Abstract
The constant exposure of rural workers to pesticides is a serious public health problem. Mancozeb (MZ) is a pesticide linked to hormonal, behavioral, genetic, and neurodegenerative effects, mainly related to oxidative stress. Vitamin D is a promising molecule that acts as a protector against brain aging. This study aimed to evaluate the neuroprotective role of vitamin D in adult male and female Wistar rats exposed to MZ. Animals received 40 mg/kg of MZ i.p. and 12.5 μg/kg or 25 μg/kg vitamin D by gavage, twice a week, for 6 weeks. The concentration of manganese had a significant increase in the hippocampus of both sexes and in the striatum of females, unlike zinc, which did not show a significant increase. MZ poisoning led to mitochondrial changes in brain tissues and promoted anxiogenic effects, especially in females. Alterations in antioxidant enzymes, mainly in the catalase activity were observed in intoxicated rats. Taken together, our results showed that exposure to MZ leads to the accumulation of manganese in brain tissues, and the behavior and metabolic/oxidative impairment were different between the sexes. Furthermore, the administration of Vitamin D was effective in preventing the damage caused by the pesticide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mancozeb (MZ) is a synthetic multisite fungicide that belongs to the class of carbamates and the subclass of dithiocarbamate pesticides. It contains a manganese (Mn) and zinc (Zn) atom coordinated with ethylene bis-dithiocarbamate [1]. It was synthesized in the 1940s as a fungicide for seed treatment [2] and demonstrated nearly seventy years of fungicidal efficacy in a wide range of agricultural and industrial applications, including as a fungicide in major agricultural crops (e. g., potato, tomato, grapevine, and citrus) for roughly 400 different plant pathogens [3] and, like most pesticides, it can be available as dust, liquid, dispersible granules, or wettable powder [4]. MZ presents low acute toxicity and short environmental persistence, according to [5]. Despite this, many studies reported environmental and health damage associated with exposure of MZ. Among these studies, genotoxicity, steatosis, and oxidative stress caused by MZ exposure have been demonstrated in different human cells [6,7,8]. Thus, MZ is one of the most widely used fungicide in Brazil, which is one of the world leaders in the use of agrochemicals [9, 10].
Several studies have already demonstrated the action of MZ increasing the production of reactive oxygen species (ROS) and mitochondrial damage mainly due to the presence of Mn in its composition. Two hypotheses can be formulated to explain this. The first is related to the accumulation of MZ, which has a lipophilic character, in the mitochondria, influencing the flow of electrons at the level of the electrogenic transport system, and the second because of the impairment of mitochondrial functions due to the inactivation of the p53 protein pathway [11].
Previous works showed that there is sexual dimorphism in the effects of pesticides in animals. In a study on the effects of organophosphate pesticide dimethoate on cultured cortical astrocytes obtained separately from male and female mouse pups, an increase in the production of ROS was observed in the astrocytes from male mice. However, estradiol reduced the production of ROS in the cells from females, suggesting that pesticides may have different neurological effects between the two sexes [12]. Another study that reports this difference between the sexes is the one of Yardimci [13], where the pesticide imidacloprid was tested in male and female Sprague–Dawley rats, and males suffered more oxidative damage in the kidney when compared to females.
Despite several studies being developed to identify treatments that can counteract MZ toxicity, not much success has been achieved so far. In this context, vitamin D is a promising group of molecules, as they act as modulators of development and protect against several CNS conditions, as brain aging [14, 15], retinal tissue [16, 17], 2021) neurodegenerative disorders [18] among others. The presence of the vitamin D receptor (VDR) in the hippocampus, hypothalamus, thalamus, cerebral cortex, and substantia nigra has raised the interest in the potential role of vitamin D in different neurological pathologies [19]. Also, vitamin D has direct and indirect antioxidant effects [20]. The major sources of vitamin D are exposure to ultraviolet radiation and diet [21,22,23].
Based on the recent and promising effects of Vitamin D in models for neurotoxicity, in this work we evaluated the potential protective effects of Vitamin D against MZ-induced behavioral, metabolic, and oxidative impairments in cerebral tissues using adult male and female Wistar rats.
Material and Methods
Chemicals
All chemicals were of analytical grade or the highest-level available pharmaceutical grade. MZ (manganese ethylene bis-dithiocarbamate with zinc salt) used was fom Unizeb Gold® (UPL do Brasil Indústria e Comércio de Insumos Agropecuários S. A.), and the vitamin D used was cholicalciferol (D3) 500 IU/drop from DePURA® (Sanofi-Aventis Pharmaceutical, S. A.).
Animals
Male and female Wistar rats were obtained from the Federal University of Rio Grande do Sul, UFRGS, Brazil, and allowed to acclimate in the animal facility of Universidade do Oeste de Santa Catarina (UNOESC) for 20 days after arrival. Rats were 6 months-old and weighed approximately 200–300 g for females and 300-500 g for males at the beginning of the study. Rats were housed standard conditions for temperature (24 ± 2 °C), humidity (30–70%), and light/dark cycle (12/12 h, lights on at 6 a.m.). Rats were kept in large cages with water and food (Neovia – Animal nutrition) ad libitum. All procedures on animals were approved by UNOESC ethics committee under n° 74/2019 protocol.
MZ and Vitamin D treatment in vivo
Male and female rats were treated with 40 mg/kg of MZ, intraperitoneally (i.p) twice a week for 6 weeks, according to Goldoni [24] with slight modifications. This dose was also confirmed by a pilot study. Control animals received 0.9% sterile saline i.p. instead. Vitamin D was administered twice a week, for 6 weeks by gavage method using a PE 190 probe at 12.5 μg/kg or 25 μg/kg, according to Salum et al. [25] and Kechrid et al. [26] and the Vitamin D administration was performed in the same days of the i.p. injections. It is important to highlight that the various recommendations for the prevention of vitamin D deficiency range in adulthood range from 400 to 800 IU/day (10–20 μg) [27]. As Vitamin D was supplied in its active form, it was not necessary to use UVB radiation for its synthesis [28]. The animals were weighed weekly.
Experimental Protocol
Separated by sex, animals were randomly allocated into the following groups: (i) control, (ii) MZ 40 mg/kg, (iii) Vit D 12.5 µg/kg, (iv) MZ 40 mg/kg + Vit D 12.5 µg/kg, and (v) MZ 40 mg/kg + Vit D 25 µg/kg. As the same groupings were made for male and female rats, there were ten experimental groups in total. Depending on the experiment, the number of animals changes and the “n” are informed in captions of the figures.
