Abstract
A water-soluble dinuclear Cu(II) complex, [Cu2(OOCC6H4Br)(OCH3)(C10H8N2)2(ClO4)2] (4-bromobenzoic acid = HOOCC6H4Br; 2-2′-bipyridyl = C10H8N2) was synthesized and characterized by X-ray crystallography. The complex was investigated as a selective catalyst for the mild oxidation of primary and secondary alcohols in the presence of hydrogen peroxide as oxidant at 70 ℃ in water. The complex proved to be an active catalyst for production of the corresponding aldehydes or ketones. Thus, cinnamyl alcohol, benzyl alcohol, 1-phenyl ethanol and 2-butanol were quantitatively oxidized in 6 h, using a low catalyst loading (1 mol%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxidation of alcohols is the key synthetic routes to obtain their aldehydes, ketones or carboxylic acids [1, 2]. To date, many catalytic systems have been investigated for these reactions, particularly with a view to the discovery of environmentally friendly chemical processes. Conventional oxidation reactions are conducted with oxidants based on heavy metals, such as RuO4, SeO2, (C5H5NH)2(Cr2O7), KMnO4, and CrO3, with associated risks of toxic waste and environmental damage [3,4,5]. To overcome these drawbacks, catalytic oxidations with hydrogen peroxide, molecular oxygen or tert-butyl hydroperoxide (t-BuOOH) are attractive in terms of both environmental and economic sustainability. In terms of solvent choice, organic solvents have obvious disadvantages compared to water, which is the ideal for green chemistry. Nevertheless, examples of catalytic alcohol oxidations in water are rare [6, 7].
Many different transition metal complexes have been used for catalytic alcohol oxidations in organic solvents, using basic auxiliary substances such as K2CO3, TEMPO, and DMAP or co-solvents [8,9,10,11,12,13,14,15]. In contrast, reports of catalytic alcohol oxidations conducted in water without any additives are rare [16, 17]. There are several reports of water-soluble complexes and their application for catalytic oxidation of alcohols in water [18,19,20,21,22]. However, the use of expensive and rare earth metals and their complexes including Pd, Ru, Rh, etc. is associated with economical concerns. Copper complexes are used effectively, especially in alcohol oxidation reactions. However, these frequently require the use of organic solvents, higher reaction temperatures, co-additives or toxic oxidants [23,24,25,26,27]. In the last few years, our research group has been interested in catalytic alcohol oxidations conducted in water using hydrogen peroxide as an oxygen source under mild conditions. In this context, we recently reported on a water-soluble copper(II) complex including a carboxylate ligand (triphenyl acetate) plus a neutral ligand (2,2′-bipyridyl). This complex was successfully used to oxidize primary and secondary alcohols to their carbonyl products using hydrogen peroxide [16]. We have also descried a water-soluble copper(II) complex of 2,2′-dipyridylamine/4-bromobenzoate ligands [17]. The catalytic activity of this complex was investigated for the oxidation of alcohols and alkenes with t-BuOOH or H2O2 in water. It was found to be especially active for the oxidation of styrene and cyclohexene with good selectivities (up to 100%).
Following on from these findings, in this paper we report on the synthesis and characterization of a new water-soluble dinuclear copper(II) complex, [Cu2(OOCC6H4Br)(OCH3)(C10H8N2)2(ClO4)2], and an investigation of its catalytic activity for the oxidation of primary and secondary alcohols in water without any additives under mild conditions.
Experimental
Materials and methods
All reagents were commercially obtained (Sigma-Aldrich) and used as received. The crystallographic data were collected with a Bruker AXS diffractometer. FTIR spectra were recorded with a Perkin Elmer Spectrum 100 Spectrometer using KBr discs in the range of 400–4000 cm−1. UV–Vis spectra were recorded on a Shimadzu UV-2450 spectrophotometer between 200 and 900 nm. Elemental analyses were obtained with an Elementar Vario EL III microanalyzer instrument. Magnetic susceptibility was measured with a Sherwood Scientific MX-I balance.
