Abstract
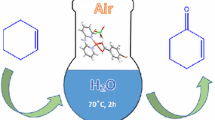
An environmentally friendly protocol is described for an economic, practical laboratory-scale oxidation of primary and secondary alcohols to aldehydes and ketones, using a bis-chloro-bridged binuclear Cu(II) complex [(HL)Cu(μ 2-Cl)2Cu(HL)]*1.5 CH3OH as catalyst. The catalyst was prepared in situ from commercially available reagents and is characterized by single crystal X-ray analysis, FT-IR, UV-visible spectra, mass spectrometry, and powder x-ray diffraction (PXRD). The geometry of the complex has been optimized using the B3LYP level of theory confirming the experimental data. Our results demonstrated well the efficiency, selectivity and stability of this new catalyst in the oxidation of alcohols in ethanol and tert-butyl hydroperoxide (tBuOOH) as a green solvent and oxidant, respectively. Turnover number and reusability have proven the high efficiency and relative stability of the catalyst.

A novel [(HL)Cu(μ2-Cl)2Cu(HL)]*1.5CH3OH complex was used as an efficient catalyst for the oxidation of alcohols. It was synthesized, using (E)-1-(((2-hydroxypropyl)imino)methyl)naphthalen-2-ol as a monoanionic and tridentate Schiff base ligand, and characterized by IR and UV-Visible spectroscopy and single-crystal X-ray analysis. The geometry of the complex was optimized using the B3LYP level of theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Development of efficient and selective catalyst for oxidation of alcohols to their corresponding carbonyl compounds is a vital prerequisite in the pharmaceutical, fragrance, flavoring and fine chemicals industries.[1,2] These reactions are performed at present using harmful oxidants and they have many drawbacks and limitations Over the past decade, it was found that catalytic methods could potentially alleviate these problems.[3] In this regard, Schiff base complexes are ideal catalysts having long-term shelf-stability and be readily prepared in situ from commercially available reagents.[4–8]
We recently reported the several complex based catalyst systems that have a broad substrate scope and are capable of using tetrabutylammonium peroxomonosulfate (TBAOX) as the oxidant.[9–15] To have practical laboratory scale and green protocol, instead of using TBAOX oxidant which is commercially unavailable, prepared from expensive reagents, very short-term shelf-stability (3 days), we use the green oxidants such as tBuOOH in this work. The present catalyst system shows considerably high catalytic activity, enabling efficient oxidation of primary and secondary alcohols with tBuOOH, without overoxidation to the carboxylic acid. All the reactions were carried out in ethanol, a green solvent. This work deals with the synthesis and characterization of a new Schiff base ligand obtained by the condensation reaction of 2-hydroxynaphthalene-1-carbaldehyde and 1-amino-2-propanol and its Cu(II) complex as catalyst (scheme 1). The coordination behavior of the Schiff base towards transition metal ions was investigated via FT-IR, UV–Vis and single crystal X-ray diffraction analysis. The geometry of the complex has been optimized using the B3LYP level of theory.
2 Experimental
2.1 Materials and measurements
CuCl2.2H2O (Merck India Ltd), 2-hydroxynaphthalene- 1-carbaldehyde and 1-amino-2-propanol(Sigma–Aldrich, USA) were purchased. All the chemicals and solvents employed for the synthesis were of analytical grade and used as received without further purification. FT-IR spectra of the ligand and the complex in the 4000–400 cm −1 region as KBr disks were recorded on a Thermo SCIENTIFIC model NICOLET iS10 spectrophotometer. The electronic spectra were recorded on a PG instruments Ltd, T70/T80 series (UV–Vis) spectrometer using HPLC grade methanol as solvent in the range 800–200 nm. NMR spectra were recorded on a Bruker Avance DPX 500 MHz instrument with TMS as the internal standard, using DMSO as the solvent. Mass spectra were recorded on a Shimadzu GC–MS- QP5050A. Purity determinations of the products were accomplished by GC-FID on a Agilent 6890 instrument by using a 30 m, 0.32 mm ID, and 1 micrometer coating capillary column and also on YL instrument by using a 60m, 0.32mm ID, and 0.5 micrometer coating capillary column.