Behavioral Tests
Rotarod
Balance and motor coordination were assessed in the accelerating Rotarod apparatus (Insight Scientific Equipments, Ribeirão Preto, SP, Brazil), which consists of a grooved metal roller (6 cm diameter × 16 cm length) separated into four 9 cm wide compartments. Firstly, the animals were placed on the stationary roller until they could stay for 30 s to adapt to the task. To confirm their ability to perform the task, they were subjected to a basal test in the Rotarod at a constant speed of 12 rpm for 1 min. Balance and motor coordination of rats were tested in the accelerated Rotarod in a single session during which the rotation speed was progressively increased with an automatic increase of 0.1 revolutions per second over a maximum period of 300 s, and the latency to fall (in seconds) from the accelerating Rotarod was determined [29].
Elevated Plus-maze test
Anxiety-related responses were evaluated on the elevated plus-maze (EPM) test. The EPM apparatus is a cross-shaped wooden maze with two opposite open arms (50 cm × 10 cm) and two enclosed arms (50 cm × 10 cm × 40 cm) spreading out from a central platform (10 cm × 10 cm) elevated at a height of 50 cm from the floor. The animals were individually placed in the center of the EPM, facing one of the enclosed arms, and were allowed to freely explore the apparatus for 5 min [30]. The following parameters were measured: frequency of open arm entries, frequency of enclosed arm entries, and time spent on the open arms. An entry was counted whenever the animal placed four paws on a given arm of the maze. An anxiogenic effect is defined as a decrease in the frequency of open arm entries and/or time spent on the open arms.
Sample Preparation for Analysis
Six weeks after the first MZ administration, animals were anesthetized with ketamine 80 mg/kg and xylazine 12 mg/kg and euthanized by decapitation. The teeth were extracted using pliers. The brains were isolated, and the hippocampus, striatum, and cerebral cortex were immediately stored at − 86 °C until biochemical analysis.
Determination of serum vitamin D concentration
Serum vitamin D concentration was measured through blood collection during euthanasia and an analysis was performed by a Commercial Kit ichroma ™ Vitamin D by fluorescent immunoassay using the ichroma ™ II equipment. Detection range for the test was between 8 ~ 70 ng / mL.
Determination of Mn and Zn by Atomic Absorption Spectrometry
According to Fitsanakis et al. [31], with slightly modifications, 50 mg of brain structures, 300 µL of serum and 50 mg of teeth were prepared for analysis. Tissues were digested in 4:1 acid mix (1.2 mL of 65% nitric acid and 300 μL perchloric acid) for 24 h at room temperature. After that, the digested samples were kept in ultrasonic bath at 70 °C for 3 h and then 3.5 mL of 1% perchloric acid was added. The final samples were filtered at 0.22 μm syringe membrane. The concentration of metals was measured by Perkin Elmer, Atomic Absorption Spectrometer, AAnalyst 800®, with standard curves for calibration. Results were expressed as μg/g of tissue.
Mitochondrial Complex I and II Activities
Brain tissues were homogenized in 10 vol of 4.4 mM potassium phosphate buffer pH 7.4, containing 0.3 M sucrose, 5 mM MOPS, 1 mM EGTA and 0.1% bovine serum albumin. The homogenates were centrifuged at 3000 × g 4 °C for 10 min and the supernatant were used for measurement of mitochondrial complex I and II activity, according to Latini et al. [32]. Complex I activity was measured by the rate of NADH-dependent ferricyanide reduction at 420 nm as previously described by Cassina and Radi [33]. The activity of succinate-2.6-dichloroindophenol (DCIP)-oxidoreductase (complex II) was determined according to Fischer et al. [34]. The activity of the respiratory chain complexes was calculated in nmol/min/mg of protein, and were measured using a microplate reader, Synergy HTX, Biotek®, with controlled temperature (37 °C).
ROS Measurement
The tissue homogenate was incubated with 20 μM of 5- (e-6) -carboxy-2′7'-dichlorodihydrofluorescein diacetate (carboxy-H-DCFDA, Image-iT Live Green, Molecular Probes, Carlsbad, CA, USA) for 30 min at room temperature. The carboxy-H DCFDA was hydrolyzed by esterases to carboxy-DCFH which, in turn, reacted with ER (oxygen and nitrogen) generating a fluorescent carboxy-DCF product [35]. Fluorescence was measured at 485 nm excitation and 538 nm emission. The results were expressed in relative fluorescence units.
Determination of Antioxidant Enzymes Glutathione Peroxidase (GPx), Glutathione Reductase (GR) and Catalase (CAT)
GR activity was determined based on the research protocol of Calberg and Mannervik [36]. GR reduces from NADPH, GSSG to GSH, whose mechanism can be seen at 340 nm. GPx activity was determined according to the research protocol of Wendel [37] by indirect measurement of NADPH consumption at 340 nm. GPx uses GSH to reduce tert-butyl hydroperoxide producing GSSG which is reduced to GSH by the GR using NADPH as a reducing agent. CAT activity was measured using the method of Aebi et al. [38]. The reaction was initiated by the addition of a 30 mM H2O2 solution. The H2O2 decomposition rate was measured by spectrophotometry at 240 nm.
Protein Determination
Protein quantification was estimated by the method described by Lowry et al. [39] using bovine serum albumin as standard.
Statistical Analysis
The results were expressed as mean ± standard error of the mean. For the analysis of body weight gain, ANOVA was used for repeated measures followed by Tukey's post-hoc test. The differences between the groups evaluated were analyzed by one-way or two-way ANOVA to verify the interaction between the group and sex, both followed by Tukey's post-hoc test. Pearson’s correlation was performed to correlate the ROS production and Mn concentration (In the correlation 10 animals from mancozeb group and control group were used to calculate, i.e., vitamin D groups were excluded for these analysis). The “n” refers to the number of animals included in each test. Only significant values were cited in the text. Differences were considered significant when P < 0.05. The analyzes were performed using the Statistica® version 7.0 program and the graphics were made using the Graph Pad Prism® software version 8 (GraphPad Software, San Diego, CA, USA).
Results
In comparison to the group that received MZ only, the measured concentration of vitamin D was higher in rats of all groups treated with it by gavage [F(3.16) = 3.86; p < 0.05] (Fig. 1B), with no difference between sexes [F(1.16) = 0.042; p = 0.838] or between groups and sexes [F(3.16) = 0.67; P = 0.58] (Fig. 1A). Also, it was observed that both males (Fig. 1C) and females (Fig. 1D) treated with MZ had a significantly lower weight gain during the early stages of the experiment, but at the end of the period, the weight gain was similar to the control group. The same was observed in the groups that received the MZ and vitamin D in the two doses. Among males, were found differences between groups [F(4,25) = 10.99; p < 0.001], interaction between treatment weeks [F(5,24) = 2.73; p < 0.05]) and between groups and treatment weeks [F(18.144) = 12.55; p < 0.0001]. Considering the females, there were differences between groups [F(4,25) = 8.10; p < 0.001], interaction between treatment weeks [F(5,24) = 0.89; p = 0.227]) and between groups and treatment weeks [F(18.144) = 2.87; p < 0.05].
