Abstract
The present study evaluated the protective effects of Centella asiatica (CA) leaf extract on behavioral deficits and neurotoxicity in adult rat exposed to lead during perinatal period. Adult Wistar rats were exposed to 0.15% lead acetate (Pb) from gestation day 6 through drinking water and the pups were exposed lactationally to Pb till weaning. Significant perturbations in locomotor activity and exploratory behavior were observed in rats exposed to Pb during perinatal period. The levels of lipid peroxidation increased significantly with a reduction in levels of glutathione and activity levels of acetylcholinesterase and antioxidant enzymes in hippocampus, cerebrum, cerebellum, and medulla of brains excised from Pb-exposed rats. Oral supplementation of CA during postweaning period provided significant protection against Pb-induced behavioral impairments and neurotoxicity, without chelating tissue Pb levels. The possible neuroprotective efficacy of CA may be due to its antioxidant potential but not by lowering effects of brain Pb content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is widely recognized as a long-lived environmental toxicant that poses a significant health threat to humans since antiquity. Exposure to Pb is becoming inevitable which alters physiological and biochemical functions. While Pb affects almost all vital systems in the body, the brain is particularly susceptible to the toxic effects of lead (Crumpton et al. 2001; Reckziegel et al. 2011). Pb is known to impair cerebral growth, alter the number of synapses per neuron (Nichols and McLachlan 1990), cause axonal and dendritic arborization (Petit et al. 1983), neural differentiation (Crumpton et al. 2001), delay in synaptic elaboration (Bull et al. 1983), and hypo-myelination of the nervous system (Krigman et al. 1980). Pb mainly impinge on central nervous system-dependent functions such as cognitive functions, execution of driving movements, memory and behavioral assessments which are localized in the cerebral cortex, cerebellum, and hippocampus of the brain respectively. Previous studies reported that Pb exposure causes behavior abnormalities, cognitive defects, learning impairment (Verina et al. 2007), and neurotoxicity (Sansar and Gamrani 2003) in experimental animals. The nervous system is the primary target for the Pb exposure and the developing brain is highly vulnerable than mature brain to Pb-induced neurotoxicity (Lidsky and Schineder 2003; Jin et al. 2011). Studies also suggest that gestation through lactation is a sensitive period for exposure to Pb leading to lifelong cognitive deficits (Ryzhavskii et al. 2008). Recent evidences indicate that multi-factorial mechanisms are involved in Pb-induced neurotoxicity and some of the well-known mechanisms are enhanced generation of reactive oxygen species and mitochondrial dysfunction. Hence, it is important to develop effective strategies to prevent Pb-induced neurotoxicity.
There is an increasing global interest concerning the use of plant drugs in the prevention and treatment of different pathologies (Ramana et al. 2014; Chikezie et al. 2015). Centella asiatica (CA) belongs to the family Apiaceae is referred to be one of the great multipurpose miracle herbs of oriental medicine. The active constituents of CA are triterpenoid saponins (Duggina et al. 2015) and phenolic acids (Marques et al. 2015). The CA has been subjected to extensive experimental and clinical investigation and the extract of this plant has been used in Indian system of medicine for different ailments. Extract of CA is also known to possess wound healing, antioxidant, anticonvulsant, anti-inflammatory, antidiabetic, hepatoprotective, antipsoriatic, antiulcer, sedative, immunostimulant, and cardioprotective properties (Orhan 2012). Earlier reports have also shown that administration of CA improved memory and cognitive functions in rats (Kumar and Gupta 2003; Rao et al. 2005; Kumar et al. 2009; Xu et al. 2012a). Considering the facts that Pb is neurotoxic (Reddy et al. 2003), and CA has the ability to improve neuronal activity (Rao et al. 2005), the present study was aimed to investigate whether oral administration of CA ameliorates behavioral perturbations, cognitive dysfunction, and acetylcholinesterase (AChE) activity in different brain regions of rats exposed to Pb during perinatal period. In addition, the present investigation is focused on the oxidative status in cerebral cortex, hippocampus, cerebellum, and medulla of brains of rats exposed to Pb. We further extended our studies to examine the effect of CA on Pb levels in the blood and different brain regions of rats.
Materials and methods
Chemicals
Lead acetate (AR grade) was purchased from E-Merck, India. Acetylcholine iodide, dithiobis-2-nitro benzoic acid, and bovine serum albumin were obtained from Sigma Chemical Co (St Louis, USA). Thiobarbituric acid and malondialdehyde were obtained from E-Merck, Germany. The remaining chemicals used in the study were of the highest purity available and obtained from Hi-Media, Qualigens, and Loba Chemie, India.