2.2 Synthesis
2.2.1 Synthesis of the Schiff base ligand (E)-1-(((2- hydroxypropyl)imino)methyl)naphthalen-2-ol (H 2 L )
The asymmetric tridentate Schiff base H2L was obtained by addition of a solution of 0.01 mol 1-amino-2-propanol (0.75 g) in methanol (10 mL) to a solution of 0.01 mol 2-hydroxynaphthalene-1-carbaldehyde (1.72 g) in 10 mL methanol and the reaction mixture was stirred and heated to reflux for 1 h. The yellow precipitate that formed was filtered off and dried in air. The crude product was recrystallized from CHCl3–hexane (1/4 v/v). Yield: 90% (1.52 g). M.p.:46–48∘C. IR (KBr disc, cm −1): 3224 (υ OH), 1636 (υ C=N), 1545 (υ C=C); 1H NMR (500 MHz, DMSO) δ ppm: 1.38 (d, J = 6.3, 3H), 3.51 (dd, J 1 = 13, J 2 = 7.5, 1H), 3.75 (dd, J 1 = 13.2, J 2 = 3.4, 1H), 4.17 (m, 1H), 6.86 (d, J = 9.2, 1H), 7.21 (t, J = 7, 1H), 7.43 (t, J = 9.1, 1H), 7.46 (d, J = 7.8, 1H), 7.53 (d, J = 9.2, 1H), 7.82 (d, J = 8.35, 1H), 8.74 (s, 1H), 14.1 (s, 1H); 13C NMR (500 MHz, DMSO) δ ppm: 21.15, 67.17, 118.34, 123.17, 124.71, 126,60, 128.41, 129.63, 138.06; MS: m/z 229 (M +).
2.2.2 Synthesis of the[(HL)Cu(μ2-Cl)2Cu(HL)]*1.5CH3 OH complex
To 0.01 mol H2L ligand (2.29 g) was added 0.01 mol dihydrate copper (II) chloride (1.704 g) in 10 mL methanol. The reaction mixture was stirred under reflux condition for 2 h and a green precipitate was removed by filtration. The resulting deep green solution was then left undisturbed. The complex was recrystallized from methanol and is quite air stable as solids and also in solution. After 7 days, dark green, block-shaped, X-ray diffraction quality single crystals appeared. The product was secured in 62% yield. M.p.: >250∘C. IR (KBr disc, cm − 1):1623(ν C=N),1543(ν C=C),560(ν Cu−O) and 480(ν Cu−N).
2.3 Crystallographic Data Collection Structure Refinement and Computational methods
Single-crystal X-ray diffraction data for the complex were collected on a Bruker APEX II equipped with a CCD area detector and utilizing Mo-K α radiation (λ = 0.71073 Å) at room temperature. Data were collected and reduced by SMART and SAINT software in the Bruker [SHELXTL.97] and then solved.[17] The structures were solved by direct methods[16] packages. CCDC-968909 contains the supplementary[18] by least squares refinement on F 2. crystallographic data which can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
The Gaussian 03W program suite[19] was used for all quantum chemistry computations. The geometry optimizations were carried out at B3LYP method with LANL2DZ basis set. Harmonic vibrational frequencies were evaluated at the same level in order to compute the zero point vibrational energy (ZPVE) correction. The nature of the intramolecular interactions was studied by means of the Bader theory of atoms in molecules (AIMs). The calculated electron density, ρ, and its second derivative, ∇2 ρ were used for describing the nature of the intramolecular interactions. The AIM2000 program was used to find BCPs and to analysis them.[20]
2.4 Oxidation Procedure
To a solution of the alcohol (0.5 mmol) and complex (scheme 1; 0.01 mmol) in ethanol (5 mL) was added tBuOOH (0.5 mmol), and the reaction mixture was stirred in air at 80∘C for the required time. GC monitored the reaction progress and the yields of the products. Further purification was achieved by silica chromatography.