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on serum Vitamin D concentration (A and B) and on body weight gain in adult male (C) and female (D) Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group). *p < 0.05 when compared to MZ group; #p < 0.05 when compared to control group (Two-way ANOVA and for repeated measurements followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
In our study, the motor changes were investigated using the Rotarod test and are shown in Fig. 2A. For females, there was a significant difference between groups [F(1.19) = 3.84; p = 0.0433], with significantly lower latency to fall in the MZ group (p < 0.05). Among males, there was not significant difference between groups, although the MZ group had lower mean latency to fall than the other groups. Additionally, for females, treatment with vitamin D prevented the motor impairment caused by the pesticide, as the latency to fall for both MZ + Vit D at 12.5 and 25 µg/kg did not differ from the control group.
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on Rotarod test (A) and on EPM test (B-D) in adult male and female Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group). *p < 0.05; **p < 0.01; ***p < 0.001 when compared to control group; (Two-way ANOVA followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
Considering the number of times, the animals entered the closed arms in the EPM task, there were no significant differences between groups (Fig. 2B). There was a significant decrease in the time spent in the open arms when comparing the MZ and the control group [F(4.77) = 6.38; p < 0.001] (Fig. 2C). The same could be observed for the percentage (%) of animals entering the open arms [F(4.77) = 6.62; p < 0.001] (Fig. 2D). Both variables show that there is a distinction between all groups when compared to the group that was treated with MZ only. Considering the interaction between sex and group, no significant differences were observed. However, for the variables referring to entry into the open arms and percentage of time in the open arms there was a difference between the categorical variable "sex" ([F(1.77) = 7.11; p = 0.0204] (Fig. 2C) and [F(1.77) = 8.23; p = 0.0053] (Fig. 2D), respectively). This result shows that, in general, females stayed longer in the open arms. However, statistical group and sex interaction analysis revealed no differences for these parameters. This means, in general terms, that these parameters did not differ according to the sex of the animals.
It is also noteworthy that the group treated with vitamin D only did not differ from the control group and was equivalent to the groups treated with MZ, which provides evidence that treatment with both doses of the vitamin D not only prevent the anxiogenic effect, but also normalized the effects when compared to the control group.
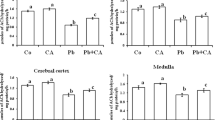
Biochemical and metabolic parameters regarding to the concentration of Mn in the cerebral cortex (A), striatum (B), hippocampus (C), serum (D), and tooth (E) of rats treated with MZ are shown in Fig. 3. It was observed a significant increase in the concentration of Mn in the hippocampi of male and female rats that received MZ and in the striatum of female rats. Moreover, there was a significant increase of in the concentration of Mn in teeth of both sexes and the treatment with vitamin D did not alter this parameter. Hippocampus: Difference between groups [F(4.41) = 6,26; p < 0.001] and interaction between the sexes [F(1.41) = 2.43; p < 0.01]; striatum: between groups [F(4.41) = 13.91; p < 0.001]; tooth: between groups [F(4.75) = 192.4; p < 0.001], between sexes [F(1.75) = 71.1; p < 0.001] and interaction between group and sex [F(4.75) = 12.4; p < 0.001].
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on cerebral cortex (A), striatum (B), hippocampus (C), serum (D) and tooth (E) Mn concentration in adult male and female Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group). *p < 0.05; ***p < 0.001 when compared to control group; #p < 0.05 when compared to MZ group (Two-way ANOVA followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
Figure 4 shows the Zn concentration in the same tissues, cerebral cortex (A), striatum (B), hippocampus (C) serum (D) and tooth (E) from intoxicated male and female Wistar rats. There was no significant difference between the groups and sexes.
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on cerebral cortex (A), striatum (B), hippocampus (C), serum (D) and tooth (E) Zn concentration in adult male and female Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group) (Two-way ANOVA followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
Exposure to MZ led to a significant inhibition of female mitochondrial complex I activity in cerebral cortex (Fig. 5A). In addition, it was possible to observe that the administration of Vitamin D prevented this inhibition in both doses [F(4.43) = 8,05; p < 0.001] and two-way ANOVA showed significant interaction between groups and sexes [F(4.90) = 19,24; p < 0.001]. On the other hand, the activity of the mitochondrial complex II and the generation of EROs did not show significant difference between the groups and sexes (Fig. 5B and 5C, respectively). Regarding to oxidative parameters, it was observed a significant reduction of GPx [F(4.19) = 3.48; p < 0.05] and GR activity [F(4.19) = 3.70; p < 0.05] from male rats treated with MZ. Other than that, CAT activity was significantly reduced in both sexes and the vitamin D treatment was effective in preventing all oxidative impairments (difference between males [F(4.15) = 3.50; p < 0.05] and females [F(4.16) = 4,20; P < 0.05] and interaction between group and sex [F(4.21) = 11.10; p < 0.05]). Additionally, all inhibitions caused by MZ were prevented by the administration of Vitamin D.
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on complex I (A) and II (B) activities, ROS generation (C), GPx (D), GR (E) and catalase (F) activities in cerebral cortex from adult male and female Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group). *p < 0.05; ***p < 0.001 when compared to control group; #p < 0.05; ###p < 0.001 when compared to MZ group (Two-way ANOVA followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
In the striatum, there were no differences in the activity of the mitochondrial complex I and II between groups. However, there was an increase in the production of ROS in females intoxicated with MZ in comparison to the control group and Vitamin D at the dose of 25 µg prevented this effect [F(4.52) = 6,01; p < 0.001]) (Figure C). In addition, there was a significant difference in the generation of ROS when comparing the MZ-treated groups by sex (difference between the sexes [F(4.90) = 34.40; p < 0.001] and interaction between groups and sexes [F(4.90) = 20.14; p < 0.001]). It was observed a significant reduction of GR activity [F(4.19) = 14.22; p < 0.05] from male rats that received MZ and two-way analysis of variance revealed significant interaction between group and sex [F(4.21) = 20.78; p < 0.001] (Fig. 6B). In addition, CAT activity showed the same pattern of decrease of males that received MZ [F(4.17) = 3.96; p < 0.05] (Fig. 6C).
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on complex I (A) and II (B) activities, ROS generation (C), GPx (D), GR (E) and catalase (F) activities in striatum from adult male and female Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group). *p < 0.05; ***p < 0.001 when compared to control group; #p < 0.05; ###p < 0.001 when compared to MZ group; &&&p < 0.001 when compared between sexes (Two-way ANOVA followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
Mitochondrial complex I activity was significantly reduced in the hippocampus from females intoxicated with MZ and the Vitamin D prevented this effect [F(4.41) = 10.66; p < 0.001] (Fig. 7A). In addition, there was a significant interaction between sexes [F(4.90) = 13.40; p < 0.001] and between groups and sexes [F(4.90) = 9.55; p < 0.05]. However, there was no significant difference in complex II activity between groups (Fig. 7B). There was also an increase in the production of ROS in the group of female rats treated with MZ and the vitamin D was effective in protecting this increase at 12.5 µ/kg [F(4.53) = 8.18; p < 0.001] (Fig. 7C). There was also a significant interaction between the sexes [F(4.90) = 52.48; p < 0.001] and between groups and sexes [F(4.90) = 15.11; p < 0.01]. A significant inhibition in CAT activity was observed in males intoxicated by MZ [F(4.18) = 5.47; p < 0.01] (Fig. 7F). GPx and GR activities were not different among groups (Fig. 7D and 7E, respectively).