Preparation of plant extract
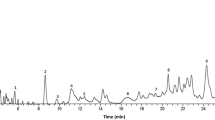
C. asiatica (L.) used in this study was obtained from the herbal gardens of Sri Venkateswara Ayurvedic Pharmacy, Narasingapuram, Tirupati, India, and the plant material was taxonomically identified and authenticated by the concerned herbarium officer, Department of Botany, S.V. University, Tirupati, India. Voucher specimen (1253) was deposited in the campus. Fresh leaves of the plant were washed in running tap water for 2 min and then with sterile distilled water for 5 min. Leaves were shade dried at room temperature in sterile conditions and pulverized using a mechanical grinder to a fine powder without producing much heat. The extract was prepared three times according to the method described earlier (Shinomol and Muralidhara 2008). In brief, 100 g of shade dried leaves was milled into fine powder and extracted in cold percolation with water for 24 h. The extract was recovered and this process was repeated four times. The extracts were pooled together, combined, and filtered using a moist muslin cloth. The filtrate was concentrated to dryness under reduced pressure in a Buchi Rotavapor. The resulting extract was air-dried, finally giving 11.09 ± 1.29 g of extract (n = 3). The triterpene content analyzed by HPLC ranged between 9 and 9.88% on dry basis and the concentration of the known active component, asiaticoside, was found to be 1.1% W/W. The HPLC analysis was performed by following the method of Inamdar et al. (1996) with minor modifications. The chromatographic separation was performed with Promosil C18 (4.6 × 250 mm, 5 μm) column with a water-acetonitrile mobile phase with UV-detection at 220 nm.
Animals and maintenance
Timed pregnant rats of Wistar/NIN (WNIN) strain were procured from an authorized vendor (M/S Raghavendra Enterprises, Bengaluru, India) and housed individually in polypropylene cages (45 cm × 25 cm × 20 cm) containing sterile paddy husk (procured locally) as bedding material. Rats were kept under standard laboratory conditions (22–25 °C, 12 h light and 12 h darkness) at the Animal Facility of the Department of Zoology, Sri Venkateswara University. The rats were fed on standard rat chow (HLL Animal feed, Bengaluru, India) and water ad libitum. All experimental investigations were done in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India (CPCSEA 2003). The protocol was approved by the Institutional Animal Ethics Committee (Regd. No. 438/01/a/CPCSEA/dt.17.07.2001) in its resolution no: 57/2012/(i)/a/CPCSEA/IAEC/SVU/ PSR-KPR dt.08-07-2012. The study was planned and organized as completely double blind.
Experimental design
The animals were randomized to two groups (one control group and treatment groups), each group consisting of eight rats. The rats in group 1 served as control and was allowed ad libitum access to tap water. Animals in group 2 were exposed to Pb through drinking water. Pb exposure was initiated on gestation day 6 with the addition of 0.15% lead acetate (819 mg lead/L) to deionized drinking water of the dam. Twenty-four hours after birth, pups were pooled and new litters consisting of eight males were randomly selected and placed with each dam. Lead exposure was continued through postnatal day 21 and stopped at weaning. Pups were never exposed to Pb directly. The dose of Pb was selected on the basis of previously published studies (Ronis et al. 1996) and our earlier results (Reshma Anjum and Reddy 2013). Food and water were freely available to all the animals throughout the study.
Experimental pups were randomly divided into two groups. Animals in the first group served as Pb-exposed group and maintained on filtered tap water and feed ad libitum. Rats in the second group were given daily aqueous extract of CA (200 mg of the crude extract/kg body weight/day) through intragastric feeding tube from PND 21 to PND 60. Control pups were also treated with aqueous extract of CA (200 mg of the crude extract/kg body weight/day) from PND 21 to PND 60. The dose of CA was selected on the basis of previously published studies (Kumar and Gupta 2003). Selected behavioral analysis in both the treated and control animals was done at 08:00 h of the day. Animals were sacrificed for biochemical analysis and determination of lead levels on postnatal day 61.
Behavioral studies
The open-field behavior was measured in a wooden box measuring 90 × 90 × 30 cm. The floor of the arena was painted white and divided into 36 equal squares by black lines. Each animal was placed in the center of the open field; the movements of the rat were scored. The number of squares crossed with all paws (crossings), standing on the hind legs (rearing), placing the nose against the wall (or) floor (sniffing), and wiping, licking, combing, or scratching of any part of the body (grooming) were recorded during a 5-min session.
The exploratory behavior was measured in a box with a hole board bottom (90 cm × 90 cm) containing three equally spaced holes (3 cm in diameter) in the floor. Each rat was placed in the center of the arena for 5 min during which time the number of head dips and head dipping duration (in seconds) were recorded. A head dip was scored if both eyes disappeared into the hole.
Morris water-maze test
Water maze is a circular tank measuring 1.85 m in diameter and 0.7 m deep similar to the basic design of Morris (1984). Four equal points are designated arbitrarily along the circumference of the water tank and thus dividing the maze into four quadrants. The pool was filled to a depth of 30 cm with water made opaque with white, inert water-based paint. A circular submerged platform (12.5 cm) remained below the surface of water. The platform was located in the north quadrant of the water maze. The starting points were changed every trial as south, east, and west. Each trial lasted until the rat had found the platform or for a maximum of 40 s. All rats were allowed to rest for 10 s after the end of each trial. Rats were trained with two trials before used in the experiments.
