalyst precursor for selective allylic cyclohexene oxidation, which results in the formation of ketone as a single product.
Herein, we present a new water-soluble copper(II)/4-bromobenzoate/2,2’-dipyridylamine complex ([Cu(OOC(\(\hbox {C}_{6}\hbox {H}_{5})\hbox {Br})(\hbox {C}_{10}\hbox {H}_{9}\hbox {N}_{3})](\hbox {ClO}_{4}))\) and its successful application for selective oxidation of alcohols and alkenes under mild reaction medium (70 \({^{\circ }}\hbox {C}\)) with \(\hbox {H}_{2}\hbox {O}_{2}\) or t-BuOOH in green solvent water.
2 Experimental
2.1 Materials and Physical measurements
All chemicals were obtained from commercial companies (\(\ge \) 98%, Sigma-Aldrich and Merck, Germany) and used without further purification. IR spectra were recorded with Jasco FT/IR-300 E Spectrophotometer using KBr pellets between 4000 and 400 \(\hbox {cm}^{-1}\). UV spectra were recorded using a Shimadzu UV-2450 spectrophotometer. Crystallographic data were collected with Bruker APEX II CCD using Mo-Ka radiation at room temperature and corrected for absorption with SADABS. A complex structure solution was performed using direct methods. Non-hydrogen atoms were refined anisotropically using full-matrix least squares on F2.
2.2 Synthesis of complex, \(([\hbox {Cu}(\hbox {OOC}(\hbox {C}_{6}\hbox {H}_{5})\hbox {Br})(\hbox {C}_{10}\hbox {H}_{9}\hbox {N}_{3})](\hbox {ClO}_{4})), \)([aqua(4-bromobenzoato)(2,2’dipyridylamine)copper(II)](perchlorate))
Neutralized solution of 4-bromobenzoic acid (100 mg, 0.49 mmol, 10 mL methanol) with sodium hydroxide (19.5 mg, 0.49 mmol) was added dropwise to the methanolic solution of copper(II) perchlorate hexahydrate (10 mL, 181 mg, 0.49 mmol) at 50 \({^{\circ }}\hbox {C}\) for 2 h. Then, 2,2’-dipyridylamine solution in methanol (84 mg, 0.49 mmol, 5 mL) was reacted overnight with a metal salt-bromobenzoate solution to obtain a copper(II) complex. Dark-blue crystals were obtained from the evaporation of the solvent after 7 days (180 mg, yield 69%, M.p.: 250 \({^{\circ }}\hbox {C}\)) FT-IR (KBr; \(\upnu \), \(\hbox {cm}^{-1})\) (s. strong; m. medium; w. weak): 3416w \(\upnu _{\mathrm{N-H}}\); 1590m \(\upnu _{\mathrm{C=N}}\); 1644m \(\upnu _{\mathrm{COOasym}}\); 1484m \(\upnu _{\mathrm{COOsym}}\); 1530m \(\upnu _{\mathrm{C=N-C=C}}\); 1114m \(\upnu _{\mathrm{O-H}}\); 1088s \(\upnu _{\mathrm{C-O}}\); 769m \(\upnu _{\mathrm{C-N}}\); 625m \(\upnu _{\mathrm{Cu-O-Cu}}\) UV–Vis \(\uplambda _{\mathrm{max}}\) nm (\(\hbox {CH}_{3}\hbox {CN}\)): 244. 295. 312. Magnetic moment (\(\upmu )\) = 1.4 B.M. at room temperature.