Effect of Mancozeb (40 mg/kg; twice/week for 6 weeks; i.p.) and/or Vitamin D (12.5 µg/kg or 25 µg/kg; twice/week for 6 weeks; gavage) administration on complex I (A) and II (B) activities, ROS generation (C), GPx (D), GR (E) and catalase (F) activities in hippocampus from adult male and female Wistar rats. The data represent mean ± standard error of the mean (n = 3 to 6 animals per group). *p < 0.05; **p < 0.01 when compared to control group; #p < 0.05; ###p < 0.001 when compared to MZ group; &&&p < 0.001 when compared between sexes (Two-way ANOVA followed by Tukey's post-hoc test). MZ: Mancozeb; Vit D: Vitamin D
Finally, Fig. 8 demonstrates a significant positive correlation between Mn deposition and ROS generation in the striatum of male (Fig. 8A; r = 0,7466) and female (Fig. 8B; 0,8366) rats exposed to MZ.
Discussion
Although dithiocarbamate derived compounds are characterized by short persistence in the environment, causing mild acute toxicity upon exposure, MZ is known to have additional long-term toxic effects of primary concern, due to its isothiocyanate skeletons, with molecular elements showing elevated binding capacity which may inhibit human enzymes, thereby affecting the interested biological systems and increasing the risk of endocrine disruption, cancer transformation, and neuronal damage [40, 41]. The production and market volumes of MZ have kept an increasing trend for several decades. This is because MZ has a broad-spectrum efficacy towards a variety of plant diseases and quite a low purchase price. From 2021, with a grace period that ended in January 2022, MZ use as pesticide has been banned within the whole European Union (EU) due to the observed reproductive toxicity and endocrine disrupting properties [42],European Food Safety Authority (EFSA) et al., [43]; European Union, [44]). Nevertheless, in several countries, MZ is still largely employed in the agriculture sector [45].
The present work provided evidence that exposure to MZ contributes to anxious behavior and to the development of oxidative and metabolic dysfunction in the brain, with different responses between sexes. There was also an increased accumulation of Mn in the tissues analyzed, especially in hippocampus, striatum, and tooth. Treatment with vitamin D provided neuroprotective effects in rats exposed to the MZ.
A significant decrease in body weight gain was observed in male and female rats treated with MZ, even though animals from all groups gained weight throughout the six weeks of experiments. A decrease of body weight gain, especially in males intoxicated with Mn, is well described [46, 47], as an increase in inflammatory proteins leads to oxidative stress, which may induce muscle loss and inhibition of appetite [48].
We found a high concentration of Mn especially in hippocampus and tooth of both male and female rats and in the striatum in female rats. On the other hand, the Zn concentration in these tissues was not modified by the treatments. A possible explanation is that Mn constitutes around 15% of the composition of MZ, while Zn corresponds to only 1.87%, which may also be related to the fact that Mn is the one most associated with the pathophysiological outcomes associated with the pesticide [49]. The increased concentration of Mn in the teeth is in accordance with previous studies. Austin et al. [50] and Liang et al. [51] validated biomarkers for Mn exposure by assessing the relationships between Mn in the teeth and a clear dose–response relationship in rats treated with Mn orally and intraperitoneally. Thus, Mn in teeth could be a good indication of cumulative exposure to MZ. Serum Mn concentration did not differ between groups because of the rapid clearance of Mn in blood, since the half-life of Mn in human blood is relatively short about four days [52].
In comparison to males, female rats exposed to MZ showed a lower latency to fall from the Rotarod apparatus. According to Peres et al. [53], male Wistar rats intoxicated with 10 and 20 mg/kg/day of MnCl2 performed worse on the Rotarod test than controls, indicating motor and balance impairments. It is known that Mn exposure can result in significant neurological and motor deficits, as it can affect dopaminergic pathways and the development of brain structures [54]. Also, the anxiety profile was observed in both sexes exposed to MZ and the administration of Vitamin D prevented these behavioral impairments. In a work with zebrafish, it was noted that changes in behavior due to exposure to MZ happened before biochemical alterations could be detected, and even at concentrations that did not lead to changes in morphology or mortality among embryos and larvae [1].
According to a meta-analysis by Rimmelzwaan et al. [55], treatment with the active hormone of vitamin D has a protective effect on dopaminergic neurons and on the symptomatology of Parkinson’s disease. Also, vitamin D has a role in the signaling of dopaminergic neurons, increasing the concentration of VDR, production of dopamine through increasing tyrosine hydroxylase, and protecting against subsequent oxidative damage. Thus, vitamin D can act in the ontogeny of dopaminergic systems [56].
Therefore, the brain is a major target organ of Mn toxicity. It is absorbed by mitochondria through uniport calcium channels, and the excess of Mn inhibits mitochondrial respiratory chain complexes and impairs ATP synthesis [57,58,59,60,61]. Mn also induces mitochondrial permeability transition (mPT) and releases cell death mediators to the cytoplasm [59, 60]. Thus, ROS formation and mitochondrial dysfunction are pointed out as the main mechanisms involved [62].
In rat brains, Mn accumulates with the highest concentration in the globus pallidus, followed by the substantia nigra pars compacta, hippocampus, and thalamus [63]. Ivleva et al. [64] performed the intranasal administration of MnCl2 in male Wistar rats and found accumulated Mn in hippocampal cells and mainly in the striatum, which led to a dysregulation of dopamine levels in these tissues. Here we found the Mn accumulation preferably in hippocampus of both sexes and in the striatum of the females.
Previous studies by Domico et al. [65] demonstrated that 15 mM of MZ and Maneb, a similar pesticide, promoted a mitochondrial electron transport chain dysfunction, and doses greater than 30 mM inhibited this process. Furthermore, when they used Nabam, another pesticide that contains Sodium (Na) in its structure, it became less toxic than MZ and Maneb, so when adding MnCl2 to Nabam, it increased its toxicity to the same extent as the other two pesticides, suggesting that Mn plays a key role in cellular toxicity.
Regarding Zn, also present in MZ composition, Costa-Silva et al. [1] did not find increased levels of this metal in carp exposed to MZ. In another work, Domico et al. [65] whose experiments were carried out with ZnCl2 associated with Nabam, did not demonstrate similar neurotoxic potential. In our study, the Zn concentration did not show significant differences between groups in all tissues evaluated.