Analgesic test
The analgesic activity was assessed by hot plate (thermal) method (Espejo and Mir 1993). Briefly, the animals were placed on a hot plate (analgesia meter, techno) maintained at a temperature of 45 °C and basal reaction time (s) was recorded to analyze the rat’s response to the heat stimulus by using adverse evoked responses (forepaw-licking, hindpaw-licking, and jumping off).
Necropsy
The body weight of rats was recorded at the time of initiation and completion of the experiment. Food and water intake were measured once a week throughout the experimental period in both control and experimental groups. Rats were fasted overnight, weighed, and euthanized by using an overdose of anesthetic ether on the day following the last treatment. All biochemical analyses were analyzed in naïve rats, which were not used for behavioral studies.
Assay of AChE activity
The hippocampus, cerebellum, cerebral hemispheres, and medulla oblongata were isolated, weighed, and homogenized in ice-cold homogenizing buffer. The synaptosomal fraction was separated and suspended in 0.32 M sucrose. The AChE activity was determined in the synaptosomal fraction of tissue homogenates following the method of Ellman et al. (1961). The AChE activity was expressed as micromoles ACh hydrolyzed/mg protein/min. Protein content in enzyme source was determined using bovine serum albumin as standard (Lowry et al. 1951).
Lipid peroxidation and glutathione levels and activities of antioxidant enzymes
Different brain regions were homogenized in ice-cold 50 mM phosphate buffer (pH 7.4) containing 0.25 M sucrose at 4 °C. The homogenates were centrifuged at 2500 rpm for 10 min at 4 °C. An aliquot of supernatant was used for the determination of lipid peroxidation and reduced glutathione levels, while the rest was further centrifuged at 12,000 rpm for 30 min at 4 °C to obtain the supernatant fraction for assays of superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and glutathione reductase.
The extent of lipid peroxidation was determined as the concentration of thiobarbituric acid reactive product, malondialdehyde (MDA), by the method of Ohkawa et al. (1979) and levels of reduced glutathione were determined using the method described by Akerboom and Sies (1981). The activity levels of SOD were assayed by the method of Misra and Fridovich (1972), while the catalase activity was measured by using the method of Aebi (1984). The activity of GPx was determined by the method of Flohe and Gulzer (1984) and glutathione reductase enzyme activity was determined according to the method of Carlberg and Mannervik (1985). All the enzyme activities were expressed per milligram protein.
Pb analysis
For blood, hippocampus, cerebellum, cerebral hemispheres, and medulla oblongata, metal concentration on postnatal day 61, wet tissue weight, and volume of blood were recorded. Digestion was performed with concentrated nitric acid. Samples were brought to a constant volume and Pb concentration was determined by using atomic absorption spectrophotometer (Shimadzu - AA Model No: 6300).
Statistical analysis
The data were analyzed using one-way analysis of variance using SPSS. The post hoc Dunnet’s multiple range tests were performed to know the significant difference among the groups. Differences were considered to be significant at p < 0.01. The data were presented as mean ± S.D.
Results
General observations
The rats were observed for responses with respect to overall appearance, body position, activity, coordination or gait, skin color, fur arrangement, color of their eyes, and behavior. No significant changes in lacrimation, urination, respiration, vocalization, and postural or gait abnormalities were observed in any of the control and treated rats. All the animals were apparently normal and no unusual behaviors (viz. head flicking, head searching, biting, licking, self-mutilation, circling, and walking backwards) were observed in any of the rats. The initial and final body weights were comparable in all the groups and there were no significant changes between control and treated groups with respect to food and water intake (data not shown).
Open-field behavior
Table 1 summarizes the crossings, rearings, sniffings, and groomings in open-field activity of control and experimental groups. Significant (p < 0.01) decrease in the crossings (− 61.19%), rearings (− 75.62%), groomings (− 77.43%), and sniffings (− 78.54%) was observed in rats exposed to Pb during perinatal period as compared with control rats. Administration of CA did not influence the open-field activity in control rats (Table 1), whereas administration of CA significantly improved the open-field activity in Pb-exposed rats. The exploratory behavior recorded in three-hole board showed significant reduction in head dip counts and head dip duration in Pb-exposed rats. The CA supplementation, however, significantly reversed the Pb-induced alterations in these behaviors (Table 1).
Water-maze behavior
Water-maze behavior (cognition) in control and experimental groups is presented in Table 1. Significant (p < 0.01) delay to find a hidden platform was observed with rats exposed to Pb during perinatal period. The time taken to reach the destination was significantly decreased after CA treatment in Pb-exposed rats when compared to lead alone exposed rats. Administration of CA alone did not influence the water-maze tests when compared to control rats. Though swim speed and the distance traveled in Morris water maze were not determined in the present study, all the animals were apparently normal and swim actively in water maze. No signs of hyperactivity and lethargy were recorded and none of the animals were excluded.