Synthesis of the complex
A solution of 4-bromobenzoic acid (105 mg, 0.52 mmol) in methanol (10 mL, neutralized with 1.04 mL of 0.5 M NaOH) was added dropwise to a solution of copper(II) perchlorate hexahydrate (192.6 mg, 0.52 mmol) in methanol (10 mL). After stirring for 30 min, a solution of 2,2′-bipyridine (81.2 mg, 0.52 mmol) in methanol (5 mL) was added. The mixture was then refluxed for 12 h. The solution was then filtered. Blue crystals were collected after 5 days of solvent evaporation (215 mg; Yield: 48% m.p.: 235 °C). The complex is soluble in water and several polar organic solvents (MeOH, EtOH, CH3CN), Anal. Calc. for C28H23BrCl2Cu2N4O11 (869.39 g/mol) C, 38.68; H, 2.64; N, 6.45%, Found: C, 38.61; H, 2.68; N, 6.50%. Significant IR bands (KBr, ν cm−1) (s, strong; m, medium; w, weak): 3092–3030w νC–Harom.; 1617 m νC=N; 1678 m νCOOasym; 1445 m νCOOsym; 1552 m νC=N–C=Csym; 1088 s νC–O; 770 m νC–N; 729 m νCu–O–Cusym; 621 m νCu–O–Cuasym. UV–Vis λmax nm (CH3CN): 241, 301, 311. Magnetic moment (μ) = 1.37 B.M. at room temperature.
X-ray crystallography
Single crystal data collection was performed on a Bruker AXS APEX CCD diffractometer equipped with an Mo Kα radiation source at 293 (2) K. The data processing was done with the Bruker SMART program package [28]. The SHELXS- 97 [30] program was used for the structure solution by direct methods. Non-hydrogen atoms were located through difference Fourier synthesis [29] and refined with full-matrix least squares methods on F2 using first isotropic and later anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms were added at the calculated positions. The molecular structures were drawn using MERCURY [31]. The PLATON [32] program was used for geometric analyses.
General procedure for catalytic oxidations
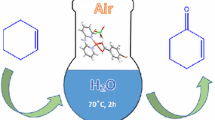
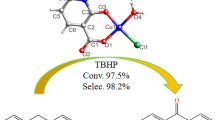
Catalytic oxidation experiments were carried out in a 50 mL round-bottomed flask fitted with a reflux condenser under open air with a magnetic stirrer at 70 °C. In a typical experiment, 5 mg of catalyst (5.3 × 10−3 mmol) was dissolved in deionized water (10 mL). The substrate (0.53 mmol) (substrate/catalyst = 100) plus hydrogen peroxide (2 mL of 30% in water, 1.95 mmol) was added and the mixture was stirred. To monitor the progress of the reaction, ten drops of reaction mixture were withdrawn at certain time intervals and analyzed by GC with an HP-5 quartz capillary column (30 m × 0.32 mm × 0.25 μm) with a flame ionization detector (FID). Each analysis was repeated twice and the peak identification was done by comparing with authentic samples.
Results and discussion
Structural analysis of the complex
The reaction between 4-bromobenzoic acid, 2,2′-bipyridine and copper(II) perchlorate resulted in dark blue cubic crystals of (Cu2(OOCC6H4Br)(OCH3)(C10H8N2)2(ClO4)2) after slow evaporation of the methanol solvent over 5 days. The crystals of the complex proved to be of the triclinic space group, P − 1. The molecular structure and molecular formula of the complex are given in Fig. 1. The crystallographic data, bond distances, bond angles and torsion angles are listed in Tables 1 and 2.
According to the X-ray crystal structure, the complex consists of a pair of Cu(II) centers, bridged by anionic carboxylate and methoxide ligands. In addition, each Cu(II) center is coordinated by two nitrogen atoms of chelating bipyridine ligands together with perchlorate anions, to form a dimeric unit. All the Cu–N bond distances are similar between 1.985 and 1.998 Å, which are also similar to the literature values [33, 34]. The methoxide and carboxylate groups are both coordinated in bidentate modes to the copper atoms, acting as bridging ligands. The Cu–O bond lengths of the carboxylate oxygens with Cu1 and Cu2, at 1.925 and 1.946 Å, are also similar to those of related complexes [35, 36]. The Cu–O bond lengths for the bridging methoxide ligand are 1.929 and 1.917 Å for Cu1–O3 and Cu2–O3, respectively [37, 38]. In the axial positions, the bond distances for the perchlorate ligands are Cu1–O4 = 2.402 Å and Cu2–O12 = 2.399 Å, indicating that they are directly coordinated to the Cu(II) centers. The Cu1–Cu2 distance is 3.127 Å.
The geometry around both Cu(II) centers is best described as distorted square-pyramidal, with coordination angles between 84.36°and 101.29° for Cu1 and 85.82°–100.83° for Cu2. The reduction of angle values from 90° for O4–Cu1–N2 and O4–Cu1–N1, at 84.36 and 88.78, respectively, can be attributed to the steric effect of the bridged methoxide ligand. The torsion angles of the bipy ligands for O12–Cu2–N3–C27 and O4–Cu1–N2–C17 are 81.55° and 88.17°, respectively. The methoxy bridge angle between Cu1 and Cu2 is 108.81°. Additionally, several intermolecular interactions can be identified between the bipy carbons and perchlorate oxygens.