3 Results and Discussion
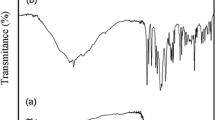
Theligand,(E)-1-(((2-hydroxypropyl)imino)methyl)naphthalen-2-ol (H2L), which is stable in air, was characterized by IR, UV-Vis, 1H NMR, 13CNMRspectra and mass spectrometry.Copper complex[(HL)Cu(μ 2-Cl)2Cu(HL)] *1.5CH3OH was prepared by the reaction of H2L with dihydrate copper (II) chloride. This new binuclear Cu(II) complex, containing two the tridentate Schiff base ligandsandchlorobridgedCu(μ-Cl)2Cu,wascharacterized by UV-visible, IR spectroscopy and single-crystal X-ray analysis. The IR spectra of complex in KBr matrix confirm that the strong absorption band at 1623 cm −1 can be assigned to the imine stretching frequency of the coordinated ligand, whereas for the free ligand molecule the same band is observed at 1636 cm−1. The shift of this band towards lower frequency on complexation with the metal suggests coordination to the metal ion through imine nitrogen atom.[21,22] A very strong band of ν (C−O) mode at about 1187 cm−1 and the C =C vibration band of the aromatic ring at 1476 cm−1 are observed. Ligand coordination to the metal center is substantiated by the single bands appearing at 560 and 480 cm−1 which correspond to the vibrations of Cu-O and Cu-N bonds, respectively.[23] On the other hand, the broad band at about 2969 cm −1 is attributable to C-H stretching vibrations of the lattice methanol molecule. The electronic spectra of the H2L ligand and the complex in methanol are in good agreement with their geometry. The two strong absorption bands in the UV region 227 and 238 nm are clearly charge transfer in origin attributed to the transition from the coordinated unsaturated ligand to the metal ion (LMCT). Again, the intense high energy bands at about 315 and 400 nm may be assigned to the intraligand π→π* and n→π* transitions, respectively. The broad low-intensity absorption band centered at 650 nm is a typical d–d band for the copper (II) ion.[24]
3.1 Crystal structure description of the complex and theoretical studies
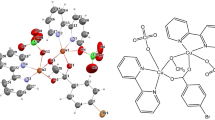
The[(HL)Cu(μ 2-Cl)2Cu(HL)]*1.5CH3OHcomplexcrystallizes as dark green single crystals in the space group P-1 as seen from table S1 (Supporting Information). The unit cell contains two distinct entities of [Cu(HL)Cl]\(_{2}^{\mathrm {\ast }}\) 1.5CH3OH. An ORTEP view of the complex is shown in figure 1. The geometry of metal complex as determined by x-ray crystal structure analysis was fully optimized at the above mentioned level of theory. The calculated bond lengths and bonds are in good agreement with the experimental values (table 1). The (E)-1-(((2-hydroxypropyl)imino)methyl)naphthalen-2-ol ligand is coordinated to the Cu(II) ion via its imine nitrogen, alkoxy oxygen and deprotonated phenolic oxygen atoms and the coordination sphere of Cu(II) is completed by the two bridging chloride anions. The crystal structure reveals that there are two complexes with three lattice methanol molecules (figure 2). The PXRD pattern of the complex has shown sharp intense peaks throughout the spectrum. It is indicating crystalline nature of the sample. The particle size has been calculated using Scherrer’s formula t = 0.9 λ/B\(\cos \theta \), where t is the particle size (same unit as λ), B is half width (in radians) of diffraction line, 𝜃 is the Bragg angle and λ is the wavelength. The particle size of Cu(II) complex was found to be around 27 nm. The XRD pattern of the complex is shown in figure S2 (Supporting Information).