Analgesic behavior
Exposure to Pb resulted in significant (p < 0.001) delay to exhibit evoked responses (forepaw-licking, hindpaw-licking, and jumping off) in rats as compared to the control rats (Table 1), whereas administration of CA resulted in partial recovery in analgesic behavior in Pb-exposed rats.
AChE activity
The activity levels of AChE in different brain regions such as hippocampus, cerebrum, cerebral hemisphere, and medulla oblongata were presented in Fig. 1. Significant decrease in AChE activity was observed in all the four regions of the brain after exposure to Pb. Administration of CA resulted in partial recovery of AChE activity in different brain regions of Pb-exposed rats, whereas administration of CA extract to control rats resulted in no significant change in AChE activity levels in different brain regions.
Lipid peroxidation and antioxidant enzyme activities
The level of lipid peroxidation was markedly high in different brain regions of rat exposed to Pb during perinatal period, though no significant change was recorded in CA alone treated group (Fig. 2). Further, a significant decrease was noted in the levels of GSH and activity levels of SOD, catalase, GPx, and glutathione reductase in different brain regions of rats treated with Pb during perinatal period, while activity levels of these enzymes and levels of GSH in CA alone treated group did not show difference in comparison to controls (Figs. 3, 4, 5, 6, and 7). Administration of CA to Pb-exposed rats, however, showed significant increases in SOD, catalase, GPx and glutathione reductase activities, and GSH levels, and a decrease in MDA level in different regions of the brain, indicating recovery from oxidative stress.
Lipid peroxidation levels in different brain regions of control (CO), lead-exposed (Pb) and Centella asiatica extract-treated control (CA), and Pb-exposed (Pb + CA) rats. Bars are means ± S. D. of eight individuals. Bars with the same superscript do not differ significantly from each other. p < 0.01
Reduced glutathione levels in different brain regions of control (CO), lead-exposed (Pb) and Centella asiatica extract-treated control (CA), and Pb-exposed (Pb + CA) rats. Bars are means ± S. D. of eight individuals. Bars with the same superscript do not differ significantly from each other. p < 0.01
Activity levels of catalase in different brain regions of control (CO), lead-exposed (Pb) and Centella asiatica extract-treated control (CA), and Pb-exposed (Pb + CA) rats. Bars are means ± S. D. of eight individuals. Bars with the same superscript do not differ significantly from each other. p < 0.01
Activity levels of glutathione peroxidase in different brain regions of control (CO), lead-exposed (Pb) and Centella asiatica extract-treated control (CA), and Pb-exposed (Pb + CA) rats. Bars are means ± S. D. of eight individuals. Bars with the same superscript do not differ significantly from each other. p < 0.01
Activity levels of glutathione reductase in different brain regions of control (CO), lead-exposed (Pb) and Centella asiatica extract-treated control (CA), and Pb-exposed (Pb + CA) rats. Bars are means ± S. D. of eight individuals. Bars with the same superscript do not differ significantly from each other. p < 0.01
Pb levels in different brain regions and blood
One of the objectives of the present study was to determine whether CA co-administration would reduce Pb burden in the tissues. The levels of Pb in blood and different brain regions were presented in Table 2. Significant accumulation of Pb was observed in blood and all the four regions of the brain after exposure to Pb. The levels of Pb in blood and different brain regions of CA-administered Pb-exposed rats are comparable with the Pb-exposed rats.
Discussion
This study was carried out to provide a comprehensive assessment of the effects of perinatal exposure to Pb or administration of CA extract alone or in combination on the behavior and neurochemistry of adult rats submitted to a wide range of tasks. The regimen of Pb exposure employed in the present study caused no effect on the weight gain of animals indicating Pb does not show acute toxicity at the dose level selected. On the contrary, a significant decrease in the locomotory, exploratory, cognitive, and analgesic behavior was observed in rats exposed to Pb during perinatal period. The present results are in consonance with earlier studies (Burdette and Goldstein 1986; Altmann et al. 1993; Rodrigues et al. 1996; Moreira et al. 2001; Reddy et al. 2003), which reported that the memory habituation in the open field is processed by the hippocampus, which is believed to be the target for Pb-induced neurotoxicity (Leret et al. 2003; Sun et al. 2005). The higher Pb levels observed in hippocampus (3.92 μg/g) compared to other brain regions in rats at PND 61 indicates greater vulnerability of hippocampus to Pb compared to other brain regions. Our results on the Pb levels in different brain regions are similar to those observed by Basha et al. (2012) who reported that Pb levels in hippocampus (4678%) and cerebellum (2829%) of rats exposed to 0.2% Pb (Pb acetate in drinking water of mother) from gestational day 6 till weaning (postnatal day 21) were significantly higher on postnatal day 60.
