Abstract
Paclitaxel-induced peripheral neuropathy (PIPN) is a very common and complex painful condition related to paclitaxel (PTX) exposure, severely impacting patients’ quality of life, and contributing to the emergence of clinical signs of anxiety and cognitive loss. At present, no sufficient treatment options are available for PIPN and its exact pathophysiology remains unclear. Based on the therapeutic potential of the 7-chloro-4-(phenylselanyl) quinoline (4-PSQ), we assessed its ability to reverse PIPN and its comorbities induced by PTX. The effect of 4-PSQ was evaluated on pathophysiological processes involved in PIPN, such as oxidative stress (oxidative damage and antioxidant enzymes), neuroinflammation (mRNA expression levels of nuclear factor-kappa B, interleukin-1beta, tumor necrosis factor-alpha, and inducible nitric oxide synthase), and calcium homeostasis (Ca2+ATPase activity) in the spinal cord, cerebral cortex, and hippocampus of mice. Male Swiss mice received PTX (2 mg/kg) or vehicle by intraperitoneal route (days 1, 2, and 3). Oral administration of 4-PSQ (1 mg/kg) or vehicle was performed on days 3 to 14. It was observed that 4-PSQ reduced the mechanical and thermal hypersensitivities induced by PTX. Likewise, 4-PSQ reduced both anxious behavior and cognitive impairment in mice with PIPN. We believe that effects of 4-PSQ may be associated, at least in part, with the modulation of oxidative stress, reduction of neuroinflammation, and normalizing Ca2+ATPase activity in the spinal cord, cerebral cortex, and hippocampus of mice with PIPN. Taken together, the 4-PSQ might be a good prototype for the development of a more effective drug for the treatment of PIPN and its comorbities.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paclitaxel (PTX) is a chemotherapeutic agent belonging to the taxane family, commonly used in the treatment of various solid tumors, including breast, ovarian, and lung [1]. This chemotherapy drug exerts its action against different types of cancers by interrupting the G2 phase of the cell cycle by binding to polymerized tubulins (α and β) stabilizing the microtubules [2]. The loss of this instability leads to the interruption of cell mitosis and, consequently, to cell death by apoptosis [1]. Although PTX is highly effective against the proliferation of cancer cells, it also can affect cells that are not in division, such as neurons. Thus, exposure to PTX can result in damage to both the central (CNS) and peripheral (PNS) nervous systems [3, 4]. Peripheral neuropathy is a common dose-limiting adverse effect associated with PTX treatment and is the major cause of ongoing pain in cancer survivors [5]. In clinical use, symptoms of neurotoxicity often occur when the PTX dose per administration is 250 mg/m2 infused over 24 h [6, 7]. Unfortunately, PTX-induced peripheral neuropathy (PIPN) is very common and is reported in approximately 90% of patients, but generally remains relatively mild until the cumulative PTX dose exceeds 1400 mg/m2 [8,9,10]. In some cases, dose reduction or early discontinuation of treatment is advised [11]. In this line, PIPN can severely impact the quality of life of cancer patients and also contribute to the development of comorbidities [5, 12].
The neurotoxicity caused by PTX can lead to damage to peripheral sensory neurons, especially in cells located in the dorsal root ganglia, and consequently, influence pain signaling [4, 13]. Alongside, PTX affects the PNS and causes a predominantly sensory peripheral axonal neuropathy with paresthesia and pain [14]. PIPN symptoms usually develop first in the feet and hands and may progress to other regions of the body [11]. At present, the mechanisms underlying PIPN remain unclear. However, some events have been proposed as responsible for the damage caused by PIPN [14]. Pathophysiological processes induced by PTX include increased intracellular Ca2+ concentration and oxidative stress; together, these events can cause cellular apoptosis and, consequently, degeneration of peripheral sensory fibers [15, 16]. In addition, the release of pro-inflammatory mediators, such as cytokines, produced by glial cells in the spinal cord is thought to contribute to the development of PIPN [12]. Peripheral nociceptors are known to respond directly to cytokines, chemokines, and other inflammatory mediators [17, 18]. Thus, PTX-induced release of tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and IL-6 promotes action potential discharge by increasing Na+ and Ca2+ currents at peripheral nociceptor terminals, resulting in increased membrane excitability, lowered pain threshold, and peripheral sensitization [19]. All these effects may damage sensory neurons such as Aδ and C fibers, resulting in neuropathic pain characterized by hyperalgesia and allodynia [19,20,21]. Therefore, sensitization of sensory neurons through the release of pro-inflammatory cytokines may play a crucial role in the development of PIPN.
Considering the complexity of PIPN and its comorbidities, as well as the risk factors contributing to the process, multifunctional molecules with two or more complementary biological activities may represent an important advance for the treatment of this pathology. In this context, 7-chloro-4-(phenylselanyl) quinoline (4-PSQ), a quinoline functionalized with an organoselenium group, has been extensively studied by our research group [22–, 23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Among these studies, it was shown that 4-PSQ reduced chronic pain by modulating oxidative stress parameters in an oxaliplatin-induced chronic peripheral neuropathy model [36]. Likewise, 4-PSQ attenuated the neurotoxicity caused by this chemotherapeutic, evidenced through the reduction of anxious behavior and cognitive impairment mainly for its ability to reestablish activity of the Na+, K+ATPase enzyme [35]. Importantly, we recently demonstrated that 4-PSQ reversed acute hypersensitivity oxaliplatin-induced and aging-aggravated by reducing oxidative damage, through modulation of antioxidant enzymes [28]. Our studies have also shown that 4-PSQ is a potent anti-inflammatory agent that reduces formalin-induced nociception and paw edema [22] and modulates mRNA expression of proteins involved in inflammatory processes, such as transcription factor NF-κB and pro-inflammatory cytokines [24, 27]. Therefore, the hypothesis of neuroprotective effects of 4-PSQ against neuropathic pain may be related to a reduction in peripheral sensitization through its ability to attenuate inflammatory and oxidative parameters. Thus, the pharmacological effects of 4-PSQ seem to be related to its antioxidant, [22, 30, 34] and anti-inflammatory effect [22, 23], as well as to its ability to modulate the serotonergic, nitrergic, glutamatergic, and cholinergic systems, demonstrating the multitarget property of this molecule [22,23,24, 29,30,31, 33]. Furthermore, it is important to highlight that 4-PSQ exerts its pharmacological action in the absence of hepatic and renal toxicities in mice [22, 29]. Indeed, a growing body of evidence demonstrates that 4-PSQ is a promising compound in the field of drug development.
Faced with the need for an effective therapy to treat PIPN and its comorbidities, in this study, we investigated the effect of 4-PSQ on the PIPN model in mice. In order to elucidate if PIPN promotes the development of comorbidities, emotional and cognitive impairments were evaluated. Finally, oxidative and neuroinflammatory stress parameters, as well as the activity of the Ca2+ATPase enzyme, were analyzed in order to investigate the effect of 4-PSQ on mechanisms involved in PIPN.
Materials and Methods
Animals and Ethical Approval
Experiments were performed with 8- to 12-week-old male Swiss mice, weighing 25–35 g. Animals were housed in collective cages at 22 ± 2 °C, with a humidity of 50–80%, under a 12-h light/dark cycle, with free access to food and water. Each cage contained 4–6 animals, and those animals were distributed using a randomized block design, to obtain groups with similar baseline withdrawal thresholds.
All experiments were performed in accordance with the National Institutes of Health Animal Care Guidelines (NIH Publications No. 8023, revised 1978), and were approved by the Committee on Care and Use of Experimental Animal Resources of the Federal University of Pelotas, Brazil (CEEA 4506–2017). The number of animals and the intensity of noxious stimuli used were the minimum necessary to demonstrate consistent effects. All efforts were made to minimize the number of animals used and their suffering.
Drugs
4-PSQ (Fig. 1) was prepared and characterized in our laboratory. Nuclear magnetic resonance analysis (1H and 13C) showed analytical and spectroscopic data in full agreement with its assigned structure. The chemical purity of 4-PSQ (99.9%) was determined by gas chromatography coupled with mass spectrometry (GC–MS) [37]0.4-PSQ and PTX were dissolved in canola oil and 5% glucose solution, respectively. PTX was obtained from Eurofarma pharmaceutical company, and according to the Brazilian Health Regulatory Agency (ANVISA), its code is M.S. 100,430,899. All other chemicals used in this study were of analytical grade and obtained from Sigma-Aldrich (St. Louis, MO, USA). Mice received treatments by oral (p.o.–intragastric gavage) or intraperitoneal (i.p.) routes, at a constant volume of 10 mL/kg of body weight.