There are two common structures for a five coordinated CuII center, square-pyramidal (SP) or trigonalbipyramidal (TBP) geometry, which can be evaluated by the addison distortion index (τ). τ defined as τ=[|𝜃−Φ|/60], where 𝜃 and Φ are the two largest coordination angles and τ=0 for perfect SP and 1 for ideal TBP.[25,26] The calculated τ of Cu(1) and Cu(2) (0.21583(3) and 0.12733(3), respectively) also confirmed the distorted SP geometry for each copper ion. It should be noted that the active Jahn–Teller distortion of the copper (II) ion causes that equatorial Cu-Cl bonds are shorter than axial bonds for each copper. For example, 2.7627(7) Å and 2.2606(6) Å are related to the axial and equatorial Cu– Cl bonds for Cu(1), respectively.
DFT calculations at the B3LYP/LANL2DZ level of theory using the GAUSSIAN 03 were carried out. The Bader’s quantum theory[27,28] of atoms in molecules (QTAIM) was also applied to get more details about the nature of the intramolecular interactions in this complex. According to this theory, when two neighboring atoms are chemically bonded, a critical point for bond formation appears between them. The sign of Laplacian for electron density at a bond critical point, ∇2 ρ, reveals whether the charge is concentrated, as in a covalent bond (∇2 ρ < 0), or depleted, as in closed shell (electrostatic) interactions (∇2 ρ > 0). The calculated electron density, ρ, and its second derivative, ∇2 ρ were used for describing the nature of the intramolecular interactions.[29–34] The molecular graph of the complex was obtained from the B3LYP/LANL2DZ wave function (figure S1, Supporting Information). The corresponding values of ρBCP and ∇2 ρBCP were presented in table S2 (Supporting Information). As shown in the molecular graph of the complex, all the critical bonds between atoms have shown bonds and critical points. The comparison between the electron density of Cu-N, Cu-O and Cu-Cl bonding have shown that ρBCP for the Cu-N bonding is higher than the corresponding value for Cu-O and Cu-Cl. As a consequence, the strength of the Cu-N bond is greater than the other. Furthermore, the calculated electron densities indicate that Cu-N, Cu-O and Cu-Cl bondings have low ρBCP (about 0.0851, 0.0797 and 0.0379, respectively) and positive ∇2 ρBCP values. These properties are typical for close shell interaction for them.
The analysis of the wave function indicates that the electron adsorption corresponds to the transition from the ground state to the first excited state and is mainly described by one- electron excitation from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). The HOMO and LUMO orbitals for the investigated complex are shown in figure 3. The HOMO of the complex has shown antibonding character at Cu-Cl. There is no electronic projection over the methanol group. The LUMO has a larger electronic projection over Cu atom in this complex.
3.2 Oxidation of alcohols
Oxidation of benzyl alcohol was studied exclusively for solvent effect, catalyst concentration, oxidant volume, reaction time and the results of the study are as follows. The oxidation of benzyl alcohol in the presence of tBuOOH without catalyst was slow (7% after 24 h). By addition 2 mol% catalyst, the conversion in reaction was enhanced markedly (50% after 24 h) and increasing the mole percentage of catalyst up to 10% did not affect the rate of reaction. The influence of axial nitrogenous bases having dramatic effects on the rate and selectivity in the oxidation reactions catalyzed by various transition metal complexes such as porphyrin[35] and salen[36] catalysts has also been studied Imidazole and pyridine as commonly used axial base were added and no improvement in the reaction rate was observed. Water and ethanol were investigated as green media for the reaction (net reaction, 2%, 50% respectively after 24 h) and ethanol was found to be the best solvent in terms of conversion rate and benzaldehyde yield. The reaction rates and products yields were found to be poor at room temperature and all the reactions were conducted at reflux temperature (80∘C). The best yield of benzaldehyde was obtained during oxidation of benzyl alcohol (1 mmol) using 2 mol% of catalyst with 1 mmol of tBuOOH in 1 mL ethanol for 1 h at 80∘C.