2.3 X-ray crystallography
Diffraction data for the complex collected with Bruker AXS APEX CCD diffractometer equipped with a rotation anode at 296 (2) K, respectively using graphite monochromated Mo K\(\alpha \) radiation at \(\lambda =0.71073\) Å. The data reduction was performed with the Bruker SMART program package. [37] The structures were solved by direct methods and the non-hydrogen atoms were located through subsequent difference Fourier syntheses. [38] Structure solution was found with the SHELXS-97 package using the direct methods and were refined SHELXL-97 [39] against \(\hbox {F}^{2}\) using first isotropic and later anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms were added to the structure model at calculated positions. The molecular drawing was obtained using MERCURY. [40] Geometric calculations were performed with PLATON. [41]
2.4 General procedure for alcohol/alkene oxidation
Alcohol or alkene (\(9.3\,\times \,10^{-4}\) mol) (substrate/catalyst ratio = 100) copper catalyst (9.3 \(\times \) 10\(^{-6}\) mol) and oxidant \(\hbox {H}_{2}\hbox {O}_{2}\) (2 mL, \(1.92\,\times \,10^{-4}\) mol) or t-BuOOH (2 mL, \(1.4\,\times \,10^{-2}\) mol) were mixed in 10 mL water in a 50 mL reaction flask with a condenser at 70 \({^{\circ }}\hbox {C}\) for 24 h. Internal samples were taken from reaction media after certain time intervals for monitoring the substrate/product(s) percentage on a GC with an HP-5 quartz capillary column (30 m \(\times \) 0.32 mm \(\times \) 0.25 \(\upmu \)m) and a flame ionization detector (FID). An unknown peak definition was made by analyzing real GC standards.
3 Results and Discussion
3.1 Structural analysis of the complex 1
The copper(II)-complex, \(([\hbox {Cu}(\hbox {OOC}(\hbox {C}_{6}\hbox {H}_{5})\hbox {Br})(\hbox {C}_{10}\hbox {H}_{9}\hbox {N}_{3})](\hbox {ClO}_{4}))\), with 4-bromobenzoate (bba) and 2,2’-dipyridylamine (dpya) ligands, was successfully synthesized via the stoichiometric reaction of reactants in ethanolic solution. Dark-blue crystals were obtained through solvent evaporation within one week. The crystals of the compound exhibited monoclinic, P21/c and symmetry. The molecular structure of the compound 1 is presented in Figure 1. Crystallographic data, bond distances, and angles relevant to the metal coordination sphere of the compound 1 are listed in Tables 1–2.
Copper(II) has a disordered square planar geometry and is coordinated with two nitrogen atoms (Cu–N = 1.928 (4) and 1.938 (4) Å) from a chelating dpya ligand and two oxygen atoms (Cu–O = 1.976 (3) and 2.017 (3) Å) from the carboxylate group of the dpya ligand in \({\mu }^{2}\) mode. The +2 charge on the copper ion is balanced with a coordinated deprotonated dpya and perchlorate anion as counter ion. The geometry is far removed from the ideal square plane, principally because of the “bite” of dpya (N1-Cu1-N2 = 95.34 (16)degr) and bba (O1-Cu1-O2 = 65.19(14)degr). Large deviations were observed from 180degr for the N1-Cu1-O2 (165.29 (15)degr) and N2-Cu1-O1 angle (161.48 (15)degr). The chelate ring adopts an approximate plane conformation, which the observed torsion angles; C5N3N1C6 and Cu1O2C11O1 are –1.0(8)degr and 1.2(4)degr, respectively.
Weak intermolecular hydrogen bonding interactions are observed between the perchlorate ion and dpya. Two mononuclear units are linked by two N-H \(^{. . .}\) O hydrogen bonds between the nitrogen atom of the coordinated dpya and the oxygen atoms of the lattice perchlorate ion, with an N3 \(^{. . .}\) O6 distance of 2.898 (6) Å (N3 –H6 \(^{. . .}\) O6). In addition, the \(\pi ^{\ldots }\pi \) interactions between the two parallel rings of the dpya and bba ligands (R1=C12C13C14C15C16C17, R2=N1C1C2C3C4, and R3=N2C6C7C8C9C10), R1 \(^{. . .}\) R2 = 4.529 Å and R1 \(^{. . .}\) R3 = 4.175 Å, form a chain along a direction (Figure 2).
3.2 Spectroscopic study
The X-ray structure of the complex is consistent with the infrared spectra. The band observed between 3042 and 3094 \(\hbox {cm}^{-1}\) is attributed to the aromatic \(\upnu (\hbox {C-H})\) vibration of two coordinated ligands. Carboxylate group asymmetric and symmetric vibration peaks were observed at 1644 and 1484 \(\hbox {cm}^{-1}\), respectively. The neutral ligand azomethine \(\upnu (\hbox {C=N})\) vibration peak difference between the coordinated and the uncoordinated form is found to be about 60 \(\hbox {cm}^{-1}\), indicating that metal center and nitrogen atom coordination occurs. Two bands encountered at 625 and 769 \(\hbox {cm}^{-1}\) are related to the nitrogen atoms of dipyridylamine and metal center coordination.