Peripheral Neuropathy Induced by PTX
PTX is one of the most employed drugs to treat solid tumors. Due to its wide use and high incidence of peripheral neuropathy (around 70%) [11, 38], we chose this antineoplastic drug to induce neuropathy. Peripheral neuropathy was induced by PTX administration according to the methodology described previously by Polomano et al. [38] and adapted for use in mice. PTX (6 mg/mL) was stored at 4 °C for a maximum of 28 days after opening. The stock solution was diluted up to 0.2 mg/mL in 5% glucose solution immediately before use. Animals received PTX intraperitoneally (2 mg/kg) on days 1, 2, and 3 of the experimental protocol, using an injection volume of 10 mL/kg. The cumulative dose of PTX was 6 mg/kg. Control animals received vehicle (5% glucose solution). The development of peripheral neuropathy was assessed 8 days after the first injection of PTX by testing the mouse sensitivity to mechanical and thermal stimuli.
Experimental Design
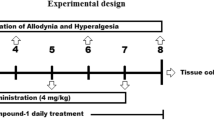
Firstly, mice were randomly divided into four groups (7 animals/group): (i) control, (ii) PTX, (iii) 4-PSQ, and (iv) PTX + 4-PSQ. On days 1, 2, and 3, mice of the control and 4-PSQ groups received a 5% glucose solution (10 mL/kg, i.p.), whereas mice of the PTX and PTX + 4-PSQ groups received PTX (2 mg/kg, i.p.). From day 3 to day 14 of the experimental protocol, mice of the control and PTX groups received canola oil (10 mL/kg, p.o.), whereas mice of the 4-PSQ and PTX + 4-PSQ groups received 4-PSQ (1 mg/kg, p.o.), once a day. The nociceptive response was evaluated on days 8, 11, and 14 of the experimental protocol. To investigate the effects of PTX and 4-PSQ on comorbidities associated with peripheral neuropathy, cognitive impairment was evaluated on days 12 and 13 and on day 15, the anxiety-like behavior of the mice (Fig. 2).
Twenty-four hours after the last treatment, the animals were euthanized by inhalation of isoflurane anesthetic. The spinal cord, cerebral cortex, and hippocampus samples were rapidly dissected, weighed, and placed on ice then used for ex vivo assays. The operators in the behavioral tests and data analysis were blinded. The dose and the protocol of 4-PSQ treatment were based on a previous study [31] since other studies evaluating the pharmacological actions of the 4-PSQ have been carried out [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36, 39].
Behavioral Analyses
Assessment of Plantar Mechanical Sensitivity
Mechanical sensitivity was carried out in mice according to the method previously described by Alamri et al. [40], with some modifications. For this test, mice were placed individually inside acrylic cages with wire grid floors 30 min before the start of testing performed in a quiet room. Before paw stimulation, the animals were quieted, without exploratory movements and not resting on their paws. The test consisted of evoking a hind paw flexion reflex with a hand-held force transducer (digital analgesimeter, Insight, São Paulo, Brazil) adapted with a polypropylene tip. The paw withdrawal threshold was measured by applying the polypropylene tip perpendicular to the middle of the plantar surface of the hind paw at constant progressive pressure until paw withdrawal, and the pressure value was automatically recorded. Mechanical sensitivity was evaluated on the 8th, 11th, and 14th days of the experimental protocol.
Assessment of Heat Sensitivity
Thermal sensitivity was tested in mice as reported by Woolfe and MacDonald [41], with some modifications. The hot plate test is a behavioral model of nociception in which behaviors such as jumping and hind paw-licking are elicited following a noxious thermal stimulus. Thermal sensitivity was evaluated on the 8th, 11th, and 14th days of the experimental protocol. For this, animals were placed in a glass box on a heated metal plate maintained at 52 ± 1 °C. The latency of nociceptive responses such as licking or shaking one of the paws or jumping was recorded as the reaction. Significant decreases of paw withdrawal latency were interpreted as indicative of heat hyperalgesia. To avoid damage to the paws of animals, time standing on the plate was limited to 45 s.
Assessment of Locomotor and Exploratory Domains
The open field was made of plywood and surrounded by 30-cm-high walls. The floor of the open field, 45 cm long and 45 cm wide, was divided by masking tape markers into 9 squares (3 rows of 3). The locomotor and exploratory domains were evaluated on the 12th day of the experimental protocol. In this test, each animal was placed at the center of the open field and observed for 4 min to record the locomotor (number of segments crossed with the four paws) and exploratory (number of rearing on the hind limbs) activities [42].
Assessment of Emotional Domain
The elevated plus maze (EPM) test is widely validated to measure anxiety in rodents [43]. The EPM apparatus consists of two opposed open arms (16 × 5 cm) and two opposed closed arms (16 × 5 × 10 cm) mounted at a 90° angle, all facing a central platform (5 × 5 cm) elevated 50 cm from the floor. This test was performed on the 15th day of the experimental protocol. Each animal was placed individually at the center of the apparatus facing one of the open arms. The frequency of entries into both open or closed arms and the time spent in the open arm was measured for 5 min. The data were expressed as a percentage of entries (with the four paws) into, and time spent in the open arms in relation to the total number of entries and time, respectively, in both open and enclosed arms. The total number of entries into the enclosed arms was also recorded. The anxiolytic effects of a drug are illustrated by a significant statistical augmentation of parameters in open arms.
Assessment of Cognitive Domain
The object recognition task was carried out according to the method previously described by Lueptow [44]. This task has been widely used to evaluate short-term (STM) and long-term (LTM) memories. The task was performed in an open-field apparatus on the 12th and 13th days of the experimental protocol. On the day of the task (the 12th day of the experimental protocol), each animal underwent a 5-min habituation session in the absence of objects, where the test to assess the locomotor and exploratory domains of the animals was performed.
After habituation on the apparatus, the mice were submitted to the training session, by being placed individually in the arena with two identical objects (object A1 and A2) for 5 min. Exploration was accounted when the animal directed its nose around 2 cm of the object while sniffing, touching, or looking at it. In the presence of a familiar object (A1) and a new object (B), 1.5 h after training, the STM of mice was evaluated. The time to explore was defined as 5 min, enough to measure learning and recognition memory. In turn, LTM was assessed 24 h after training. For this, the mice were placed to explore a familiar object (A1) and a new object (C) for 5 min. Likewise, the time spent exploring each object was recorded. The objects used were placed in a symmetrical position inside the arena. The A1 and A2 objects were two identical balls, the B object was a cube, and the C object was a square. The objects used were made of plastic material, measuring 10 × 10 cm (length × height), and had the following color patterns: blue, red, and yellow. The arena and objects were cleaned between trials with 70% ethanol to remove residues and smells. Data were expressed as a percentage of the exploratory preference and calculated as follows: Training = (A2 / (A1 + A2)) × 100; STM = (B / (A1 + B)) × 100; LTM = (C / (A1 + C)) × 100.
Ex Vivo Assays
Tissue Processing
Motivated by behavioral test results, ex vivo assays were performed to extend the knowledge about the factors that determine the development of PTX-induced peripheral neuropathy and that contribute to the therapeutic effect of 4-PSQ. Twenty-four hours after the last treatment, animals were killed by inhalation of isoflurane anesthetic. The spinal cord, cerebral cortex, and hippocampus were collected to determine oxidative stress markers, such as glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities, as well as reactive oxygen species (ROS), thiobarbituric acid reactive species (TBARS), and nitrate and nitrite (NOx) levels as indicator of nitric oxide (NO) production. Furthermore, the involvement of the Ca2+ATPase enzyme in the spinal cord, cerebral cortex, and hippocampus was evaluated. For these analyses, samples were homogenized in 50 mM TrisHCl pH 7.4 and centrifuged at 900 × g for 10 min to yield a supernatant (S1). Specifically for NOx levels, samples were homogenized in 200 mM ZnSO4 and acetonitrile (96%), centrifuged at 13,000 × g at 4 °C for 30 min, and the S1 was collected. Moreover, samples of spinal cord, cerebral cortex, and hippocampus were collected to determine nuclear factor-kappa B (NF-κB), TNF-α, IL-1β, and inducible nitric oxide synthase (iNOS) expressions using the qRT-PCR technique. Important, for this quantification, all collected tissues were snap-frozen in liquid nitrogen and stored at − 80° C until the mRNA expression levels were evaluated.
Involvement of Oxidative Stress
Oxidative Damage
ROS Levels
The ROS levels were determined using a spectrofluorimetric method, using 2′,7′-dichlorofluorescein diacetate (DCHF-DA) assay according to Loetchutinat et al. [45]. S1 (50 μL) was incubated with 20 μL of DCHF-DA (1 mmol/L) and 2430 μL of Tris HCl (10 mmol/L) in pH 7.4. The oxidation of DCHF-DA to fluorescent dichlorofluorescein (DCF) was measured for the detection of intracellular ROS. The DCF fluorescence intensity emission was recorded at 525 nm (with 488-nm excitation) 30 min after the addition of DCHF-DA to the medium (Shimadzu RF-5301PC fluorometer). ROS levels were expressed as arbitrary units of fluorescence.
TBARS Levels
Levels of TBARS were used as an indicator of lipid peroxidation [46]. An aliquot of S1 was added to the reaction mixture containing 0.8% thiobarbituric acid (TBA), 8.1% sodium dodecyl sulfate (SDS), and acetic acid buffer (pH 3.4) and after incubated at 95 °C for 2 h. Malondialdehyde (MDA) reacts with TBA to generate a colored product that can be optically measured at 532 nm. Results were reported as nmol MDA/mg protein.