Spectroscopic studies
The FTIR spectrum of the complex is given in Figure S1. The peaks between 3030 and 3092 cm−1 are assigned to the aromatic ʋ(C–H) vibrations of the ligands. Symmetric and asymmetric vibration peaks of the carboxylate ligand were observed at 1678 and 1445 cm−1, respectively. The azomethine ʋ(C=N) vibration of the bipyridine ligand shifted from 1589 to 1617 cm−1 upon complexation, consistent with coordination of the N atom. Peaks observed at 729 and 621 cm−1 are assigned to Cu–N bond vibrations.
The UV–Vis spectrum of the complex was recorded in acetonitrile solution between 200 and 800 nm. In total, three absorption bands were observed at 241, 301 and 311 nm (Figure S2). The band at 241 can be assigned to carboxylate-based π → π* intraligand transitions. The bands at 301 and 311 nm arise from bipyridine ligand n → π* transitions. No d → d transitions were observed for the Cu centers.
According to room temperature measurement of the complex magnetic susceptibility with a Gouy balance, the magnetic moment of the complex is 1.37 B.M. This value is consistent with one unpaired electron, although lower than the theoretical value of 1.73 B.M. due to the magnetic interactions between the ligands and the metal centers [39].
Catalytic studies
The homogenous catalytic oxidation of primary and secondary alcohols in the presence of this dinuclear copper(II) complex under open air using H2O2 (30% in water) as an oxidant was investigated at 70 ℃ in water as solvent. The results are given in Table 3 and Fig. 2.
According to the results, the copper complex was an effective catalyst for the selected alcohol oxidations, giving high TON values (up to 100). It is noteworthy that no traces of carboxylic acid were detected during or after the reactions; furthermore, the C=C bonds were not affected. The complex was generally found to be more effective as a catalyst for benzylic and cyclic alcohols rather than 1-heptanol as a primary aliphatic alcohol. The total substrate conversions ranged from 17 to 100% for a range of different alcohols with a 6 h reaction time (Table 3, Entries 2–7), whilst the complete oxidation of cinnamyl alcohol required only 2 h (Table 3, Entry 1). The oxidation of cinnamyl alcohol gave both cinnamaldehyde (11%) and benzaldehyde (11%) in the first minute of the reaction and the total product formation was found to be 22% with a TOF value of 1320. The substrate was fully converted to aldehydes after 2 h (TON = 100), with overall 88% selectivity for benzaldehyde. The oxidation of benzyl alcohol resulted in selective product formation, with only benzaldehyde obtained in 100% conversion for a 6-h reaction time; no other oxidation product was observed (Entry 2). Cyclohexanol was oxidized to cyclohexanone in a moderate yield of 53% after the same time period (Entry 3). Cyclopentanol oxidation gave a higher yield of product compared to cyclohexanol, with a 71% yield of cylopentanone after 6 h (Entry 4). The unactivated primary aliphatic alcohol 1-heptanol gave the lowest product conversion value of 17% as heptaldehyde (Entry 7). According to these findings, the reactivities of aromatic alcohols were much higher than aliphatic ones, in agreement with previous studies [40].
The selectivity of this catalytic system was tested between a primary and a secondary alcohol, or between a cyclic alcohol and an aliphatic alcohol at 70 ℃ for 6 h reaction time, with the results given in Table 4. In the catalytic oxidation of benzyl alcohol plus 1-phenyl ethanol mixture, 16.4% benzaldehyde was obtained together with 62.4% acetophenone (Entry 1). Hence, this catalytic system shows a tendency to act on the secondary alcohol.
The oxidation of a mixture of a linear secondary alcohol (2-butanol) and a cyclic alcohol (cyclopentanol) resulted in lower conversions for both (Table 4, Entry 2), compared to yields of 100 and 71% for 2-butanone and cyclopentanone obtained from the initial experiments, on single substrates.
Overall, these experiments indicated that the competition between the mixtures of alcohols results in lower product yields compared to the pure substrates under the same conditions.
Conclusions
In summary, we have described the synthesis and characterization of a dinuclear, water-soluble copper (II) complex. The complex showed good homogenous catalytic activity for the oxidation of both primary and secondary alcohols under mild conditions (70 ℃) with hydrogen peroxide in water. This system was found to give moderate to good yields for benzylic and cyclic alcohols, whereas 1-heptanol as a representative aliphatic alcohol gave poorer results. No over-oxidation products could be detected in these reactions.