The UV-Vis spectrum of the complex shows three absorption bands at 244, 295 and 312 nm in acetonitrile solution. The lower wavelength band is dedicated to anionic carboxylate group \(\uppi \rightarrow \uppi \)* transitions. The other two absorption bands are related to the \(\hbox {n}\rightarrow \uppi \)* transitions of dpya ligand. Additionally, \(\hbox {d}\rightarrow \hbox {d}\) transitions were not observed.
Based on the magnetic measurements of the solid Cu(II) complex at room temperature, the magnetic moment value is 1.4 B.M., and it is consistent with one unpaired electron system.
3.3 Catalytic experiments
The catalytic oxidation reactions of primary/secondary alcohol and alkene were performed in a 50 mL round-bottom flask with a temperature-controlled magnetic stirrer at 70 \({^{\circ }}\hbox {C}\). The experiments were carried out in water and \(\hbox {H}_{2}\hbox {O}_{2}\) or TBHP as the oxygen source. Comparison of catalytic results was presented in Tables 3–4 and Figures 3–4. A blank experiment showed that the uncatalyzed oxidation of the studied alcohols and alkenes were negligible under the applied conditions.
The results indicated that a synthesized compound 1 has a catalytic effect on both the studied alcohol and alkene oxidations in water. The activity of the \(\hbox {Cu(II)}/\hbox {H}_{2}\hbox {O}_{2}/\hbox {H}_{2}\hbox {O}\) catalytic system is found to be higher in aromatic alcohols than in aliphatic ones. In a 6 h reaction time, the total % conversions of 90, 86, 71, 29, and 13 were obtained for cinnamyl alcohol, 1-phenylethanol, benzyl alcohol, cyclohexanol, and 1-heptanol, respectively. Benzaldehyde was detected as a major product of both benzyl alcohol and cinnamyl alcohol oxidations (Table 3, Entries-2, 4, 1, 3, 5).
In benzyl alcohol oxidation, the substrate selectively converted to one product, benzaldehyde, with 71% formation in 6 h reaction time (Entry 1). Additionally, formation of the over oxidation product, benzoic acid, was not observed even when the reaction was prolonged to 24 h. In the oxidation of secondary alcohol phenyl ethanol, a TOF value of 1200 was reached at the first minute of the catalytic oxidation with 20% acetophenone formation. After a 6 h reaction time, acetophenone was the only product observed at 86% (Entry 4). The highest TOF value was obtained in allylic-type alcohol, cinnamyl alcohol oxidation (2100 \(\hbox {h}^{-1}\) at the first minute of the reaction with 35% total conversion). After a 4 h reaction time, conversion increased to 84%, and the aldehyde selectivity was found to be 88% as benzaldehyde (Entry 2). Cinnamaldehyde is a minor product with a selectivity value of 12% after 4 h. The result that needs to be emphasized is cinnamaldehyde carboxylation, which is converted to benzaldehyde through the carboxylation process as the reaction proceeds. As a cyclic alcohol, cyclohexanol was poorly oxidized to the corresponding ketone, and 29% cyclohexanone formation was obtained at 6 h reaction time (Entry 3). The lowest conversion rate was observed in 1-heptanol oxidation as the primary aliphatic alcohol (13% total conversion, Table 3, Entry 5). Generally, aliphatic alcohols are less reactive than aromatic alcohols, and these findings are in agreement with published results.[42, 43]
In alkene oxidation reactions, hydrogen peroxide has been found to be an ineffective oxygen source. That is why TBHP was used as oxidant. The Cu(II)/TBHP/\(\hbox {H}_{2}\hbox {O}\) catalytic system shows good activities of styrene, ethylbenzene, and cyclohexene oxidation with reasonably good selectivity (Table 4) at 70 \({^{\circ }}\hbox {C}\). In styrene oxidation, acetophenone (2%) and benzaldehyde (85%, selectivity 98%) were observed in the initial period of reaction with a TON value of 87. After 4 h of reaction time, complete conversion of styrene to benzaldehyde was observed as the sole product. When the reaction continued, the over-oxidation product, benzoic acid, was obtained (77% after 24 h) (Entry 1). In ethylbenzene oxidation, acetophenone was obtained as a major product and changed from 62% (TOF = 64.4) to 97% (TOF = 17 \(\hbox {h}^{-1}\).) within a 1–6 h reaction time (overall selectivity \(\sim \)97%) (Entry 2). Among the studied alkenes, cyclohexene oxidation is the fastest catalytic reaction (in the first 15 min of the reaction time, 71% yield and TOF = 280 \(\hbox {h}^{-1}\)). In cyclohexene, the oxidation of the carbon-carbon double bond does not convert to cyclohexene oxide (epoxide) with TBHP, whereas the oxidation of the allylic C-H bond results in 2-cyclohexene-1-ol, which is further oxidized to 2-cyclohexene-1-one as one product within a reaction time of 2 h (TOF = 50 \(\hbox {h}^{-1}\)) (Table 2, Entry 3).