NOx Levels
NOx levels were assayed spectrophotometrically according to a previously published study by Miranda et al. [47]. Spinal cord, cerebral cortex, and hippocampus samples were weighed and homogenized; the S1 was used to determine the nitrate and nitrite content, an indicator of NO production. NOx content was estimated in a medium containing 2% VCl3 (in 5% HCl), 0.1% N-(1-naphthyl) ethylene-diamine dihydrochloride, and 2% sulfanilamide (in 5% HCl). After incubating at 37 °C for 60 min, nitrite levels were determined spectrophotometrically at 540 nm, based on the reduction of nitrate to nitrite by VCl3. Tissue nitrate/nitrite levels were expressed as nmol of NOx/g of tissue.
Antioxidant Enzymes
GPx Activity
GPx activity was assayed spectrophotometrically by the method of Wendel [48], which involves monitoring the dismutation of hydrogen peroxide (H2O2) in the presence of S1 at 340 nm. S1 (50 μL) was added in a system composed by reduced glutathione (GSH)/NADPH/glutathione reductase (GR), and the enzymatic reaction was initiated by the addition of H2O2 (100 μL). In this assay, the enzymatic activity is indirectly measured by NADPH decay. H2O2 is reduced and generates oxidized glutathione (GSSG) from GSH. GSSG is regenerated back to GSH by the GR present in the analysis medium at the expense of NADPH. The enzymatic activity was expressed as nanomoles per minute per milligram of protein.
SOD Activity
This method is based on the capacity of SOD to inhibit the autoxidation of epinephrine. SOD activity was measured spectrophotometrically according to Misra and Fridovich’s method [49]. S1 (6, 12, or 18 μL) was added to a 0.05 mol/L Na2CO3 buffer, and the enzymatic reaction was started by adding epinephrine (30 μL). The color reaction was measured at 480 nm. One unit of the enzyme was defined as the amount of enzyme required to inhibit the rate of epinephrine autoxidation by 50% at 26 °C. The enzymatic activity was expressed as units per milligram of protein.
Involvement of Ca2+ATPase Activity
The activity of Ca2+ATPase was measured as previously described [50] with minor modifications [51]. Briefly, the assay medium consisted of 30 mM Tris–HCl buffer (pH 7.4), 50 mM NaCl, 5 mM KCl, 0.1 mM EDTA, 3 mM MgCl2, and 100 μg of protein in the presence or absence of 0.4 mM CaCl2. The reaction was started by the addition of adenosine triphosphate (ATP) to a final concentration of 12 mM. After 50 min at 37 °C, the reaction was stopped by the addition of 50% (w/v) trichloroacetic acid. The samples were then centrifuged; an aliquot was removed, and color reagent was added (2% ammonium molybdate). The amount of inorganic phosphate (Pi) released was quantified by colorimetric analysis, as previously described [52]. The Ca2+ATPase activity was determined by subtracting the activity measured in the presence of Ca2+ from that determined in the absence of Ca2+ (no added Ca2+ plus 0.1 mM EDTA) and expressed in nmol of Pi/min/mg of protein.
RNA Extraction, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total mRNA was extracted from thawed samples of the spinal cord, cerebral cortex, and hippocampus weighing between 50 and 100 mg using TRIzol reagent (Invitrogen™, Carlsbad, USA), followed by DNase treatment with DNase I Amplification Grade (Invitrogen™, Carlsbad, USA) to ensure minimum DNA contamination of the samples. The total RNA isolated was quantified, and its purity (260/280 and 260/230 ratios) was examined using a NanoVue spectrophotometer (GE, Fairfield, CT, USA).
The cDNA synthesis was performed using High Capacity cDNA Reverse Transcription kit (AppliedBiosystems™, UK) according to the manufacturer’s protocol. For reverse transcription, 1 μg of total RNA was used in a reaction volume of 20 μL. The amplification was made with GoTaq® qPCR Master Mix (Promega, Madison, WI) using the LightCycler® 96 Real-Time PCR System (Roche Molecular Systems Inc., CA, USA), and the sequence of primers used are indicated in Table 1. The qPCR conditions were as follows: 10 min at 95 °C to activate the hot-start Taq polymerase, followed by 35 cycles of denaturation for 15 s at 95 °C, primer annealing for 60 s at 60 °C, and extension for 30 s at 72 °C (fluorescence signals were detected at the end of every cycle). Baseline and threshold values were automatically set by the LightCycler® 96 Software.
The number of PCR cycles required to reach the fluorescence threshold in each sample was defined as the Ct value. The 2−ΔΔCT method was used to normalize the fold change in gene expressions [58], using GAPDH as housekeeping gene.
Protein Determination
The protein concentration was measured spectrophotometrically at 595 nm by the method of Bradford [59], using bovine serum albumin as the standard. The reaction mixture contained S1 (50 μL) and Coomassie brilliant blue (2.5 mL). The reaction mixture was incubated for 10 min. The protein level was expressed as milligrams of protein per milliliter.
Data and Statistical Analysis
Initially, we used a dedicated software for statistical power analyses (G*Power freeware from Heinrich-Heine-University Düsseldorf, v3.1.9.4) to determine the number of samples [60]. According to the statistical parameters provided, such as effect size = 0.7, power 80%, actual power 82.8%, and α = 0.05, G*Power suggests the use of 7 animals in each group (4 groups), totaling a sample size of the N = 28 in this study.
The normality of the data was evaluated using the D’Agostino and Pearson omnibus normality test. Statistical analysis was performed using GraphPad Prism 8.0 software (San Diego, CA, USA). Data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey test when appropriated for parametric data. Data were expressed as mean ± standard error of the mean (SEM). Post hoc tests were performed only when the F value achieved the necessary level of statistical significance (P < 0.05) and when there was no significant variance in homogeneity. Additionally, the data and statistical analysis of this study comply with the recommendations on experimental design and analysis in pharmacology [61].
Results
4-PSQ Reverses the PTX-Induced Thermal and Mechanical Sensitivities in Mice
PTX treatment caused a marked decrease in the paw withdrawal threshold after mechanical stimuli starting 8 days after the first PTX injection and persisting for at least 14 days. Reductions of 47% (day 8) (F(3,24) = 403.4, P < 0.0001), 44% (day 11) (F(3,24) = 333.1, P < 0.0001), and 44% (day 14) (F(3,24) = 575.8, P < 0.0001) in the paw withdrawal threshold in the mice exposed to PTX were verified, characterizing a mechanical hypersensitivity (Fig. 3). Likewise, thermal sensitivity was influencedl animals that received PTX showed a decrease in latency on the hot plate test on days 8 (around 63%) (F(3,24) = 40.75, P < 0.0001), 11 (around 62%) (F(3,24) = 50.25, P < 0.0001), and 14 (around 56%) (F(3,24) = 36.92, P < 0.0001), characterizing thermal hypersensitivity in mice and corroborating with the results of mechanical sensitivity (Fig. 4).
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the paw withdrawal threshold to mechanical stimulus in the von Frey test on the B 8th, C 11th, and D 14th day of the experimental protocol. Each point represents the mean of 7 mice in each group. ****P < 0.0001 denotes significance levels when compared with the control group; ####P < 0.0001 denotes significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the latency to thermal stimulus in the hot plate test on the B 8th, C 11th, and D 14th day of the experimental protocol. Each point represents the mean of 7 mice in each group. ****P < 0.0001 denotes significance levels when compared with the control group; ####P < 0.0001 denotes significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
To date, there are no effective and safe treatment options for chemotherapy-induced peripheral neuropathy (CIPN). For this reason, the effect of 4-PSQ was evaluated on PIPN. As shown in Fig. 3, daily treatment with 4-PSQ significantly attenuated the development of mechanical hypersensitivity induced by PTX (Fig. 3). The mechanical sensitivity of animals in the PTX + 4-PSQ group was similar to the control group. No significant difference was observed in the 4-PSQ group when compared to the control group (Fig. 3).
The 4-PSQ treatment effect on the hot plate test is demonstrated in Fig. 4. These results revealed that the treatment with 4-PSQ protected against PTX-induced thermal hypersensitivity (Fig. 4).
Locomotor and Exploratory Domains Were Not Altered by 4-PSQ and PTX
The number of crossings and rearing in the open field is presented in Fig. 5. The data analysis revealed that the treatment of mice with 4-PSQ and/or PTX did not cause any significant change in the number of crossings (F(3,24) = 1.453, P = 0.2522) or rearings (F(3,24) = 0.3525, P = 0.7841).
4-PSQ Attenuated the Emotional Impairment Caused by PIPN
On day 15 of the experimental protocol, the anxious-like behavior of the mice was assessed in the EPM test. The PTX-treated animals that showed painful stimuli in the previous tests also showed an anxious-like behavior. This behavior can be evidenced by the lower interaction of animals with open arms when compared to animals with the control group (Fig. 6). The data revealed that exposure to PTX decreased the percentage of time spent in the open arms (around 80%) (F(3,24) = 13.91, P < 0.0001) (Fig. 6A) and the percentage of entries in the open arms (around 68%) (F(3,24) = 21.84, P < 0.0001) (Fig. 6B) in the EPM test when compared with the control group.