In agreement with these findings, we observed an inhibition of mitochondrial complex I activity in brain tissues such as the hippocampus and cerebral cortex of female rats alongside Mn accumulation in the hippocampus of both sexes and striatum of the females. Also, it was found an increase in ROS generation and administration of Vitamin D prevented these effects. Costa-Silva et al. [1] also detected increased levels of ROS in brain tissues of carp exposed to MZ.
Another study by Zhang et al. [61], suggested that Mn associated with the organic complex ethylene bis-dithiocarbamate, despite promoting inhibition dose-dependent of complex I and complex II, preferentially inhibited the mitochondrial function of complex III in vitro. They also observed that this association causes selective striatal dopamine efflux, as well as dopaminergic neurodegeneration and suggested the hypothesis that this process is linked to the observed mitochondrial inhibition. However, we did not found changes on mitochondrial complex II activity in all investigated tissues.
In another study, Morales-Ovalles et al. [2], demonstrated the neurochemical variations of the hypothalamus after i.p. administration of MZ in mice, both in low (30 mg/kg) and high doses (90 mg/kg), for six weeks. They observed the development of cytotoxicity and excitotoxicity in the hypothalamus of young adult mice, mechanisms by which nerve cells are damaged and killed by over-stimulation of neurotransmitters such as glutamate and aspartate. Thus, the cytotoxicity caused by MZ can be explained through its action on extrinsic and intrinsic pathways, which is associated with intracellular stress such as DNA damage, oxidative stress, cytosolic calcium overload, and excitotoxicity, among others, and is known that MZ can induce oxidative stress, catalyze the production of ROS, and inhibit mitochondrial respiration. In this regard, a significant positive correlation was found in this study between the deposition of Mn and the generation of ROS, especially in striatum for male and female rats.
Another important factor, related to oxidative stress, is the activity of antioxidant enzymes that provide protection against this process. Antioxidant molecules are recruited to protect the biological system by preventing oxidative processes. Glutathione is the first line of defense against oxidative stress that protects cells from ROS. Also, superoxide dismutase (SOD) which is responsible for the disproportionation of superoxide anion and catalase which converts hydrogen peroxide into oxygen and water, are the main antioxidant enzymes in the brain [66].
Patel et al. [67] found an increase of CAT activity in the striatum when Swiss albino mice were concomitantly exposed, i.p., to the pesticides Maneb and Paraquat, which was not observed when administered individually. In our work, on the other hand, CAT was the most sensitive enzyme to inhibition, reinforcing the role of oxidative stress in MZ-induced toxicity [1]. Overproduction of ROS in this case might be related to the loss of neural cells, culminating in neurobehavioral deficits [68]. Thus, different sources of ROS by Mn include the oxidation of Mn2+ to Mn3+, wich catalyzes DA oxidation with the formation of toxic and reactive intermediaries [69]. Also, Mn can increase the proportion of Fe (II), wich can then prompt oxidative stress via the Fenton reaction [70]. Thus, the accumulation of Mn caused by MZ exposure could contribute to oxidative damage and behavioral impairment in flies by some of the mentioned mechanisms.
The neurological role of vitamin D can be evidenced by the presence of nuclear VDR in brain tissues of adult rats. In tests carried out by Bayo-Olugbami [71] with VDR knockout mice, it was shown that the animals had impaired motor function in behavioral tests, demonstrating that these receptors are important for neuronal control. Da Silva et al. [72] demonstrated in their study that vitamin D may also be related to a neuronal protection mechanism against Mn. They found that vitamin D acts on the brain by inducing the expression of a manganese-carrying enzyme called ZnT10, which, in reduced concentrations, is associated with the occurrence of manganism. This transporter provides neurological protection against Mn by promoting its efflux. In our study, Vitamin D was able to prevent the mitochondrial complex I inhibition and the increase of ROS, as well as the decrease of the antioxidant capacity in the evaluated brain tissues, which was most evident in the cerebral cortex.
Our study also found relevant differences between sexes. According to Schmitz et al. (2019), liver damage was identified in female rats intoxicated with subacute Mn, showing a different accumulation pattern of Mn in the body of males and females, with a more expressive accumulation among females.
Oxidative stress and mitochondrial functions are sexually dysmorphic depending on the affected brain area, normally women have greater defense by antioxidant enzymes and less ROS production [73]. In a study with midbrain cells exposed to oxidopamine, males had higher levels of ROS and less expression of mitochondrial proteins when compared to females [74]. In our study, there was increase in ROS generation predominantly in female´s striatum and hippocampus.
In addition, in vivo and in vitro experiments have shown that females have higher levels of PON2 gene expression in mitochondria and that these receptors are modulated by estrogen. This relationship was demonstrated with a reduction in the expression of PON2 among ovariectomized females and with an increase among males treated with estradiol, which appears to act as a potent antioxidant [75].
It has been shown that female rats aged 23 to 24 weeks can begin to present irregularities in the estrous cycle corroborating to the loss of neuronal protection among females [76]. Also, the environmental exposure to the pesticides can induce changes in the estrous cycle that can lead to early reproductive senescence, in addition to changes that have already been demonstrated in oocytes and ovarian follicles [77].
A general impairment of Wistar rats estrous cycle treated with MZ 500 at 800 mg/kg for 30 days was observed in comparison with controls [78]. Morphometric analysis of albino rats, intoxicated with oral MZ (700 mg/kg) showed a time-dependent destruction of the estrous cycle and an increase in atretic follicles and pathological changes in the gonads and uterus [78]. Our study did not evaluate hormonal and gonadal parameters, but the available evidence suggests that these parameters could explain the differences found in females.
Even though animals had the same age, the males expectedly had a greater body mass. This might limit our conclusions in three aspects: (i) increased body fat might alter MZ and vitamin D pharmacodynamics, with potential effects on absorption and distribution; (ii) the different basal body weight between sexes possibly had an impact in the differences in weight variation along the study; and (iii) even with the same training protocol, males might have had more difficulty to adapt to the Rotarod apparatus, due to their larger and heavier bodies.