Supplementary material
CCDC 1830893 contains the supplementary crystallographic data for complex. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
References
Choudary BM, Kantam ML, Santhi PL (2000) Catal Today 57:17–32
Vinod CP, Wilson K, Lee AF (2011) J Chem Technol Biotechnol 86:161–171
Lu Z, Ladrak T, Roubeau O, Toorn J, Teat SJ, Massera C (2009) Gamez p, Reedijk. J Dalton Trans. 18:3559–3570
Rong M, Liu C, Han J, Sheng W, Zhang Y, Wang H (2008) Catal Lett 125:52–56
Karthikeyan P, Bhagat PR, Kumar SS (2012) Chin Chem Lett 23:681–684
Sheldon RA (2015) Catal Today 247:4–13
Brink GJ, Arends IWCE, Sheldon RA (2002) Adv Synth Catal 2002(344):3–4
Sun N, Zhang X, Jin L, Hu B, Shen Z, Hu X (2017) Catal Commun 101:5–9
Zhang G, Zhang YZ, Lo W-F, Jiang J, Golen JA, Rheingold AL (2016) Polyhedron 103:227–234
Kopylovich MN, Gajewska MJ, Mahmudov KT, Kirillova MV, Figiel PJ, Silva FCG, Sanchizd Hernandez BG, Pombeiro AJL (2012) New J Chem 36:1646–1654
Gao J, Ren ZG, Lang JP (2015) J Organomet Chem 792:88–92
Mannam S, Alamsetti SK, Sekar G (2007) Adv Synth Catal 349:2253–2258
Samantha S, Das S, Samantha PK, Dutta S, Biswas P (2013) RSC Adv 3:19455–19466
Jiang N, Ragauskas AJ (2005) Org Lett 7(17):3689–3692
Liu L, Ma J, Sun Z, Zhang J, Huag J, Li S, Tong Z (2011) Can J Chem 89:68–71
Unver H, Kani I (2017) Polyhedron 134:257–262
Unver H, Kani I (2018) J Chem Sci 130:33
Brink GJ, Arends IWCE, Sheldon RA (2002) Adv Synth Catal 344(3–4):355–369
Buffin BP, Clarkson JP, Belitz NL, Kundu A (2005) J Mol Catal A: Chem 225:111–116
Pagliaro M, Campestrini S, Crimina R (2005) Chem Soc Rev 34:837–845
Liu L, Yu M, Wayland BB, Fu X (2010) Chem Commun 46:6353–6355
Wang X, Wang C, Liu Y, Xiao J (2016) Green Chem 18:4605
Samanta S, Das S, Samanta PK, Dutta S, Biswas P (2013) RSC Adv 3:19455–19466
Mannam S, Alamsetti SK (2007) Adv Synth Catal 349:2253–2258
Dijksman A, Arends IWCE, Sheldon RA (2001) Synlett 1:102–104
Cecchetto A, Fontna F, Minisci F, Recupero F (2001) Tetrahedron Lett 42:6651–6653
Jiang N, Ragauskas AJ (2006) J Org Chem 71:7087–7090
MART, Bruker AXS, (2000)
Sheldrick GM (2008) Acta Crystallogr. Sect. A 64:112
Sheldrick GM (1997) SHELXS-97, Program for Crystal Structure Solution. University of Gottingen, Gottingen
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) J Appl Crystallogr 39:453
Spek AL (2005) PLATON—a multipurpose crystallographic tool Utrecht. Utrecht University, Utrecht
Pap JS, Taheri N, Fadel A, Goger S, Bogath D, Molnar M, Giorgi M, Speier G, Imaan AJ, Kaizer J (2014) Eur J Inorg Chem 17:2829–2838
Molcanov K, Juric M, Prodic BK (2013) Dalton Trans 42:15756–15765
Jennifer SJ, Muthiah PT, Muthukumaran G (2013) Inorg Chim Acta 406:100–105
Bickey J, Law RPB, Martines MAB, Steiner A (2004) Inorg Chim Acta 357:891–894
Osanai K, Ishida T, Nogami T (2005) Chem Lett 34(4):620–621
Graham B, Hearn MTW, Junk PC, Kepert CM, Mabbs FE, Moubaraki M, Murray KS, Spiccia L (2001) Inorg Chem 40(7):1536–1543
Masoud MS, Enein SAAE, Ayad ME, Goher AS (2004) Spectrochim. Acta A 60:77–87
Kaneda K, Yamashita T, Matsushita T, Ebitani K (1998) J Org Chem 63:1750–1751
Acknowledgements
The author thankfully acknowledges the Medicinal Plants and Medicine Research Centre of Anadolu University, Eskişehir, Turkey for the use of X-ray Diffractometer and Anadolu University, Chemistry Department, Eskişehir, Turkey for the other spectroscopic measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ünver, H. Crystal structure of an alkoxide bridged dinuclear copper(II) complex: mild and selective oxidation of primary and secondary alcohols in water. Transit Met Chem 43, 641–646 (2018). https://doi.org/10.1007/s11243-018-0252-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0252-2