The decrease in the activity levels of AChE in different brain regions observed in the present study indicates the reduction in cholinergic synapses in the brain of Pb-exposed rats. Neurochemical, histochemical, and electrophysiological data have also indicated the inhibitory function of Pb on both cholinergic and aminergic systems (Shih and Hanin 1978; Reddy et al. 2003). Therefore, the observed decrease in behavioral alterations can be attributed to the perturbations in the cholinergic and aminergic systems in Pb-exposed brain. The present results, however, contradict the reports of increased locomotory activity observed in Pb-exposed rats in the open field (Rodrigues et al. 1996).
It is well known that Pb is a bioaccumulative and relentless neurotoxic contaminant in the environment. Once enters into the body, it gets access to different regions of the brain either as free ion (Pb-OH+) or complexes with low molecular weight ligands (Bradbury and Deane 1993; Williams et al. 2000). The mean Pb levels in blood and hippocampus, cerebellum, cerebral hemispheres, and medulla oblongata were significantly elevated in rats exposed to Pb during perinatal period. The neuroanatomical and functional development of the rat hippocampus extends up to the second week. It was reported that the hippocampus which makes up only 13.4% of the fresh weight of the brain contains approximately 50% of the total Pb (Fjerdingstad et al. 1974). It is well known that hippocampus is involved in the behavioral and cognitive manifestation (Day et al. 1991; Ma et al. 1999; File et al. 2000) and the elevated Pb levels in the hippocampus might be responsible for the observed alterations in behavior.
In the current study, we observed significant decrease in antioxidant enzyme activities along with GSH levels in all regions of the brain of rats exposed to Pb during perinatal period. Conversely, the levels of lipid peroxidation increased in all regions of the brain of rats exposed to Pb during perinatal period. Several studies have demonstrated increased lipid peroxidation and reduced antioxidant enzyme activities in the tissues after exposure to Pb (Hermes-Lima et al. 1991; Sandhir et al. 1994; Antonio et al. 1999; Prasanthi et al. 2010). Enzymatic antioxidants (SOD, catalase, GPx, and glutathione reductase) form the first line of antioxidant defense mechanism to protect the deleterious effects reactive oxygen species (ROS). SOD catalyzes the dismutation of superoxide radical to hydrogen peroxide (H2O2). Catalase degrades H2O2 into water and oxygen and the GPx/reductase system has been shown to catalyze the degradation of H2O2 and lipid peroxides by using GSH. Glutathione is one of the most prominent non-enzymatic antioxidants and a powerful nucleophile, critical for cell proliferation, such as detoxification of ROS and conjugation and excretion of toxic molecules. It is well known that depletion of tissue GSH levels enhances cellular damage caused by oxidative stress. The reduction in the levels of GSH and activities of these enzymes reflects the inability of cells to eliminate ROS generated after exposure to Pb. The mechanism of action of Pb on the reduction in free radical scavenging enzymes remains unclear. In the present study, Pb level was significantly elevated in all regions of the brain of rats exposed to Pb during perinatal period. It was established that Pb passes through blood-brain barrier easily during the developmental period whereas in adults, such transfer of Pb is stopped and the accumulated Pb acts on the enzymes. The reduction in SOD and catalase is mainly attributed to the high affinity of Pb for sulfhydryl groups in these enzymes leading to inhibition of these enzyme activities. Further, SOD, which requires Cu2+ and Zn2+ for its activity was found to be decreased in Pb-exposed rats (Sivaprasad et al. 2002, 2004). This could be due to the Pb-induced Cu2+ and Zn2+ deficiency as Pb competes and replaces Cu2+ and Zn2+ in their binding sites (Mylroie et al. 1984). Besides, Pb is known to reduce the absorption of Fe in the gastrointestinal tract and inhibit the heme biosynthesis leading to reduction in catalase activity (Sivaprasad et al. 2004), since heme is the prosthetic group of catalase.
The findings of the present study also indicate that administration of CA improves behavior and increases brain AChE activity in Pb-exposed rats. Increased AChE activity levels might reflect the enhancement of acetylcholine release which would facilitate in synaptic transmission of neurons. The enhancement of cognitive functions by CA may be attributed to its ability to elevate brain AChE activity. Despite the fact that C. asiatica is an important medicinal plant, its efficacy in relation to protecting animals from the neurotoxic effects of metals or metalloids has not been thoroughly studied. C. asiatica has been mentioned in ancient Ayurvedic text of the Indian system of medicine for its properties to promote intelligence (Chopra et al. 1994). Earlier, it has been shown that CA supplementation increased cognitive functions in rats (Kumar and Gupta 2003; Rao et al. 2005). It was also reported that CA enhances learning and memory and promotes the growth of neurons (Kumar et al. 2009). Employing intracerebroventricular colchicine model of Alzheimer’s disease rats, Kumar et al. (2009) reported that administration of aqueous extract of C. asiatica could prevent the cognitive deficits. Interestingly, elevated AChE activity is reported to be sufficient to reverse memory deficits (Reddy et al. 2003).