Conclusions
This study showed that MZ administration contributes to anxious-like behavior, motor impairments, and metabolic and oxidative dysfunction in cerebral tissues. Female rats appeared to be more sensitive to develop anxious behavior and inhibition of mitochondrial complexes, while males demonstrated greater impairments in the activity of enzymes. Vitamin D was able to protect against most outcomes. It is important to re-evaluate the abundant use of pesticides in agriculture and the consequences of lifetime human exposure to prevent the potential damage on health associated with this practice, which represents a serious problem in public health. On the other hand, our work also brings novelty in the scenario, providing more evidence on the neuroprotective role of vitamin D, although this role has already been demonstrated against different neurotoxicity mechanisms, in our work, for the first time, against MZ-induced toxicity, providing new perspectives on pesticides intoxication treatment.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Costa-Silva DG, Lopes AR, Martins IK, Leandro LP, Nunes MEM, de Carvalho NR, Rodrigues NR, Macedo GE et al (2018) Mancozeb exposure results in manganese accumulation and Nrf2-related antioxidant responses in the brain of common carp Cyprinus carpio. Environ Sci Pollut Res Int 25:15529–15540. https://doi.org/10.1007/s11356-018-1724-9
Morales-Ovalles Y, Miranda-Contreras L, Peña-Contreras Z, Dávila-Vera D, Balza-Quintero A, Sánchez-Gil B, Mendoza-Briceño RV (2018) Developmental exposure to mancozeb induced neurochemical and morphological alterations in adult male mouse hypothalamus. Environ Toxicol Pharmacol 64:139–146. https://doi.org/10.1016/j.etap.2018.10.004
Runkle J, Flocks J, Economos J, Dunlop AL (2017) A systematic review of Mancozeb as a reproductive and developmental hazard. Environ Int 99:29–42. https://doi.org/10.1016/j.envint.2016.11.006
Al-Alam J, Bom L, Chbani A, Fajloun Z, Millet M, (2016) Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption Spectrometry. J Chromatogr Sci chromsci;bmw198v1. https://doi.org/10.1093/chromsci/bmw198
US Environmental Protection Agency (2005) Reregistration eligibility decision for mancozeb, List B, case No. 0643. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-014504_20-Sep-05.pdf. Accessed 13 Mar 2023
Balaji B, Rajendar B, Ramanathan M (2014) Quercetin protected isolated human erythrocytes against mancozeb-induced oxidative stress. Toxicol Ind Health 30:561–569. https://doi.org/10.1177/0748233712462465
Pirozzi AVA, Stellavato A, La Gatta A, Lamberti M, Schiraldi C (2016) Mancozeb, a fungicide routinely used in agriculture, worsens nonalcoholic fatty liver disease in the human HepG2 cell model. Toxicol Lett 249:1–4. https://doi.org/10.1016/j.toxlet.2016.03.004
Srivastava AK, Mishra S, Ali W, Shukla Y (2016) Protective effects of lupeol against mancozeb-induced genotoxicity in cultured human lymphocytes. Phytomedicine 23:714–724. https://doi.org/10.1016/j.phymed.2016.03.010
Pignati WA, Lima FAN de S e, Lara SS de, Correa MLM, Barbosa, JR, Leão LH da C, Pignatti MG, (2017) Distribuição espacial do uso de agrotóxicos no Brasil: uma ferramenta para a Vigilância em Saúde. Ciênc. saúde coletiva 22:3281–3293. https://doi.org/10.1590/1413-812320172210.17742017
Rigotto RM, e Vasconcelos DP, Rocha MM (2014) Pesticide use in Brazil and problems for public health. Cad Saúde Pública 30:1360–1362. https://doi.org/10.1590/0102-311XPE020714
Iorio R, Castellucci A, Rossi G, Cinque B, Cifone MG, Macchiarelli G, Cecconi S (2015) Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol In Vitro 30:438–445. https://doi.org/10.1016/j.tiv.2015.09.018
Astiz M, Acaz-Fonseca E, Garcia-Segura LM (2014) Sex differences and effects of estrogenic compounds on the expression of inflammatory molecules by astrocytes exposed to the insecticide dimethoate. Neurotox Res 25:271–285. https://doi.org/10.1007/s12640-013-9417-0
Yardimci M, Sevgiler Y, Rencuzogullari E, Arslan M, Buyukleyla M, Yilmaz M (2014) Sex-, tissue-, and exposure duration-dependent effects of imidacloprid modulated by piperonyl butoxide and menadione in rats. Part I: oxidative and neurotoxic potentials. Arh Hig Rada Toksikol 65:387–398. https://doi.org/10.2478/10004-1254-65-2014-2554
Groves NJ, McGrath JJ, Burne THJ (2014) Vitamin D as a Neurosteroid Affecting the Developing and Adult Brain. Annu Rev Nutr 34:117–141. https://doi.org/10.1146/annurev-nutr-071813-105557
Lee PW, Selhorst A, Lampe SG, Liu Y, Yang Y, Lovett-Racke AE (2020) Neuron-Specific Vitamin D Signaling Attenuates Microglia Activation and CNS Autoimmunity. Front Neurol 11:19. https://doi.org/10.3389/fneur.2020.00019
Lazzara F, Amato R, Platania CBM, Conti F, Chou T-H, Porciatti V, Drago F, Bucolo C (2021) 1α,25-dihydroxyvitamin D3 protects retinal ganglion cells in glaucomatous mice. J Neuroinflammation 18:206. https://doi.org/10.1186/s12974-021-02263-3
Lazzara F, Longo AM, Giurdanella G, Lupo G, Platania CBM, Rossi S, Drago F, Anfuso CD et al (2022) Vitamin D3 preserves blood retinal barrier integrity in an in vitro model of diabetic retinopathy. Front Pharmacol 13:971164. https://doi.org/10.3389/fphar.2022.971164
Kumar RR, Singh L, Thakur A, Singh S, Kumar B (2022) Role of Vitamins in Neurodegenerative Diseases: A Review. CNS Neurol Disord Drug Test 21:766–773. https://doi.org/10.2174/1871527320666211119122150
Moretti R, Morelli ME, Caruso P (2018) Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int J Mol Sci 19:E2245. https://doi.org/10.3390/ijms19082245
Wimalawansa SJ (2019) Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 8:30. https://doi.org/10.3390/biology8020030
Hafez AA, Naserzadeh P, Ashtari K, Mortazavian AM, Salimi A (2018) Protection of manganese oxide nanoparticles-induced liver and kidney damage by vitamin D. Regul Toxicol Pharmacol 98:240–244. https://doi.org/10.1016/j.yrtph.2018.08.005
Mokhtari Z, Hekmatdoost A, Nourian M (2016) Antioxidant efficacy of vitamin D. J Parathyr Dis 5:11–16
Özerkan D, Özsoy N, Akbulut KG, Güney Ş, Öztürk G (2017) The protective effect of vitamin D against carbon tetrachloride damage to the rat liver. Biotech Histochem 92:513–523. https://doi.org/10.1080/10520295.2017.1361549
Goldoni A, Klauck C, Da Silva S, Da Silva M, Ardenghi P, Da Silva LB (2014) DNA damage in Wistar rats exposed to dithiocar-bamate pesticide mancozeb. Folia Biol 60:202–204
Salum E, Kals J, Kampus P, Salum T, Zilmer K, Aunapuu M, Arend A, Eha J et al (2013) Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res Clin Pract 100:243–249. https://doi.org/10.1016/j.diabres.2013.03.008