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on behavioral parameters in the elevated plus-maze test in mice. A Percentage of time spent in the open arms and B percentage of entries in the open arms. Each column represents the mean ± SEM of 7 mice in each group. **P < 0.01 and ****P < 0.0001 denote significance levels when compared with the control group; ###P < 0.001 and ####P < 0.0001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
In turn, treatment with 4-PSQ was effective in reversing the changes caused by PTX (Fig. 6). The results presented in Fig. 6A demonstrate that treatment with 4-PSQ increased the time spent in open arms (F(3,24) = 13.91, P < 0.0001), reversing the change caused by PTX. Furthermore, the results presented in Fig. 6B demonstrate that treatment with 4-PSQ reversed the decrease of open arms entries (F(3,24) = 21.84, P < 0.0001) induced by PTX. This result confirms the potential anxiolytic-like effect of 4-PSQ [24, 29].
4-PSQ Attenuated the Cognitive Impairment Caused by PIPN
In the object recognition task, during the training phase, there was no significant difference among groups in the percentage of exploratory preference (F(3,24) = 0.4095, P = 0.7476) (Fig. 7A). Cognitive impairment of the animals was evaluated using the object recognition task on days 12 and 13. The data evidence that animals in the PTX group demonstrated a significant reduction in exploratory preference for the new object during the object recognition task (Fig. 7). This behavior demonstrates that the animals had a cognitive impairment, both in short-term (around 42%) (F(3,24) = 33.38, P < 0.0001) (Fig. 7B) and long-term (around 57%) (F(3,24) = 59.95, P < 0.0001) (Fig. 7C) memory when compared with the control group.
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the A training phase, B short-term, and C long-term memories in the object recognition task. Each column represents the mean ± SEM of 7 mice in each group. ****P < 0.0001 denotes significance levels when compared with the control group; ####P < 0.0001 denotes significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
In contrast, the administration of the 4-PSQ (1 mg/kg, p.o.) compound was able to reverse the cognitive impairment caused by PTX in mice since this group of animals preferred to explore more the new object during the task (Fig. 7). Thus, the results demonstrate that the treatment with 4-PSQ reversed the impairment of PTX-induced short-term memory (F(3,24) = 33.38, P < 0.0001) (Fig. 7B). Similarly, in long-term memory, treatment with 4-PSQ reversed the damage caused by PTX, increasing the exploratory preference for the new object (F(3,24) = 59.95, P < 0.0001) (Fig. 7C).
Involvement of Oxidative Stress on PIPN and the Antioxidant Effect of 4-PSQ in Mice
Several studies have explored the contributory role of oxidative stress to PTX-induced peripheral neuropathy [62, 63]. Thus, the contribution of oxidative stress on PTX-induced peripheral neuropathy was evaluated by means of oxidative damage markers (ROS, TBARS, and NOx levels), as well as by the activity of antioxidant enzymes (GPx and SOD) in the spinal cord, cerebral cortex, and hippocampus of mice. Increased levels of ROS were detected in the spinal cord (around 14%) (Fig. 8A), cerebral cortex (around 48%) (Fig. 8B), and hippocampus (around 12%) (Fig. 8C) of mice exposed to PTX when compared with the control group. Importantly, the treatment with 4-PSQ reversed the increase in the ROS levels induced by PTX exposure in the spinal cord (F(3,24) = 7.494, P = 0.0010), cerebral cortex (F(3,24) = 11.41, P < 0.0001), and hippocampus (F(3,24) = 7.351, P = 0.0012) of mice (Fig. 8).
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the ROS levels in the spinal cord (A), cerebral cortex (B), and hippocampus (C) of mice. Each column represents the mean ± SEM of 7 mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001 denote significance levels when compared with the control group; ##P < 0.01 and ###P < 0.001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
PTX administration also increased TBARS levels in the spinal cord (around 19%), cerebral cortex (around 22%), and hippocampus (around 44%) of mice compared with the control group (Fig. 9). On the other hand, the results showed that treatment with 4-PSQ reversed the increase in the TBARS levels induced by PTX in the spinal (F(3,24) = 16.89, P < 0.0001), cerebral cortex (F(3,24) = 9.377, P = 0.0003), and hippocampus (F(3,24) = 8.110, P = 0.0007) of mice when compared with the PTX group (Fig. 9).
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the TBARS levels in the spinal cord (A), cerebral cortex (B), and hippocampus (C) of mice. Each column represents the mean ± SEM of 7 mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001 denote significance levels when compared with the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
To determine whether especially reactive species of nitrogen play a key role in PTX-induced peripheral neuropathy, NOx levels were evaluated. Mice treated with PTX demonstrated a significant increase in NOx levels in the spinal cord (around 157%), cerebral cortex (around 55%), and hippocampus (around 71%) when compared with the control group (Fig. 10). In turn, the animals treated with 4-PSQ showed a significant reduction in NOx levels in the spinal cord (F(3,24) = 11.48, P < 0.0001) (Fig. 10A), cerebral cortex (F(3,24) = 11.93, P < 0.0001) (Fig. 10B), and hippocampus (F(3,24) = 9.612, P = 0.0002) (Fig. 10C) of mice when compared with the PTX group.
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the NOx levels in the spinal cord (A), cerebral cortex (B), and hippocampus (C) of mice. Each column represents the mean ± SEM of 7 mice in each group. **P < 0.01 and ***P < 0.001 denote significance levels when compared with the control group; ##P < 0.01 and ###P < 0.001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
These findings indicate that 4-PSQ was able to reverse the increased ROS, TBARS, and NOx levels induced by PTX in all tissues evaluated, indicating that the antioxidant effect of 4-PSQ can be involved in its analgesic action. Based on this, on the next step, we examined whether antioxidant enzymes GPx and SOD contribute to PTX and 4-PSQ effects. Figure 11 summarizes the results obtained regarding the activity of the enzyme GPx analyzed after the administration of PTX and/or treatment with 4-PSQ. Exposure to PTX increased the GPx activity in the spinal cord (around 46%), cerebral cortex (around 34%), and hippocampus (around 52%) (Fig. 11) of mice. Notably, daily treatment with 4-PSQ normalized the activity of the GPx enzyme in the spinal cord (F(3,24) = 5.059, P = 0.0075) (Fig. 11A), cerebral cortex (F(3,24) = 13.92, P < 0.0001) (Fig. 11B), and hippocampus (F(3,24) = 8.218, P = 0.0006) (Fig. 11C) of mice exposed to PTX. Likewise, the administration of PTX at the cumulative dose of 6 mg/kg, i.p., promoted a significant increase in SOD activity in all evaluated structures compared with the control group (Fig. 12). Thus, in the spinal cord (Fig. 12A), this increase was 20%, in the cerebral cortex 24% (Fig. 12B), and in the hippocampus, it was 35% (Fig. 12C). In turn, data analysis revealed that 4-PSQ treatment was able to attenuate SOD activity in the spinal cord (F(3,24) = 7.573, P = 0.0010), cerebral cortex (F(3,24) = 12.10, P < 0.0001), and hippocampus (F(3,24) = 6.273, P = 0.0027) of PTX-treated mice (Fig. 12).
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on glutathione peroxidase activity in the spinal cord (A), cerebral cortex (B), and hippocampus (C) of mice. Each column represents the mean ± SEM of 7 mice in each group. *P < 0.05 and ***P < 0.001 denote significance levels when compared with the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on superoxide dismutase activity in the spinal cord (A), cerebral cortex (B), and hippocampus (C) of mice. Each column represents the mean ± SEM of 7 mice in each group. *P < 0.05, **P < 0.01, and ****P < 0.0001 denote significance levels when compared with the control group; #P < 0.05, ##P < 0.01, and ###P < 0.001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
Inflammatory Response Contributes to PIPN and 4-PSQ Exerts an Anti-inflammatory Effect in Mice
Inflammatory response has been regarded as an important trigger on PIPN. For this reason, to investigate the neuroinflammatory changes during the chronic phase of PIPN, analyses of the mRNA expression levels of NF-κB, IL-1β, TNF-α, and iNOS were performed on the 15th day after the start of treatment with PTX.
NF-κB is an important transcription factor involved in controlling the expression of several genes linked to the inflammatory response. The results presented display that the mRNA expression levels of NF-κB were significantly upregulated in the spinal cord (around 22%) (Fig. 13A), cortex cerebral (around 13%) (Fig. 14A), and hippocampus (around 14%) (Fig. 15A) of PTX-treated mice when compared with the control group. In contrast, the mice which received 4-PSQ plus PTX demonstrated mRNA expression levels of NF-κB normalized in the spinal cord (F(3,20) = 12.05, P = 0.0001) (Fig. 13A), cortex cerebral (F(3,20) = 8.116, P = 0.0010) (Fig. 14A), and hippocampus (F(3,20) = 5.801, P = 0.0051) (Fig. 15A) when compared with the PTX group.