The adopted Cu(II)/TBHP(or \(\hbox {H}_{2}\hbox {O}_{2})/\hbox {H}_{2}\hbox {O}\) system was simpler, greener, and more efficient for alcohol and especially alkene oxidation under mild reaction conditions. A clean oxidant \(\hbox {H}_{2}\hbox {O}_{2}\) was used since it produces water, and more importantly, using water as a solvent is an important feature of a green chemical reaction.
It should be emphasized that such level of yields is extremely good in the field of alkene (particularly cyclohexene) oxidation, especially for the inertness of the saturated hydrocarbon and mild reaction conditions.[44, 45] The obtained products and yields are also comparable with or even superior to those achieved using other related copper catalysts bearing carboxylate and N-based ligands.[32,33,34,35,36]
4 Conclusions
We have developed a new water-soluble copper(II)-complex (1) as a catalyst precursor bearing 4-bromobenzoate and neutral 2,2’-dipyridylamine ligands. The compound has been fully characterized using X-ray and other spectroscopic techniques. It is found that compound is an active catalyst for the peroxidative oxidation of alkenes and alcohols with \(\hbox {H}_{2}\hbox {O}_{2 }\) and/or TBHP under mild conditions without any promoter in water, a green solvent system. In particular, high conversions for alkene oxidation, and an excellent selectivity for 2-cyclohexe-1-one for cyclohexene oxidation in water were obtained. Using a low-cost catalyst, TBHP/\(\hbox {H}_{2}\hbox {O}_{2}\), and water as solvent made our catalytic system attractive and environmentally sustainable.
References
Jessop P G 1999 Homogenous catalysis in supercritical fluids Chem. Rev. 99 475
Wassercheid P and Keim W 2000 Ionic Liquids-New “Solutions” for Transition Metal Catalysis Angew Chem. Int. Edit. 39 3772
Wolf E and Koten G 1999 Florous phase separation techniques in catalysis Chem. Soc. Rev. 28 37
Azemi T F, Kondaveti L and Bisht K S 2002 Solventless Enantioelective Ring-Opening Polymerization of Substituted \(\varepsilon \)-Caprolactones by Enzymatic Catalysis Macromolecules 35 3380
Ramin M, Jutz F Grunwaldt J D and Baiker A 2005 Solventless synthesis of propylene carbonate catalysed by chromium–salen complexes: Bridging homogeneous and heterogeneous catalysis J. Mol. Catal. A 242 24232
Suzuka T, Sueyoshi H and Ogihara K 2017 Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN\(_{3}\) as Ammonia Equivalent Catalysts 7 107
Sindhu K S, Ujwaldev S M, Krishana K K and Anilkumar G 2017 A green approach for arylation of phenols using iron catalysis in water under aerobic conditions J. Catal. 348 146
Sharma S K and Jashra R V 2015 Aqueous phase catalytic hydroformylation reactions of alkenes Catal. Today 247 70
Shabbir S, Lee S, Lim M, Lee H, Ko H, Lee Y and Rhee H 2017 Pd nanoparticles on reverse phase silica gel as recyclable catalyst for Suzuki-Miyaura cross coupling reaction and hydrogenation in water J. Organomet. Chem. 846 296
Anna M M D, Mali M, Mastrolilli P, Cotugno P and Monopoli A 2014 Oxidation of benzyl alcohols to aldehydes and ketones under air in water using a polymer supported palladium catalyst J. Mol. Catal. A 386 114
Ng Y H, Ikeda S, Harada T, Morita Y and Matsumura M 2008 An efficient and reusable carbon-supported platinum catalyst for aerobic oxidation of alcohols in water Chem. Commun. 3181