Primary benzylic alcohols bearing a variety of electron donating and electron withdrawing groups undergo efficient and selective oxidation to the aldehyde, without overoxidation to the carboxylic acid. It represents a practical alternative to traditional reagents and methods for the oxidation of primary and secondary alcohols Benzylic alcohols are generally excellent substrates for this catalyst (table 2, entries 1–7). Electronic and steric effects do not seem to have a significant effect on the isolated yields for electronrich and electron-poor benzylic alcohols. When we applied this catalytic system for the oxidation of secondary alcohols, good/high yields of ketones were secured (table 2, entries 8–11). The good conversion of less reactive primary and secondary aliphatic alcohols to their corresponding carbonyl compound without overoxidation to carboxylic products is a notable feature of this oxidation method.
To prove the high efficiency and relative stability of this noble catalyst, the turnover number (TON) and turnover frequency (TOF) in the oxidation of benzylic alcohol were investigated. In this oxidation protocol, 5000:10000:1 molar ratio was used for substrate/oxidant/catalyst and impressive turnover frequency (TOF = 1640/h) and turnover number (TON = 5000 in 24 h) were obtained.
After completion of the oxidation of benzylic alcohol, 2 mol% of catalyst with 1 mmol of tBuOOH was added again to reaction media and was stirred for another 1 h. After repeating three times, the solvent was dried under vacuum washed with diethyl ether and used directly for the next round of reaction without further purification. Comparison of the IR spectra of the used [(HL)Cu(μ2-Cl)2Cu(HL)] *1.5CH3OH (figure 4) with fresh catalyst showed that the structure and morphology of the catalyst remained almost intact after four recoveries.
Thus, the methodology described herein is cost effective and environmentally benign due to the use of ethanol and tBuOOH as a reaction medium and oxidant. Further, catalyst is stable and the reaction does not need nitrogen donors. These advantages of this high yielding oxidation method offered ready scalability. For example the use of a semi scale-up procedure (10 mmol) for the oxidation of benzylic alcohol in the presence of [(HL)Cu(μ 2-Cl)2Cu(HL)] *1.5CH3OH led to the isolation of the related aldehyde in 91% yield.
4 Conclusions
A novel Cu(II) complex, [(HL)Cu(μ 2-Cl)2Cu(HL)] *1.5CH3OH was synthesized, using (E)-1-(((2-hydroxypropyl)imino)methyl)naphthalen-2-ol (H2L) as tridentate OON donor Schiff base ligand and characterized by IR, UV-Visible spectroscopy and single-crystal X-ray analysis. This complex was used as a new catalyst for a practical laboratory-scale oxidation strategy using tBuOOH and ethanol as oxidant and green solvent, respectively. The high turnover number and turnover frequency have confirmed the stability and efficiency of the catalyst.