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the levels of mRNA expression of nuclear factor-kappa B (NF-κB) (A), interleukin-1beta (IL-1β) (B), tumor necrosis factor-alpha (TNF-α) (C), and inducible nitric oxide synthase (iNOS) (D) in the spinal cord of mice. Each column represents the mean ± SEM of 6 mice in each group. **P < 0.01 and ***P < 0.001 denote significance levels when compared with the control group; #P < 0.05, ##P < 0.01, and ####P < 0.0001 denote significance levels when compared with the PTX group; + P < 0.05 and + + P < 0.01 denote significance levels when compared with the 4-PSQ group (one-way ANOVA followed by Tukey’s test)
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the levels of mRNA expression of nuclear factor-kappa B (NF-κB) (A), interleukin-1beta (IL-1β) (B), tumor necrosis factor-alpha (TNF-α) (C), and inducible nitric oxide synthase (iNOS) (D) in the cerebral cortex of mice. Each column represents the mean ± SEM of 6 mice in each group. *P < 0.05, **P < 0.01, and ****P < 0.0001 denote significance levels when compared with the control group; #P < 0.05, ##P < 0.01, and ###P < 0.001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on the levels of mRNA expression of nuclear factor-kappa B (NF-κB) (A), interleukin-1beta (IL-1β) (B), tumor necrosis factor-alpha (TNF-α) (C), and inducible nitric oxide synthase (iNOS) (D) in the hippocampus of mice. Each column represents the mean ± SEM of 6 mice in each group. *P < 0.05, ***P < 0.001, and ****P < 0.0001 denote significance levels when compared with the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 denote significance levels when compared with the PTX group; + + + P < 0.001 denote significance levels when compared with the 4-PSQ group (one-way ANOVA followed by Tukey’s test)
Pro-inflammatory cytokines IL-1β and TNF-α play important role in initiating the inflammatory response. The IL-1β mRNA expression levels were found markedly upregulated in the spinal cord (around 98%) (Fig. 13B), cerebral cortex (around 68%) (Fig. 14B), and hippocampus (around 92%) (Fig. 15B) of PTX-treated mice when compared with the control group. On the other hand, the analysis of the results revealed that the increase in the IL-1β mRNA expression levels induced by PTX in mice was normalized in the spinal cord (F(3,20) = 6.499, P = 0.0030) (Fig. 13B) and cerebral cortex (F(3,20) = 14.83, P < 0.0001) (Fig. 14B) and reversed partially in the hippocampus (F(3,20) = 15.99, P < 0.0001) (Fig. 15B) after 4-PSQ treatment. Similarly, the TNF-α mRNA expression levels were upregulated in the spinal cord (around 67%) (F(3,20) = 15.96, P < 0.0001) (Fig. 13C) and hippocampus (around 43%) (F(3,20) = 38.31, P < 0.0001) (Fig. 15C) of PTX-treated mice when compared with the control group. Here, the treatment with 4-PSQ also was able to reverse this increase in the spinal cord (F(3,20) = 15.96, P < 0.0001) (Fig. 13C) and hippocampus (F(3,20) = 38.31, P < 0.0001) (Fig. 15C) of PTX-exposed mice. In turn, TNF-α mRNA expression levels in the cerebral cortex (F(3,20) = 1.783, P = 0.1828) of mice were not changed by treatments (Fig. 14C).
The pro-inflammatory enzyme iNOS also was upregulated in the spinal cord (around 73%) (Fig. 13D), cerebral cortex (around 55%) (Fig. 14D), and hippocampus (around 75%) (Fig. 15D) of the mice exposed to PTX when compared with the control group. On the other hand, the analysis of the results revealed that the increase in expression mRNA levels of the iNOS induced by PTX was decreased in the spinal cord (F(3,20) = 18.32, P < 0.0001) (Fig. 13D), cerebral cortex (F(3,20) = 9.126, P = 0.0005) (Fig. 14D), and hippocampus (F(3,20) = 12.17, P < 0.0001) (Fig. 15D) after 4-PSQ treatment in mice.
4-PSQ Normalizes Ca2+ATPase Activity in Mice with PIPN
Intracellular Ca2+ dysregulation has been associated with PIPN [16]. For this reason, the effect of PTX and/or 4-PSQ on the activity of the Ca2+ATPase ‒ an enzyme responsible for transporting calcium ions across membranes ‒ was investigated in the spinal cord, cerebral cortex, and hippocampus of mice. The results shown in Fig. 16 reveal that the administration of PTX increased the activity of the Ca2+ATPase enzyme in the spinal cord (around 188%) (F(3,24) = 43.81, P < 0.0001), cerebral cortex (around 126%) (F(3,24) = 18.77, P < 0.0001), and hippocampus (around 152%) (F(3,24) = 13.28, P < 0.0001) of mice when compared with the control group. In turn, the increased activity of this enzyme caused by exposure to PTX was normalized by treatment with 4-PSQ in the spinal cord (F(3,24) = 43.81, P < 0.0001) (Fig. 16A), cerebral cortex (F(3,24) = 18.77, P < 0.0001) (Fig. 16B), and hippocampus (F(3,24) = 13.28, P < 0.0001) (Fig. 16C) of mice.
Effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) (1 mg/kg, p.o.) and paclitaxel (PTX) (2 mg/kg, i.p.) on Ca.2+ATPase activity in the spinal cord (A), cerebral cortex (B), and hippocampus (C) of mice. Each column represents the mean ± SEM of 7 mice in each group. ***P < 0.001 and ****P < 0.0001 denote significance levels when compared with the control group; ###P < 0.001 and ####P < 0.0001 denote significance levels when compared with the PTX group (one-way ANOVA followed by Tukey’s test)
Discussion
The results presented here provide compelling evidence that 4-PSQ treatment was able to ameliorate the behavioral and biochemical changes observed in mice with painful peripheral neuropathy induced by PTX. Here, we reveal that 4-PSQ was effective in attenuating mechanical and thermal sensitivity in mice with PIPN. Moreover, it is noted that pain, cancer diagnosis, and treatment become distressing situations that can promote emotional and cognitive impairments that severely affect the quality of life of cancer survivors [64]. In this sense, our study demonstrates that treatment with PTX promoted emotional and cognitive impairments in mice. In turn, treatment with 4-PSQ attenuated anxiety-like behavior and cognitive impairment in mice with PIPN. PTX promoted important biochemical changes such as increased oxidative stress and neuroinflammation. Meanwhile, 4-PSQ treatment modulate markers of oxidative damage (ROS, NOx, and TBARS levels) and antioxidant enzymes (SOD and GPx). Likewise, 4-PSQ modulates the expression of inflammation-associated genes (NF-κB, IL-1β, TNF-α, and iNOS). Of note, that PTX promoted changes in Ca2+ homeostasis, increasing Ca2+ATPase activity. On the other hand, 4-PSQ showed an important role, normalizing the activity of this enzyme in mice with PIPN. Currently, drug combinations and multitarget therapies have been proposed as a promising therapeutic strategy for improving the adverse effects of chemotherapy such as PIPN. In this sense, these results highlight the potential pharmacological action of 4-PSQ for the treatment of comorbidities: peripheral neuropathy, anxiety, and memory impairment associated with exposure to PTX.
Taxane-induced toxicity affects the somatosensory nerves leading to the development of neuropathic pain [3]. Herein, it was demonstrated that the administration of 3 consecutive doses of PTX (2 mg/kg, i.p.) promoted mechanical and thermal hypersensitivities in mice, an outstanding feature of CIPN. Although the exact mechanism of PIPN is not fully understood, the consequence of this PTX-induced pathophysiological condition has been shown to be related to processes of neuroinflammation, oxidative stress, and alterations of mitochondrial function and excitability of peripheral neurons [3, 5].
PTX interacts with the mitochondrial permeability transition pore, and these changes are accompanied by increased production of ROS in the nervous system [63]. In fact, in our study, the administration of PTX (at a cumulative dose of 6 mg/kg, i.p.) promoted an increase in the levels of oxidative damage markers, such as ROS, TBARS, and NOx, on the CNS structures of mice. The balance between reactive species production and antioxidant defenses determines the degree of oxidative stress. Indeed, the burden of reactive species production is largely neutralized by a complex antioxidant system. SOD speeds the conversion of superoxide to H2O2, whereas GPx converts hydroperoxides such as H2O2 to water [65]. Considering the obtained results, it was observed that PTX exposure induced an increase in GPx and SOD activities in all tissues evaluated. These results are similar to those reported by Dugget et al. [63] which demonstrated that together with elevated ROS levels, GPx and SOD antioxidant enzyme activities are increased mainly at the peak pain (23–31 days) induced by PTX. In this study, Dugget et al. [63] attributed this increase to an inadequate and delayed response of the endogenous antioxidant system, and thus, excessive ROS would increase pain signaling, leading to the development of persistent pain. Herein, ex vivo analyses were performed 15 days after the first administration of PTX, so this phenomenon also seems to be occurring during this period. On the other hand, another hypothesis that we cannot rule out is that the increased activity of GPx (spinal cord, cerebral cortex, and hippocampus) and SOD (spinal cord, cerebral cortex, and hippocampus) in animals exposed to PTX may be associated with a compensatory mechanism, that is, the system antioxidants would be acting in synergy to control potential damage caused by the accumulation of ROS. This compensatory mechanism has been observed together with the increase in SOD expression, whose expression level adapts to changes caused by oxidative stress [66, 67]. Indeed, when the oxidative damage is severe, survival is dependent on the ability of the cell to adapt and resist the damage, as well as to repair or replace the damaged molecules [67]. Hence, several oxidative stress response mechanisms have evolved to help the cells and organism adapt to oxidative damage [68]. The isoform manganese superoxide dismutase (MnSOD) has demonstrated a crucial role in protecting cells against oxidative stress, especially in relation to the maintenance of cell physiology in response to genotoxic conditions, such as oxidative stress [66, 69]. Although we have not evaluated the activity and expression of this isoform, here we report an increase in SOD activity in the CNS, and we believe that this phenomenon may be associated with the adaptive role of the MnSOD isoform.