Aliende C, Manrique M P, Jalon F A, Manzano B R, Rodriguez A M and Espino G 2012 Arene Ruthenium Complexes as Versatile Catalysts in Water in both Transfer Hydrogenation of Ketones and Oxidation of Alcohols. Selective Deuterium Labeling of rac-1-Phenylethanol Organometallics 31 6106
Wu J, Liu Y, Ma X, Liu P, Gu C and Dai B 2016 Highly selective copper-catalyzed oxidation of benzyl alcohols to aromatic aldehydes in water at room temperature Appl. Organomet. Chem. 30 577
Monfared H H, Nather C, Winkler H and Janiak C 2012 Highly selective and “green” alcohol oxidations in water using aqueous 10% \(\text{ H }_{2}\text{ O }_{2}\) and iron-benzenetricarboxylate metal–organic gel Inorg. Chim. Acta 391 75
Chen G, Chen L, Ma L, Kwong H K and Lau T C 2016 Photocatalytic oxidation of alkenes and alcohols in water by a manganese(V) nitrido complex Chem. Commun. 52 9271
Figiel P J, Sibaouih A, Ahmad J U, Nieger M, Raisanen M T, Leskela M and Repo T 2009 Aerobic Oxidation of Benzylic Alcohols in Water by 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)/Copper(II) 2-N-Arylpyrrolecarbaldimino Complexes Adv. Synth. Catal. 351 2625
Kopylovich M N, Mahmudov K T, Haukka M, Figiel P J, Mizar A, da Silva A L and Pombeiro A J L 2011 Water-Soluble Cobalt(II) and Copper(II) Complexes of 3-(5-Chloro-2-hydroxy- 3-sulfophenylhydrazo)pentane-2,4-dione as Building Blocks for 3D Supramolecular Networks and Catalysts for TEMPO-Mediated Aerobic Oxidation of Benzylic Alcohols Eur. J. Inorg. Chem. 4175
Zhang G F, Han X W, Luan Y X, Wang Y, Wen X, Xu L, Ding C G and Gao J R 2013 Copper-catalyzed aerobic alcohol oxidation under air in neat water by using a water-soluble ligand RSC Adv. 3 19255
Xie J B, Bao J J, Li H X, Tan D W, Li H Y and Lang J P 2014 An efficient approach to the ammoxidation of alcohols to nitriles and the aerobic oxidation of alcohols to aldehydes in water using Cu(II)/pypzacac complexes as catalysts RSC Adv. 4 54007
Bullock R M 2007 Ein Eisenkatalysator zur Hydrierung von Ketonen unter milden Bedingungen Angew. Chem. 119 7504
Gamez P, Arends I W C E and Sheldon R A 2004 Room Temperature Aerobic Copper-Catalysed Selective Oxidation of Primary Alcohols to Aldehydes Adv. Synth. Catal. 346 805
Uber J S, Vogels Y, Van den Helder D, Mutikainen I, Turpeinen U, Fu W T, Roubeau O, Gamez P and Reedijk J 2007 Pyrazole-Based Ligands for the [Copper–TEMPO]-Mediated Oxidation of Benzyl Alcohol to Benzaldehyde and Structures of the Cu Coordination Compounds Eur. J. Inorg. Chem. 26 4197
Meder M B and Gade L H 2004 Coordination Chemistry of 1,3-Bis(2-pyridylimino)- and 1,3-Bis- (2-thiazolylimino)isoindole Copper Complexes: Investigation of Their Catalytic Behavior in Oxidation Reactions Eur. J. Inorg. Chem. 2716
Murphy E F, Mallat T and Baiker A 2000 Allylic oxofunctionalization of cyclic olefins with homogeneous and heterogeneous catalysts Catal. Today 57 115
Jiang D, Mallat T, Meier D M, Urakawa A and Baiker A 2010 Copper metal–organic framework: Structure and activity in the allylic oxidation of cyclohexene with molecular oxygen J. Catal. 270 26
Dutta B, Bera R and Koner S 2007 Anchoring of Copper Complex in MCM-41 Matrix: A Highly Efficient Catalyst for Epoxidation of Olefins by tert-BuOOH Langmuir 23 2492
Dutta B, Jana S, Bera R, Saha P K and Koner S 2007 Immobilization of copper Schiff base complexes in zeolite matrix: Preparation, characterization and catalytic study Appl. Catal. A 318 89