References
Busacca C A, Fandrick D R, Song J J and Senanayake C H 2011 Adv. Synth. Catal. 353 1825
Arends W C E and Sheldon R A 2010 In Modern Oxidation Methods 2nd ed. J E Bäckvall (ed.) (Weinheim: Wiley–VCH) pp. 147–185
Hoover J M, Steves J E and Stahl S S 2012 Nature Protocols 7 1161
Fatiadi A J 1976 Synthesis 65
Taylor R J K, Reid M, Foot J and Raw S A 2005 Acc. Chem. Res. 38 851
Pfitzner K E and Moffatt J G 1963 J. Am. Chem. Soc. 85 3027
Mancuso A J, Brownfain D S and Swern D 1979 J. Org. Chem. 44 4148
Tidwell T T 1990 Synthesis 857
Moreno A L, Tejeda D C, Calbo J, Naeimi A, Bermejo F A, Ortí E and Pérez E M 2014 Chem. Commun. 50 9372
Aguiló J, Naeimi A, Bofill R, Bunz H M, Llobet A, Escriche L, Sala X and Albrech M 2014 New J. Chem. 38 1980
Rezaeifard A, Jafarpour M, Naeimi A and Haddad R 2012 Green Chem. 14 3386
Rezaeifard A, Jafarpour M, Naeimi A and Salimi M 2012 Inorg. Chem. Commun. 15 230
Rezaeifard A, Jafarpour M and Naeimi A 2011 Catal. Commun. 16 240
Rezaeifard A, Jafarpour M, Naeimi A and Mohammadi K 2012 J. Mol. Catal. A: Chem. 357 141
Rezaeifard A, Jafarpour M, Naeimi A and Kaafi S 2011 Catal. Commun. 12 761
Bruker, 1999 SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA
Burla M C, Caliandro R, Camalli M, Carrozzini B, Cascarano G L, De Caro L, Giacovazzo C, Polidori G and Spagna R 2005 J. Appl. Crystallogr. 38 381
Sheldrick G M 1997 SHELXL97. Program for crystal structure refinement. University of Gottingen, Germany
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr , Vreven T., Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A 2004 Gaussian 03, Revision E.01 (Gaussian, Inc.: Wallingford, CT)
Biegler-Konig F., AIM 2000 version 1.0, 1998 University of Applied. Science, Bielefeld, Germany
Tarafder M, Kasbollah A, Crouse K, Ali A, Yamin B and Fun H K 2001 Polyhedron 20 2363
Vafazadeh R, Khaledi B, Willis A C and Namazian M 2011 Polyhedron 30 1815
Trzesowska-Kruszynska A 2012 J. Mol. Struc. 1017 72
Thakurta S, Roy P, Rosair G, Gómez-García C J, Garribba E and Mitra S 2009 Polyhedron 28 695
Hathaway B, Wilkinson G, Gillard R and McCleverty J 1987 In Comprehensive Coordination Chemistry Vol. 5 (Oxford: Pergamon)
Addison A W, Rao T N, Reedijk J, van Rijn J and Verschoor G C 1984 J. Chem. Soc., Dalton Trans. 1349
Bader R F 1991 Chem. Rev. 91 893
Bader R F 1990 In Atoms in Molecules: A Quantum Theory (London: Oxford University Press)
Raissi H, Yoosefian M, Mollania F and Khoshkhou S 2013 Struct. Chem. 24 123
Raissi H, Yoosefian M and Mollania F 2012 Int. J. Quantum Chem. 112 2782
Fazli M, Jalbout A, Raissi H, Ghiassi H and Yoosefian M 2009 J. Theor. Comput. Chem. 8 713
Yoosefian M, Barzgari Z and Yoosefian J 2014 Struct. Chem. 25 9
Raissi H, Khanmohammadi A, Yoosefian M and Mollania F 2013 Struct. Chem. 24 1121
Raissi H, Yoosefian M, Mollania F, Farzad F and Nowroozi A R 2011 Comput. Theor. Chem. 966 299
Meunier B 1992 Chem. Rev. 92 1411
McGarrigle E M and Gilheany D G 2005 Chem. Rev. 105 1563
Acknowledgements
We are thankful to University of Jiroft and Vali-e-Asr University of Rafsanjan Research Council for their support on this work. We also thank Dr. Emilio Perez for his valuable comments to improve our project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
Figures S1 and S2 and tables S1 and S2 are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
NAEIMI, A., SAEEDNIA, S., YOOSEFIAN, M. et al. A novel dinuclear schiff base copper complex as an efficient and cost effective catalyst for oxidation of alcohol: Synthesis, crystal structure and theoretical studies. J Chem Sci 127, 1321–1328 (2015). https://doi.org/10.1007/s12039-015-0896-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0896-9