Kechrid Z, Hamdi M, Naziroğlu M, Flores-Arce M (2012) Vitamin D supplementation modulates blood and tissue zinc, liver glutathione and blood biochemical parameters in diabetic rats on a zinc-deficient diet. Biol Trace Elem Res 148:371–377. https://doi.org/10.1007/s12011-012-9383-z
Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, Grant WB, Pludowski P et al (2018) Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front Endocrinol 9:373. https://doi.org/10.3389/fendo.2018.00373
Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Calou IBF, Andrade GM, Viana GSB (2018) Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflammation 15:249. https://doi.org/10.1186/s12974-018-1266-6
Jiang C, Wan X, Jankovic J, Christian ST, Pristupa ZB, Niznik HB, Sundsmo JS, Le W (2004) Dopaminergic Properties and Experimental Anti-Parkinsonian Effects of IPX750 in Rodent Models of Parkinson Disease. Clin Neuropharmacol 27:63–73. https://doi.org/10.1097/00002826-200403000-00004
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. https://doi.org/10.1016/0165-0270(85)90031-7
Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M (2008) Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci 103:116–124. https://doi.org/10.1093/toxsci/kfn019
Latini A, da Silva CG, Ferreira GC, Schuck PF, Scussiato K, Sarkis JJ, Dutra Filho CS, Wyse ATS et al (2005) Mitochondrial energy metabolism is markedly impaired by d-2-hydroxyglutaric acid in rat tissues. Mol Genet Metab 86:188–199. https://doi.org/10.1016/j.ymgme.2005.05.002
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316. https://doi.org/10.1006/abbi.1996.0178
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JMF, Veerkamp JH, Stadhouders AM, Sengers RCA, Janssen AJM (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36. https://doi.org/10.1016/0009-8981(85)90135-4
Ali SF, LeBel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13:637–648
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490. https://doi.org/10.1016/s0076-6879(85)13062-4
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333. https://doi.org/10.1016/s0076-6879(81)77046-0
Aebi H, Wyss SR, Scherz B, Skvaril F (1974) Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem 48:137–145. https://doi.org/10.1111/j.1432-1033.1974.tb03751.x
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Costa C, Teodoro M, Rugolo CA, Alibrando C, Giambò F, Briguglio G, Fenga C (2020) MicroRNAs alteration as early biomarkers for cancer and neurodegenerative diseases: New challenges in pesticides exposure. Toxicol Rep 7:759–767. https://doi.org/10.1016/j.toxrep.2020.05.003
Dallagnol JC, Ferri Pezzini M, Suarez Uribe N, Joveleviths D (2021) Systemic effects of the pesticide mancozeb – A literature review. Eur Rev Med Pharmacol Sci 25:4113–4120. https://doi.org/10.26355/eurrev_202106_26054
Bao J, Zhang Y, Wen R, Zhang L, Wang X (2022) Low level of mancozeb exposure affects ovary in mice. Ecotoxicol Environ Safety 239:113670. https://doi.org/10.1016/j.ecoenv.2022.113670
European Food Safety Authority (EFSA), Abdourahime H, Anastassiadou M, Arena M, Auteri D, Barmaz S, Brancato A, Bura L, et al, (2020) Peer review of the pesticide risk assessment of the active substance mancozeb. EFS2 18. https://doi.org/10.2903/j.efsa.2020.5755
European Union (2020) Commission Implementing Regulation (EU) 2020/2087. OJ L 423:50-52. https://eur-lex.europa.eu/eli/reg_impl/2020/2087/oj. Accessed 13 Mar 2023
Panis C, Candiotto LZP, Gaboardi SC, Gurzenda S, Cruz J, Castro M, Lemos B (2022) Widespread pesticide contamination of drinking water and impact on cancer risk in Brazil. Environ Int 165:107321. https://doi.org/10.1016/j.envint.2022.107321
Huang P, Chen C, Wang H, Li G, Jing H, Han Y, Liu N, Xiao Y et al (2011) Manganese effects in the liver following subacute or subchronic manganese chloride exposure in rats. Ecotoxicol Environ Saf 74:615–622. https://doi.org/10.1016/j.ecoenv.2010.08.011
Richter Schmitz CR, Eichwald T, Branco Flores MV, Varela KG, Mantovani A, Steffani JA, Glaser V, de Carvalho D et al (2019) Sex differences in subacute manganese intoxication: Oxidative parameters and metal deposition in peripheral organs of adult Wistar rats. Regul Toxicol Pharmacol 104:98–107. https://doi.org/10.1016/j.yrtph.2019.03.005
Nihi MM, Manfro RC, Martins C, Suliman M, Murayama Y, Riella MC, Lindholm B, do Nascimento MM (2010) Associação entre gordura corporal, inflamação e estresse oxidativo na hemodiálise. J Bras Nefrol 32:11–17. https://doi.org/10.1590/S0101-28002010000100003
Ksheerasagar RL, Kaliwal BB (2003) Temporal effects of mancozeb on testes, accessory reproductive organs and biochemical constituents in albino mice. Environ Toxicol Pharmacol 15:9–17. https://doi.org/10.1016/j.etap.2003.08.006
Austin C, Richardson C, Smith D, Arora M (2017) Tooth manganese as a biomarker of exposure and body burden in rats. Environ Res 155:373–379. https://doi.org/10.1016/j.envres.2017.03.004
Liang G, Zhang L, Ma S, Lv Y, Qin H, Huang X, Qing L, Li Q et al (2016) Manganese accumulation in hair and teeth as a biomarker of manganese exposure and neurotoxicity in rats. Environ Sci Pollut Res Int 23:12265–12271. https://doi.org/10.1007/s11356-016-6420-z
Baker MG, Stover B, Simpson CD, Sheppard L, Seixas NS (2016) Using exposure windows to explore an elusive biomarker: blood manganese. Int Arch Occup Environ Health 89:679–687. https://doi.org/10.1007/s00420-015-1105-3
Peres T, Eyng H, Lopes S, Colle D, Gonçalves G, Venske D, Lopes M, Ben J, et al, (2015) Developmental exposure to manganese induces lasting motor and cognitive impairment in rats. Neurotoxicol 50. https://doi.org/10.1016/j.neuro.2015.07.005
Cordova FM, Aguiar AS, Peres TV, Lopes MW, Gonçalves FM, Pedro DZ, Lopes SC, Pilati C et al (2013) Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch Toxicol 87:1231–1244. https://doi.org/10.1007/s00204-013-1017-5
Rimmelzwaan LM, van Schoor NV, Lips P, Berendse H, Eekhoff EM (2016) Systematic Review of the Relationship between Vitamin D and Parkinson’s Disease. J Parkinson’s Dis. https://doi.org/10.3233/JPD-150615
Pertile RAN, Cui X, Eyles DW (2016) Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience 333:193–203. https://doi.org/10.1016/j.neuroscience.2016.07.020
Malecki EA (2001) Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull 55:225–228. https://doi.org/10.1016/S0361-9230(01)00456-7
Ommati MM, Heidari R, Ghanbarinejad V, Aminian A, Abdoli N, Niknahad H (2020) The neuroprotective properties of carnosine in a mouse model of manganism is mediated via mitochondria regulating and antioxidative mechanisms. Nutr Neurosci 23:731–743. https://doi.org/10.1080/1028415X.2018.1552399