In the present study, CA treatment caused a marked decrease in lipid peroxidation in different brain regions of rats exposed to Pb. The antioxidant activity of CA have been attributed to various mechanisms, such as prevention of chain initiation, binding of transition metal ion, decomposition of peroxides, and radical scavenging power (Sugunabai et al. 2015). Administration of CA to Pb rats increased the activities of SOD, catalase, GPx and glutathione reductase, and GSH levels in the different brain regions and may help to regulate the levels of ROS, indicating CA-offered protection to cells against oxidative stress. Our results are in agreement with earlier reports indicating antioxidant properties of CA (Veerendra Kumar and Gupta 2003; Hussin et al. 2007; Flora and Gupta 2007). The increased activities of antioxidant enzymes may act as an added compensation mechanism to maintain the cell integrity and protection against ROS. This may due to the presence of many antioxidants such as phenols, tannins, terpenoids, and flavonoids in CA (Orhan 2012). Though there was a significant recovery from Pb-induced neurotoxicity by CA, the levels of Pb in blood and different regions of the brain were comparable with Pb group indicating CA is not a Pb chelator.
The improvement of neurological and behavioral parameters by CA has been reported earlier by several workers (Wang et al. 2003; Wijeweera et al. 2006). CA contains high amounts of triterpenoid glycosides, such as asiatic acid, asiaticoside, madecassic acid, madecassoside, madasiatic acid, betulinic acid, thankunic acid, isothankunic acid, noxyasiaticoside, and centellosides (Inamdar et al. 1996; Shukla et al. 1999; James and Dubery 2009), flavonoid derivatives, such as quercetin, kaempferol, patuletin, rutin, apigenin, castilliferol, castillicetin, and myricetin (Kuroda et al. 2001; Orhan 2012), and several phenolic acids, for example, p-hydroxybenzoic acid, vanillic acid, p-coumaric acid, o-coumaric acid, and transcinnamic acid (Orhan 2012). Asiaticoside, active constitute of C. asiatica, has been reported as dementia-treating agent and cognitive enhancer (Wijeweera et al. 2006; Krishnamurthy et al. 2009; Xu et al. 2012a, 2012b). It has also been found to have remedial value against β-amyloid neurotoxicity (Wang et al. 2003). Although asiaticoside is found to be the major contributor for neuroprotection in CA, we are of the opinion that further investigations to determine the mechanism(s) of action of asiaticoside for its noted effects need to be conducted in order to optimize its usage in humans.
In conclusion, it could be concluded from the present results that administration of aqueous extract of CA offers significant protection from neurotoxic effects as evidenced by increased motor activity, cognitive function, and AChE activity and reduced oxidative stress in different brain regions of rat exposed to Pb during perinatal period without chelating the metal. However, future research is warranted, for the better understanding of the mechanism of action of CA by which it modulates neuronal activity in depressed condition. The present study also provides a perinatal Pb-exposed rat model with depressed neuronal functions.
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:125–126
Akerboom TP, Sies H (1981) Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Meth Enzymol 77:373–382
Altmann L, Weinsberg F, Sveinsson K, Lilienthal H, Wiegand H, Winneke G (1993) Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol Lett 66:105–112
Antonio MT, Corpas I, Leret ML (1999) Neurochemical changes in new born rat’s brain after gestational cadmium and lead exposure. Toxicol Lett 104:1–9
Basha DC, Usha Rani M, Devi CB, Ram Kumar M, Reddy GR (2012) Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: reversal effect of calcium co-administration. Int J Dev Neurosci 30:343–350
Bradbury MW, Deane R (1993) Permeability of the blood-brain barrier to lead. Neurotoxicology 14:131–136
Bull RJ, McCauley PT, Taylor DH, Croten KM (1983) The effects of lead on the developing central nervous system of the rat. Neurotoxicology 4:1–17
Burdette LJ, Goldstein R (1986) Long-term behavioral and electrophysiological changes associated with lead exposure at different stages of brain development in the rat. Brain Res 394:101–110
Carlberg I, Mannervik B (1985) Glutathione reductase. Meth Enzymol 113:484–490
Chikezie PC, Chiedozie P, Ibegbulem O, Mbagwu FN (2015) Bioactive principles from medicinal plants. Res J Phytochem 9:88–115
Chopra RN, Nayer SL, Chopra IC, Asolkar LV, Kakkar KK (1994) Glossary of Indian medicinal plants. CSIR Publications, New Delhi
CPCSEA (2003) Committee for the Purpose of Control And Supervision of Experiments on Animals: guidelines for laboratory animal facility. Indian J Pharm 35:257–274
Crumpton T, Atkin DS, Zawia NH, Barone JRS (2001) Lead exposure to pheochromocytoma (PC12) cells alters neural differentiation and Sp1 DNA-binding. Neurotoxicology 22:49–62