Mukherjee S, Samanta S, Roy B C and Bhaumik A 2006 Efficient allylic oxidation of cyclohexene catalyzed by immobilized Schiff base complex using peroxides as oxidants Appl. Catal. A 301 79
Mizar A, Fátima M, Silva C G, Kopylovich M N, Mukherjee S, Mahmudov K T and Pombeiro A J L 2012 Water-Soluble Copper(II) Complexes with a Sulfonic-Functionalized Arylhydrazone of \(\upbeta \)-Diketone and Their Application in Peroxidative Allylic Oxidation of Cyclohexene Eur. J. Inorg. Chem. 2305
Hu Z and Kerton F M 2012 Room temperature aerobic oxidation of alcohols using CuBr2 with TEMPO and a tetradentate polymer based pyridyl-imine ligand Appl. Catal. A 413 332
Ünver H and Kani I 2017 Homogeneous oxidation of alcohols in water catalyzed with Cu(II)- triphenyl acetate/bipyridyl complex Polyhedron 134 257
Kirillova M V, Paiva P T, Carvalho W A, Mandelli D and Kirillov A M 2017 Mixed-ligand aminoalcohol-dicarboxylate copper(II) coordination polymers as catalysts for the oxidative functionalization of cyclic alkanes and alkenes Pure Appl. Chem. 89 61
Shul’pin G B 2016 New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review Catalysts 6 50
Kirillov A M, Kirillova M V and Pombeiro A J L 2012 Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes Coord. Chem. Rev. 256 2741
Dias S P, Kirillova M V, André V, Kłak J and Kirillov A M 2015 New tricopper(II) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes Inorg. Chem. Front. 525
Fernandes T A, André V, Kirillov A M and Kirillova M V 2017 Mild homogeneous oxidation and hydrocarboxylation of cycloalkanes catalyzed by novel dicopper(II) aminoalcohol-driven cores J. Mol. Catal. A Chem. 426 357
SMART, Bruker AXS 2000
Sheldrick G M 2008 A Short history of SHELX Acta Crystallogr. Sect. A 64 112
Sheldrick G M 1997 SHELXS-97, Program for crystal structure solution. University of Gottingen
Macrae C F, Edgington P R, McCabe P, Pidcock E, Shields G P, Taylor R, Towler M and Streek J 2006 Mercury: visualization and analysis of crystal structures J. Appl. Crystallogr. 39 453
Spek AL PLATON - A Multipurpose Crystallographic Tool Utrecht Utrecht University The Netherlands 2005
Samantha S, Das S, Samantha P K, Dutta S and Biswas P 2013 A mononuclear copper(II) complex immobilized in mesoporous silica: an efficient heterogeneous catalyst for the aerobic oxidation of benzylic alcohols RSC Adv. 3 19455
Conejo M M, Avila P, Alvarez E and Galindo A 2017 Synthesis and structure of nickel and copper complexes containing the N-allyl-o-hydroxyacetophenoniminato ligand and the application of copper complex as catalyst for aerobic alcohol oxidations Inorg. Chim. Acta 455 638
Banu A P, Sugisaki C, Gharsa T, Marty J D, Gascon I, Krimer M, Pozzi G, Desbat B, Quici S, Lattes I R and Mingotaud C 2005 monolayers of salen derivatives as catalytic planes for alkene oxidation in water Chem. Eur. J. 11 6032
Basle O and Li C J 2007 Copper catalyzed oxidative alkylation of \(\text{ sp }^{3}\) C–H bond adjacent to a nitrogen atom using molecular oxygen in water Green Chem. 9 1047
Acknowledgements
Authors thankfully acknowledge the Medical Plants and Medicine Research Centre of Anadolu University, Eskişehir, Turkey for using X-ray Diffractometer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ünver, H., Kani, I. Homogeneous oxidation of alcohol and alkene with copper (II) complex in water. J Chem Sci 130, 33 (2018). https://doi.org/10.1007/s12039-018-1439-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1439-y