Noteworthy, it has been reported that oxidative stress is also involved in the activation of signaling pathways that lead to increased gene expression and NF-κB release, initiating inflammatory and cytokine cascades, and this inflammatory response results in neuropathic pain [20]. Kamei et al. revealed that activation of the NF-κB pathway evoked PTX-induced hyperalgesia [70]. Moreover, evidence suggests that PTX treatment can lead to pain sensitivity with the release of pro-inflammatory cytokines including TNF-α and IL-1β which directly or indirectly induce neuropathic pain [14]. Our study showed that PTX increased NF-κB expression in the spinal cord, cerebral cortex, and hippocampus. We also detected an increased expression of TNF-α and IL-1β in mice exposed to PTX after 15 days. Here, we reported an increase in RS production in animals exposed to PTX; this increase may be linked to the high amount of H2O2 formed, which can rapidly diffuse into extracellular spaces, affecting neighboring cells and contributing to oxidative stress and consequently activation of NF-κB. Alongside, it has been demonstrated that peroxynitrite, a product of the reaction of the superoxide anion radical and NO, is a potent pro-inflammatory and pronociceptive species implicated in pain of different etiologies [71, 72]. Corroborating with this, Doyle et al. [72] demonstrated that the overproduction of peroxidonitrite contributes to an increase in pro-inflammatory cytokines such as TNF-α and IL-1β. Therefore, we believe that the increase in the expression of these inflammatory mediators is associated, at least in part, with the increase in oxidative damage caused by PTX.
iNOS, which produces NO, is the enzyme primarily responsible for activating immune and inflammatory processes [73]. Here, we also documented that the animals exposed to PTX showed high levels of NOx in all tissues evaluated. Similarly, mice that demonstrated mechanical and thermal hypersensitivity induced by PTX demonstrated significant upregulation of iNOS mRNA levels in the spinal cord, cerebral cortex, and hippocampus. An important role in the initiation and maintenance of the nociceptive response has been attributed to NO [71]. In fact, nitroxidative species can directly increase the excitability of nociceptive neurons by phosphorylation of N-methyl-D-aspartate (NMDA) receptors [71]. Likewise, iNOS may play a role in the development and sensation of inflammatory and neuropathic pain [71]. Thus, the increase in NOx levels and iNOS expression observed in our study may also be contributing to the activation of the NF-κB transcription factor and, consequently, the expression of pro-inflammatory cytokines such as TNF-α and IL-1β. Taken together, these changes caused by PTX seem to influence the perception of pain and contribute to the increase in mechanical and thermal sensitivities observed in our study.
To explore other mechanisms involved in the PIPN, possible changes in Ca2+ATPase activities on CNS were evaluated. Calcium is a key regulatory ion in many cellular and physiological processes. In this line, the deregulation of Ca2+ homeostasis and Ca2+ signaling can influence membrane excitability, neurotransmitter release, and gene expression of neuronal and glial cells, and consequently contribute to the development of PIPN [5, 74]. Our results revealed, for the first time, that animals exposed to PTX significantly increased Ca2+ATPase activity in the CNS. It has been reported that PTX causes a rapid decline in mitochondrial membrane potential and a loss of mitochondrial Ca2+ [75]. Alongside, Boehmerle et al. [74] present the hypothesis that the Ca2+ oscillation is induced by the binding of PTX to neuronal Ca2+ sensor 1 (NCS-1) and subsequent positive modulation of inositol 1,4,5-trisphosphate receptor (InsP3R). These effects, in turn, lead to the activation of Ca2+-activated proteases resulting in increased damage to peripheral neurons, leading to cell death [74]. Pro-nociceptive mediators can stimulate the activity of the Ca2+ATPase enzyme, keeping intracellular Ca2+ concentration low and increasing the excitability of sensory neurons that conduct the nociceptive responses to the CNS [76]. Considering the importance of cellular homeostasis in the regulation of the nociceptive response, inhibition of the Ca2+ATPase enzyme could be an interesting target to reverse the painful stimuli induced by PTX.
To increase our understanding of PTX-induced toxicity, the relationship between nociceptive and affective/cognitive symptoms needs to be considered. Thus, possible comorbidities such as anxiety and cognitive deficit were investigated, and interestingly, we observed that the animals that showed painful stimuli induced by PTX also showed emotional and cognitive impairments. Studies have suggested that PTX can cross the blood–brain barrier, since it has been shown that this chemotherapeutic drug accumulates in low concentrations in the brain of mice following tail vein injection [77, 78]. Given the hypothesis that low concentrations of PTX can cross the blood–brain barrier, the impairments demonstrated here may be associated with mechanisms of neuroinflammation and/or induction of central neurotoxicity. This hypothesis may be raised because PTX promoted alterations in both neuroinflammatory processes and oxidative stress in the CNS of mice. We demonstrated the cognitive loss caused by PTX by means of the object recognition task. This test assesses episodic and non-spatial memory in rodents, and the hippocampus is an important brain structure for this type of memory. In turn, it has been shown that hippocampus-dependent episodic memory is vulnerable to PTX [79]. Moreover, Huehnchen et al. [80] have revealed the hypothesis that hippocampal neurogenesis is impaired by PTX. Therefore, it is possible that the mice that received PTX presented some impairment in hippocampal neurogenesis, which culminated in the significant cognitive loss. Additionally, the increased inflammatory responses and oxidative stress induced by PTX may have contributed to the development of peripheral neuropathy and consequently to the affective and cognitive impairments observed in the mice.
The most common neurotoxicity associated with PTX administration is predominantly sensory peripheral neuropathy, characterized by an increase in mechanical and thermal sensitivities [12]. Indeed, here PTX produced a significant increase in the mechanical and thermal sensitivities in mice. The management of the PIPN generally focuses on treating symptoms, since the cause of the pain still cannot be treated. Given the importance of the search for new therapeutic strategies to improve this adverse effect caused by chemotherapy, 4-PSQ was investigated for the first time as a therapeutic target for the treatment of PIPN.
The promising pharmacological effects of compound 4-PSQ are well established in the literature, including antinociceptive [22], anxiolytic-like [24, 29], antidepressant-like [27] actions, as well as prevention of cognitive deficit [31]. Recently, 4-PSQ has also been shown to reverse chronic [36] and acute [28] oxaliplatin-induced peripheral neuropathy, as well as signs of anxiety and cognitive loss [35]. 4-PSQ exerts its action primarily through its antioxidant [25, 26, 30], anti-inflammatory [23], and neuroprotective properties [39]. It is important to highlight that in the present study, 4-PSQ treatment reversed the mechanical and heat thermal hypersensitivities induced by PTX exposure, without impairing the locomotor and exploratory capacity of the animals.
Reinforcing the antioxidant potential of 4-PSQ, we observed that the PTX-induced oxidative changes were reversed in response to 4-PSQ treatment, evidenced by the normalization of ROS content, lipid peroxidation, and NOx levels in the spinal cord, cerebral cortex, and hippocampus. In addition, the PTX-exposed mice exhibited increased GPx and SOD activities, which also were normalized by treatment with 4-PSQ in all structures evaluated. These results demonstrate that treatment with 4-PSQ is able to reverse neuronal oxidative damage and modulate the antioxidant defense system. PIPN involves multiple mechanisms, including oxidative stress [14]. Based on this, we cannot affirm whether 4-PSQ acts directly on the redox state or whether it modulates other pathways, reducing the process of oxidative stress. However, we can assume that 4-PSQ may be acting on different lines of antioxidant defense, attenuating oxidative damage and restoring the antioxidant defense system, and consequently reducing the PIPN, possibly preventing damage to sensory neurons.