Day J, Damsma G, Fibiger HC (1991) Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav 38:723–729
Duggina P, Kalla CM, Varikasavu SR, Bukke S, Tartte V (2015) Protective effect of Centella triterpene saponins against cyclophosphamide-induced immune and hepatic system dysfunction in rats: its possible mechanisms of action. J Physiol Biochem 71:435–454
Ellman GL, Courtney KD, Andres VRM, Featherstone RM (1961) A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Espejo EF, Mir D (1993) Structure of rat’s behavior in the hot plate test. Behav Brain Res 56:171–176
File SE, Kenny PJ, Cheeta S (2000) The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66:65–72
Fjerdingstad EJ, Danscher G, Fjerdingstad E (1974) Hippocampus: selective concentration of lead in the normal rat brain. Brain Research 80(2):350–354
Flohe L, Gulzer WA (1984) Glutathione peroxidase. Methods Enzymol 105:114–121
Flora SJS, Gupta R (2007) Beneficial effects of Centella asiatica aqueous extract against arsenic-induced oxidative stress and essential metal status in rats. Phytother Res 21:980–988
Hermes-Lima M, Pereira B, Bechara EJH (1991) Are free radicals involved in lead poisoning? Xenobiotica 21:1085–1090
Hussin M, Abdul-Hamid A, Mohamad S, Saari N, Ismail M, Bejo MH (2007) Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem 100:535–541
Inamdar PK, Yeole RD, Ghogare AB, De Souza NJ (1996) Determination of biologically active constituents in Centella asiatica. J Chromatogr 742:127–130
James JT, Dubery IA (2009) Pentacyclictriterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 14:3922–3941
Jin Y, Yu F, Liao Y, Liu S, Liu M, Xu J, Yang J (2011) Therapeutic efficiency of succimer used with calcium and ascorbic acid in the treatment of mild lead poisoning. Environ Toxicol Pharmacol 31:137–142
Krigman M, Bouldin TW, Mushak P (1980) Lead. In: Spencer P, Schaumburg H (eds) Experimental and clinical neurotoxicology. Williams & Wilkins, Baltimore, pp 490–507
Krishnamurthy RG, Senut MC, Zemke D, Min J, Frenkel MB, Greenberg EJ, Yu SW, Ahn N, Goudreau J, Kassab M, Panickar KS, Majid A (2009) Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res 87:2541–2550
Kumar MH, Gupta YK (2003) Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethanopharmacol 79:253–260
Kumar A, Dogra S, Prakash A (2009) Neuro protective effects of Centella asiatica against intra cerebro-ventricular colchicine induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 2009:1–8
Kuroda M, Mimaki Y, Harada H, Sakagami H, Sashida Y (2001) Five new triterpene glycosides from Centella asiatica. Nat Med 55:134–138
Leret ML, Antonia JSM, Antonia MT (2003) Perinatal exposure to lead and cadmium affects anxiety-like behavior. Behav Toxicol 186:125–130
Lidsky TI, Schineder JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Lowry LH, Rosebrough NI, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Ma T, Chen HH, Ho IK (1999) Effects of chronic lead (Pb) exposure on neurobehavioral function and dopaminergic neurotransmitter receptors in rats. Toxicol Lett 105:111–121
Marques NF, Stefanello ST, Froeder AL, Busanello A, Boligon AA, Athayde ML, Soares PA, Fachinetto R (2015) Centella asiatica and its fractions reduces lipid peroxidation induced by quinolinic acid and sodium nitroprusside in rat brain regions. Neurochem Res 40:1197–1210
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23:489–495
Morris RGM (1984) Developments of water maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Mylroie AA, Umbles C, Kyle J (1984) Effects of dietary copper supplementation on erythrocyte superoxide dismutase activity, ceruloplasmin and related parameters in rats ingesting lead acetate. In: Hemphill DD (ed) Trace substances in environmental health, vol 18. University of Mussouri Press, Columbia, pp 497–504
Nichols DM, McLachlan DRXC (1990) Issues of lead toxicity. In: Yasumura S (ed) In vivo body composition studies. Plenum Press, New York, pp 237–246
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Orhan IE (2012) Centella asiatica (L.): from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Altern Med 2012:1–8
Petit TL, Alfano DP, Leboutillier JC (1983) Early lead exposure and the hippocampus: a review and recent advances. Neurotoxicology 4:79–94
Prasanthi RPJ, Devi CB, Basha RM, Reddy GR (2010) Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci 28:161–167
Ramana KD, Singhal SS, Reddy AB (2014) Therapeutic potential of natural pharmacological agents in the treatment of human diseases. BioMed Res Int 1–4, Article ID 573452
Rao KGM, Rao MS, Rao SG (2005) Centella asiatica (Linn) induced behavioural changes during growth spurt period in neonatal rats. Neuroanatomy 4:18–23
Reckziegel P, Dias VT, Benvegnu D, Boufleur N, Barcelos RCS, Segat HJ, Pase CS, Dos Santos CMM, Flore EMM, Burger ME (2011) Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol Lett 203:74–81
Reddy GR, Basha MR, Devi CB, Suresh A, Baker JL, Shafeek A, Heinz J, Chetty S (2003) Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int J Dev Neurosci 21:347–352.