Rao KVR, Norenberg MD (2004) Manganese Induces the Mitochondrial Permeability Transition in Cultured Astrocytes. J Biol Chem 279:32333–32338. https://doi.org/10.1074/jbc.M402096200
Verity MA (1999) Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology 20:489–497
Zhang S, Zhou Z, Fu J (2003) Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ Res 93:149–157. https://doi.org/10.1016/S0013-9351(03)00109-9
da Silva EB, Eichwald T, Glaser V, Varela KG, Baptistella AR, de Carvalho D, Remor AP (2022) Protective Effects of Probucol on Different Brain Cells Exposed to Manganese. Neurotox Res 40:276–285. https://doi.org/10.1007/s12640-021-00458-3
Robison G, Zakharova T, Fu S, Jiang W, Fulper R, Barrea R, Marcus MA, Zheng W et al (2012) X-ray fluorescence imaging: a new tool for studying manganese neurotoxicity. PLoS One 7:e48899. https://doi.org/10.1371/journal.pone.0048899
Ivleva I, Pestereva N, Zubov A, Karpenko M (2020) Intranasal exposure of manganese induces neuroinflammation and disrupts dopamine metabolism in the striatum and hippocampus. Neurosci Lett 738:135344. https://doi.org/10.1016/j.neulet.2020.135344
Domico LM, Cooper KR, Bernard LP, Zeevalk GD (2007) Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology 28:1079–1091. https://doi.org/10.1016/j.neuro.2007.04.008
Garg G, Singh S, Singh AK, Rizvi SI (2018) N-acetyl-l-cysteine attenuates oxidative damage and neurodegeneration in rat brain during aging. Can J Physiol Pharmacol 96:1189–1196. https://doi.org/10.1139/cjpp-2018-0209
Patel S, Singh V, Kumar A, Gupta YK, Singh MP (2006) Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: Mechanism of neurodegeneration. Brain Res 1081:9–18. https://doi.org/10.1016/j.brainres.2006.01.060
Behl C, Moosmann B, (2002) Oxidative Nerve Cell Death in Alzheimers Disease and Stroke: Antioxidants as Neuroprotective Compounds. Biol Chem 383. https://doi.org/10.1515/BC.2002.053
Diazveliz G (2004) Behavioral effects of manganese injected in the rat substantia nigra are potentiated by dicumarol, a DT-diaphorase inhibitor. Pharmacol Biochem Behav 77:245–251. https://doi.org/10.1016/j.pbb.2003.10.016
Fernsebner K, Zorn J, Kanawati B, Walker A, Michalke B (2014) Manganese leads to an increase in markers of oxidative stress as well as to a shift in the ratio of Fe(ii)/(iii) in rat brain tissue. Metallomics 6:921. https://doi.org/10.1039/c4mt00022f
Bayo-Olugbami A, Nafiu AB, Amin A, Ogundele OM, Lee CC, Owoyele BV, (2020) Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr Neurosci 1–12. https://doi.org/10.1080/1028415X.2020.1815331
da Silva TC, Hiller C, Gai Z, Kullak-Ublick GA (2016) Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. J Steroid Biochem Mol Biol 163:77–87. https://doi.org/10.1016/j.jsbmb.2016.04.006
Vegeto E, Villa A, Della Torre S, Crippa V, Rusmini P, Cristofani R, Galbiati M, Maggi A et al (2020) The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr Rev 41:bnz005. https://doi.org/10.1210/endrev/bnz005
Misiak M, Beyer C, Arnold S (2010) Gender-specific role of mitochondria in the vulnerability of 6-hydroxydopamine-treated mesencephalic neurons. Biochim Biophys Acta 1797:1178–1188. https://doi.org/10.1016/j.bbabio.2010.04.009
Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, Costa LG (2013) Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radic Biol Med 58:98–108. https://doi.org/10.1016/j.freeradbiomed.2013.01.019
Ishii M, Yamauchi T, Matsumoto K, Watanabe G, Taya K, Chatani F (2012) Maternal age and reproductive function in female Sprague-Dawley rats. J Toxicol Sci 37:631–638. https://doi.org/10.2131/jts.37.631
Bianchi S, Nottola SA, Torge D, Palmerini MG, Necozione S, Macchiarelli G (2020) Association between Female Reproductive Health and Mancozeb: Systematic Review of Experimental Models. Int J Environ Res Public Health 17:E2580. https://doi.org/10.3390/ijerph17072580
Baligar PN, Kaliwal BB (2001) Induction of gonadal toxicity to female rats after chronic exposure to mancozeb. Ind Health 39:235–243. https://doi.org/10.2486/indhealth.39.235
Acknowledgements
The authors are grateful to Universidade do Oeste de Santa Catarina (UNOESC) and Programa de Mestrado em Biociências e Saúde (PPGBS) for financial support. This work was also supported by grants from CAPES (Coordenação de Aperfeiçoamento de Pessoal e Nível Superior) by CAPES Pró-Equipamentos/2014 and FAPESC (Fundação de Amparo a Pesquisa e Inovação do Estado de Santa Catarina) by FAPESC nº 06/2017—Apoio a Grupos de Pesquisa das Instituições do Sistema ACAFE. Also, the authors are grateful to Dr. Samuel Patterson, a native speaker from UK by language revision.
Funding
This work was supported by grants from CAPES Pró-Equipamentos/2014 and FAPESC nº 06/2017—Apoio a Grupos de Pesquisa das Instituições do Sistema ACAFE.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. J.C.F. designed, planned, and performed the animal experimental model; carried out biochemical measurements and data analyses; prepared the initial figures; and revised the manuscript. A.B. performed the animal experimental model; carried out the behavioral tests and revised the manuscript. A.R.B. performed statistics and revised the manuscript. A.M. performed the Mn and Zn concentration analysis. M.C.F. performed the animal experimental model; wrote and revised the manuscript. D.C. performed the animal experimental model; carried out the behavioral tests; performed the statistical analyses; wrote and revised the manuscript. A.P.R. coordinated all experimental procedures and data analyses, prepared the final version of the figures, wrote the manuscript, was responsible for financial support acquisition, and provided the required infrastructure.
Corresponding author
Ethics declarations
Ethics Approval
All procedures on animals were approved by UNOESC animal ethics committee under n° 74/2019 protocol.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose. The authors report no conflicts of interest. The authors are responsible for the content and writing of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Favarin, J.C., Basotti, A., Baptistella, A.R. et al. Neuroprotective Effect of Vitamin D on Behavioral and Oxidative Parameters of Male and Female Adult Wistar Rats Exposed to Mancozeb (manganese/zinc ethylene bis-dithiocarbamate). Mol Neurobiol 60, 3724–3740 (2023). https://doi.org/10.1007/s12035-023-03298-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03298-8