The pathophysiology of PIPN is related to perturbed of inflammatory pathways that include cytokines and their receptors, besides NF-κB-related signaling pathways [3]. Interestingly, data of our study demonstrated that daily treatment with 4-PSQ downregulated the expression of neuroinflammatory parameters of mice exposed to PTX, such as NF-κB, TNF-α, and IL-1β. Notably, the inflammatory process can induce oxidative stress; the oxidative stress can also induce inflammation through activation of multiple pathways [81]. Elevated ROS levels can induce inflammation through activation of the NF-κB transcription factor [81]. Therefore, based on previous data, we suggested that the effect of 4-PSQ in modulating neuroinflammation is associated with the attenuation of oxidative stress in the spinal cord, cerebral cortex, and hippocampus of mice. Evidence has pointed to significant connections between inflammation and oxidative stress, both processes contributing to fuel the other one, thereby establishing a vicious cycle able to perpetuate and propagate the inflammatory response [82, 83]. Thus, these results demonstrate the importance of the therapeutic use of 4-PSQ, since it was able to attenuate neuroinflammation and oxidative stress, reducing the painful symptoms induced by PTX.
Another significant finding was that treatment with 4-PSQ normalized NOx levels as well as iNOS mRNA expression levels in the CNS of mice that received PTX. Expression of iNOS and NOx production is associated with inflammatory conditions in which TNF-α and IL-1β cytokines are produced. NOx is an inflammation-associated oxidant that demonstrates an important role in central sensitization, acting on the phosphorylation of NMDA receptors and consequently increasing the excitability of nociceptive neurons [71]. Importantly, previous studies revealed that treatment with 4-PSQ was shown to reduce glutamate uptake in the brain of mice [29]. Moreover the glutamatergic system, in particular the NMDA receptor, is strongly involved in the pharmacological effects of 4-PSQ [23]. In view of these results, we believe that the reduced levels of NOx by 4-PSQ treatment were also a consequence of its ability to attenuate iNOS expression. Furthermore, considering the involvement of the glutamatergic system in the effect of 4-PSQ, we hypothesized that this compound may be attenuating the excitability of sensory neurons and thus promoting analgesic action by reducing mechanical and thermal hypersensitivities.
Enzyme Ca2+ATPase plays an important role in the maintenance of levels of intracellular Ca2+, essential to the functioning of neurons [84]. Ca2+ is an ion responsible for regulating many cellular and physiological processes, but the increase of these levels indicate excitotoxic events, which can influence membrane excitability [85]. Here, we demonstrated for the first time that the daily administration of 4-PSQ normalizes the activity of the Ca2+ATPase; thus, we believe that 4-PSQ may be promoting regulation of Ca2+ homeostasis. Indeed, administration of Ca2+ channel antagonists has been shown to promote a reduction in paclitaxel-induced neuropathic pain [86]. Considering this, we suggest that the antinociceptive effect of 4-PSQ is also related to the maintenance of cellular ionic concentration, attenuating the excitotoxicity involved in PIPN.
Neuropathic pain caused by chemotherapy can result in profound emotional and cognitive changes, drastically affecting the quality of life of cancer survivors [87, 88]. Therefore, there is a need to improve the quality of life of patients undergoing PTX treatment by identifying new targets that prevent or treat these adverse effects. In this sense, we investigated the effect of 4-PSQ on anxiety and cognitive-deficit comorbidities caused by PIPN. We highlight that 4-PSQ attenuated anxiety-like behavior and cognitive impairment in animals treated with PTX. Based on this, our results reinforce that 4-PSQ has an anxiolytic-like action [24, 29, 35] and memory improvement [31, 33, 35], and proves the pharmacological effect of 4-PSQ in the treatment of comorbidities observed in PIPN. We can suggest that 4-PSQ appears as a promising strategy for the management of PIPN and its comorbidities, attenuating behavioral changes due to its ability to regulate molecules involved in oxidative stress and the neuroinflammation process.
The findings of this study are highly relevant, as there are no effective strategies to prevent and treat PIPN. Given the complex pathophysiology of this condition caused by chemotherapy, combined therapies may be necessary and interesting for the more effective management of PIPN [89]. Notably, it is possible to reaffirm that 4-PSQ is a promising molecule as a therapeutic strategy for PIPN, as it has been shown to act on multiple targets involved in the development of this pathology. Besides, this multitargeted molecule also attenuated the anxiety and cognitive loss associated with PTX-induced pain. In this sense, the versatility of 4-PSQ deserves special attention, as complex disorders, such as PIPN, can be more easily attenuated by the simultaneous modulation of multiple targets.
Although promising results have been obtained, it is important to emphasize that this study presents some limitations, including the absence of data comparing the effect of treatments in female mice, since sex is an important biological and experimental variable. In addition, the hypothesis that 4-PSQ could interact with PTX, interfering in the antineoplastic effect, cannot be completely ruled out. We understand that the evaluation of these conditions is important and relevant, so this point is the main objective of our research group for future studies.
Conclusion
In conclusion, this study was the first to report that 4-PSQ-based therapy reduced PTX-induced mechanical and thermal hypersensitivity. Similarly, 4-PSQ was found to be effective in attenuating comorbidities associated with PIPN, such as anxiety and cognitive impairment. Based on the findings obtained, we hypothesize that the beneficial effects of 4-PSQ on PIPN are related to its ability to modulate oxidative stress and neuroinflammatory parameters, as well as its ability to restore electrolyte homeostasis by normalizing the activity of the enzyme Ca2+ATPase. Therefore, the results of the present study support the use of 4-PSQ as a promising multitarget prototype for the treatment of PTX-induced painful peripheral neuropathy and its concomitant diseases.
Data Availability
All datasets generated and analyzed during this study are included in this article. Materials are available upon request.
References
Xie S, Ogden A, Aneja R, Zhou J (2016) Microtubule-binding proteins as promising biomarkers of paclitaxel sensitivity in cancer chemotherapy. Med Res Rev 36:300. https://doi.org/10.1002/MED.21378
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277(5698):665–667. https://doi.org/10.1038/277665a0
da Costa R, Passos GF, Quintão NLM et al (2020) Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol 177:3127–3146. https://doi.org/10.1111/BPH.15086
Tasnim A, Rammelkamp Z, Slusher AB et al (2016) Paclitaxel causes degeneration of both central and peripheral axon branches of dorsal root ganglia in mice. BMC Neurosci 17:47. https://doi.org/10.1186/S12868-016-0285-4
Starobova H, Vetter I (2017) Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 10:174. https://doi.org/10.3389/fnmol.2017.00174
Park SB, Goldstein D, Krishnan AV et al (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63:419–437. https://doi.org/10.3322/CAAC.21204
Scripture CD, Figg WD, Sparreboom A (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol 4:165. https://doi.org/10.2174/157015906776359568
Lipton RB, Apfel SC, Dutcher JP et al (1989) Taxol produces a predominantly sensory neuropathy. Neurology 39:368–368. https://doi.org/10.1212/WNL.39.3.368
van Gerven JMA, Moll JWB, van den Bent MJ et al (1994) Paclitaxel (taxol) induces cumulative mild neurotoxicity. Eur J Cancer 30:1074–1077. https://doi.org/10.1016/0959-8049(94)90459-6
Pace A, Nisticò C, Cuppone F et al (2007) Peripheral neurotoxicity of weekly paclitaxel chemotherapy: a schedule or a dose issue? Clin Breast Cancer 7:550–554. https://doi.org/10.3816/CBC.2007.N.010
Seretny M, Currie GL, Sena ES et al (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. PAIN® 155:2461–2470. https://doi.org/10.1016/J.PAIN.2014.09.020
Staff NP, Fehrenbacher JC, Caillaud M et al (2020) Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol 324:113121. https://doi.org/10.1016/J.EXPNEUROL.2019.113121
Velasco R, Bruna J (2015) Taxane-induced peripheral neurotoxicity. Toxics 3:152–169. https://doi.org/10.3390/TOXICS3020152
Klein I, Lehmann HC (2021) Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics 9.https://doi.org/10.3390/TOXICS9100229
Fidanboylu M, Griffiths LA, Flatters SJL (2011) Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS ONE 6:25212. https://doi.org/10.1371/JOURNAL.PONE.0025212
Siau C, Bennett GJ (2006) Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg 102:1485. https://doi.org/10.1213/01.ANE.0000204318.35194.ED