Reshma Anjum M, Reddy PS (2013) Effect of perinatal exposure to lead acetate on testicular lipid peroxidation adult rats. Int J Pharm Bio Sci 4:893–898
Rodrigues ALS, Rocha MA, Souza DA, Mello CF (1996) Effect of perinatal lead exposure on rat behaviour in open field and two way avoidance tasks. Pharmacol Toxicol 79:150–156
Ronis MJ, Badger TM, Robertson PK, Sheikh F (1996) Reproductive toxicity and growth effects in rats exposed to lead at different periods during development. Toxicol Appl Pharmacol 136:361–371
Ryzhavskii B, Lebedko OA, Belolyubskaya DS, Baranova SN (2008) Long-term consequences of prenatal exposure to lead on brain development in rats. Neurosci Behav Physiol 38:145–148
Sandhir R, Julka D, Gill KD (1994) Lipoperoxidative damage on lead treatment in rat brain and its implications on membrane bound enzymes. Pharmacol Toxicol 74:66–71
Sansar W, Gamrani H (2003) The pharmacological effect of Artemisia absinthium extract in protecting adult rats against lead neurotoxicity. J Neurol Sci 333:e598
Shih TM, Hanin I (1978) Effects of chronic lead exposure on levels of acetylcholine and choline and on acetylcholine turnover rate in rat brain areas in vivo. Psychopharmacology 58:263–269
Shinomol GK, Muralidhara K (2008) Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and it’s in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine 15:971–984
Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN (1999) In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol 65:1–11
Sivaprasad R, Nagaraj M, Varalakshmi P (2002) Lipoic acid in combination with a chelator ameliorates lead-induced peroxidative damages in rat kidney. Arch Toxicol 76:437–441
Sivaprasad R, Nagaraj M, Varalakshmi P (2004) Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem 15:18–23
Sugunabai J, Jeyaraj M, Karpagam T (2015) Analysis of functional compounds and antioxidant activity of Centella asiatica. World J Pharm Pharmceu Sci 4:1982–1993
Sun L, Zhao ZY, Hu J, Zhou XL (2005) Potential association of lead exposure during early development of mice with alteration of hippocampus nitric oxide levels and learning memory. Biomed Environ Sci 18:375–378
Veerendra Kumar MH, Gupta YK (2003) Effect of Centella asiatica on cognition and oxidative stress in an intra-cerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol 30:336–342
Verina T, Rohde CA, Guilarte TR (2007) Environmental Pb2+ exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145:1037–1047
Wang XS, Dong Q, Zuo JP, Fang N (2003) Structure and potential immunological activity of a pectin from Centella asiatica (L.) Urban. Carbohydr Res 338:2393–2402
Wijeweera P, Arnason JHT, Koszycki D, Merali Z (2006) Evaluation of anxiolytic properties of gotukola (Centella asiatica) extracts and asiaticoside in rat behavioral models. Phytomedicine 13:668–676
Williams K, Wilson MA, Bressler J (2000) Regulation and developmental expression of the divalent metal ion transporter in the rat brain. Cell Mol Biol 46:563–571
Xu MF, Xiong YY, Liu JK, Qian JJ, Zhu L, Gao J (2012a) Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharm Sin 33:578–587
Xu CL, Wang QZ, Sun LM, Li XM, Deng JM, Li LF, Zhang J, Xu R, Ma PS (2012b) Asiaticoside: attenuation of neurotoxicity induced by MPTP in a rat model of Parkinsonism via maintaining redox balance and upregulating the ratio of Bcl-2/Bax. Pharmacol Biochem Behav 100:413–418
Acknowledgements
The authors thank Dr. K.V.S. Sarma, Professor (Statistics), S.V. University, Tirupati, for providing assistance in the statistical analysis. KPR and CHS are grateful to the University Grants Commission, New Delhi, for financial support in the form of UGC-BSR (RFMS) fellowships.
Author information
Authors and Affiliations
Contributions
PSR conceived the idea, provided facilities, and supervised the work. KPR maintained rat colony and treatments and performed behavioral studies and assayed AChE activity. CHS prepared CA extract, analyzed the extract, and determined the Pb levels. KPR prepared rough draft of manuscript and all the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
All experimental investigations were done in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India (CPCSEA 2003). The protocol was approved by the Institutional Animal Ethics Committee (Regd. No. 438/01/a/CPCSEA/dt.17.07.2001) in its resolution no: 57/2012/(i)/a/CPCSEA/IAEC/SVU/ PSR-KPR dt.08-07-2012. The study was planned and organized as completely double blind.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Chintapanti, S., Pratap Reddy, K. & Sreenivasula Reddy, P. Behavioral and neurochemical consequences of perinatal exposure to lead in adult male Wistar rats: protective effect by Centella asiatica. Environ Sci Pollut Res 25, 13173–13185 (2018). https://doi.org/10.1007/s11356-018-1500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1500-x