Huang ZZ, Li D, Liu CC et al (2014) CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun 40:155–165. https://doi.org/10.1016/J.BBI.2014.03.014
Zhang H, Li Y, De Carvalho-Barbosa M et al (2016) Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy induced peripheral neuropathy. J Pain 17:775. https://doi.org/10.1016/J.JPAIN.2016.02.011
Wang XM, Lehky TJ, Brell JM, Dorsey SG (2012) Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine 59:3–9. https://doi.org/10.1016/J.CYTO.2012.03.027
Flatters SJL, Bennett GJ (2006) Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122:245–257. https://doi.org/10.1016/J.PAIN.2006.01.037
Postma TJ, Vermorken JB, Liefting AJM et al (1995) Paclitaxel-induced neuropathy. Ann Oncol 6:489–494. https://doi.org/10.1093/OXFORDJOURNALS.ANNONC.A059220
Pinz M, Reis AS, Duarte V et al (2016) 4-Phenylselenyl-7-chloroquinoline, a new quinoline derivative containing selenium, has potential antinociceptive and anti-inflammatory actions. Eur J Pharmacol 780:122–128. https://doi.org/10.1016/j.ejphar.2016.03.039
Silva VDG, Reis AS, Pinz MP et al (2017) Further analysis of acute antinociceptive and anti-inflammatory actions of 4-phenylselenyl-7-chloroquinoline in mice. Fundam Clin Pharmacol 31:513–525. https://doi.org/10.1111/fcp.12295
Paltian JJ, dos Reis AS, de Oliveira RL et al (2020) The anxiolytic effect of a promising quinoline containing selenium with the contribution of the serotonergic and GABAergic pathways: modulation of parameters associated with anxiety in mice. Behav Brain Res 393:112797. https://doi.org/10.1016/J.BBR.2020.112797
Lemos BB, Da Motta KP, Paltian JJ et al (2021) Role of 7-chloro-4-(phenylselanyl) quinoline in the treatment of oxaliplatin-induced hepatic toxicity in mice. Can J Physiol Pharmacol 99:378–388. https://doi.org/10.1139/CJPP-2020-0134
da Motta KP, Lemos BB, Paltian JJ et al (2021) 7-Chloro-4-(phenylselanyl) quinoline reduces renal oxidative stress induced by oxaliplatin in mice. Can J Physiol Pharmacol 99:1102–1111. https://doi.org/10.1139/CJPP-2021-0090
de Oliveira RL, Voss GT, da C. Rodrigues K et al (2022) Prospecting for a quinoline containing selenium for comorbidities depression and memory impairment induced by restriction stress in mice. Psychopharmacol 2021 2391 239:59–81. https://doi.org/10.1007/S00213-021-06039-8
Reis AS, Martins CC, da Motta KP et al (2022) Interface of aging and acute peripheral neuropathy induced by oxaliplatin in mice: target-directed approaches for Na+, K+—ATPase, oxidative stress, and 7-chloro-4-(phenylselanyl) quinoline therapy. Mol Neurobiol 1:1–15. https://doi.org/10.1007/S12035-021-02659-5/FIGURES/8
Reis AS, Pinz M, Duarte LFB et al (2017) 4-Phenylselenyl-7-chloroquinoline, a novel multitarget compound with anxiolytic activity: contribution of the glutamatergic system. J Psychiatr Res 84:191–199. https://doi.org/10.1016/j.jpsychires.2016.10.007
Vogt AG, Voss GT, de Oliveira RL et al (2018) Organoselenium group is critical for antioxidant activity of 7-chloro-4-phenylselenyl-quinoline. Chem Biol Interact 282:7–12. https://doi.org/10.1016/j.cbi.2018.01.003
Pinz MP, Dos Reis AS, Vogt AG et al (2018) Current advances of pharmacological properties of 7-chloro-4-(phenylselanyl) quinoline: prevention of cognitive deficit and anxiety in Alzheimer’s disease model. Biomed Pharmacother 105:1006–1014. https://doi.org/10.1016/j.biopha.2018.06.049
Voss GT, Oliveira RL, de Souza JF et al (2018) Therapeutic and technological potential of 7-chloro-4-phenylselanyl quinoline for the treatment of atopic dermatitis-like skin lesions in mice. Mater Sci Eng C Mater Biol Appl 84:90–98. https://doi.org/10.1016/j.msec.2017.11.026
Barth A, Vogt AG, dos Reis AS et al (2019) 7-Chloro-4-(phenylselanyl) quinoline with memory enhancer action in aging rats: modulation of neuroplasticity, acetylcholinesterase activity, and cholesterol levels. Mol Neurobiol 56:6398–6408. https://doi.org/10.1007/s12035-019-1530-5
Luchese C, Barth A, da Costa GP et al (2020) Role of 7-chloro-4-(phenylselanyl) quinoline as an anti-aging drug fighting oxidative damage in different tissues of aged rats. Exp Gerontol 130:110804. https://doi.org/10.1016/J.EXGER.2019.110804
Reis AS, Paltian JJ, Domingues WB et al (2020) Pharmacological modulation of Na+, K+-ATPase as a potential target for OXA-induced neurotoxicity: correlation between anxiety and cognitive decline and beneficial effects of 7-chloro-4-(phenylselanyl) quinoline. Brain Res Bull 162:282–290. https://doi.org/10.1016/j.brainresbull.2020.06.021
Reis AS, Paltian JJ, Domingues WB et al (2020) Advances in the understanding of oxaliplatin-induced peripheral neuropathy in mice: 7-chloro-4-(phenylselanyl) quinoline as a promising therapeutic agent. Mol Neurobiol 57:5219–5234. https://doi.org/10.1007/S12035-020-02048-4/FIGURES/9
Duarte LFB, Barbosa ES, Oliveira RL et al (2017) A simple method for the synthesis of 4-arylselanyl-7-chloroquinolines used as in vitro acetylcholinesterase inhibitors and in vivo memory improvement. Tetrahedron Lett 58:3319–3322. https://doi.org/10.1016/j.tetlet.2017.07.039
Polomano RC, Mannes AJ, Clark US, Bennett GJ (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94:293–304. https://doi.org/10.1016/S0304-3959(01)00363-3
Rodrigues KC, Bortolatto CF, da Motta KP et al (2021) The neurotherapeutic role of a selenium-functionalized quinoline in hypothalamic obese rats. Psychopharmacology 238:1937–1951. https://doi.org/10.1007/s00213-021-05821-y
Alamri FF, Al SA, Biggers A et al (2018) Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behav Brain Res 336:250–255. https://doi.org/10.1016/J.BBR.2017.09.008
WOOLFE, G. (1944) The evaluation of the analgesic actions of pethidine hydrochlodide (Demerol). J Pharmacol Exp Ther 80:300–307
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504. https://doi.org/10.1037/0033-2909.83.3.482
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. https://doi.org/10.1016/0165-0270(85)90031-7
Lueptow LM (2017) Novel object recognition test for the investigation of learning and memory in mice. JoVE (Journal Vis Exp 2017:e55718. https://doi.org/10.3791/55718
Loetchutinat C, Kothan S, Dechsupa S et al (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat Phys Chem 72:323–331. https://doi.org/10.1016/j.radphyschem.2004.06.011
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Miranda KM, Espey MG, Yamada K et al (2001) Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem 276:1720–1727. https://doi.org/10.1074/jbc.m006174200
Wendel A (1981) Glutathione peroxidase. Methods Enzym 77:325–333. https://doi.org/10.1016/s0076-6879(81)77046-0
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1016/s0021-9258(19)45228-9
Rohn TT, Hinds TR, Vincenzi FF (1993) Ion transport ATPases as targets for free radical damage: protection by an aminosteroid of the Ca2+ pump atpase and Na+/K+ pump ATPase of human red blood cell membranes. Biochem Pharmacol 46:525–534. https://doi.org/10.1016/0006-2952(93)90530-A
Trevisan G, Maldaner G, Velloso NA et al (2009) Antinociceptive effects of 14-membered cyclopeptide alkaloids. J Nat Prod 72:608–612. https://doi.org/10.1021/NP800377Y/SUPPL_FILE/NP800377Y_SI_001.PDF
Fiske CH, Subbarow YJ (1925) The colorimetric determination of phosphorus. J Biol Chem 66(2):375–400
Kim HS, Jung YY, Do SI (2014) Hepatic inducible nitric oxide synthase expression increases upon exposure to hypergravity. Braz J Med Biol Res 47:940–946. https://doi.org/10.1590/1414-431X20143834
Silverman HA, Dancho M, Regnier-Golanov A et al (2014) Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Mol Med 20:601–611. https://doi.org/10.2119/MOLMED.2014.00147
Zhao B, Ren B, Guo R et al (2017) Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol 109:505–516. https://doi.org/10.1016/J.FCT.2017.09.050
Li X, Su L, Zhang X et al (2017) Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res 39:367–373. https://doi.org/10.1080/01616412.2017.1286541
Bruckert G, Vivien D, Docagne F, Roussel BD (2016) Normalization of reverse transcription quantitative PCR data during ageing in distinct cerebral structures. Mol Neurobiol 53:1540–1550. https://doi.org/10.1007/S12035-015-9114-5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/METH.2001.1262
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. https://doi.org/10.3758/BF03193146
Amrita Ahluwalia C, Journal B, Curtis MJ et al (2018) Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Wiley Online Libr 175:987–993. https://doi.org/10.1111/bph.14153
Shim HS, Bae C, Wang J et al (2019) Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol Pain 15.https://doi.org/10.1177/1744806919840098
Duggett NA, Griffiths LA, McKenna OE et al (2016) Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 333:13–26. https://doi.org/10.1016/J.NEUROSCIENCE.2016.06.050
Miaskowski C, Mastick J, Paul SM et al (2018) Impact of chemotherapy-induced neurotoxicities on adult cancer survivors’ symptom burden and quality of life. J Cancer Surviv 12:234. https://doi.org/10.1007/S11764-017-0662-8
Harwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150. https://doi.org/10.1042/BST0351147
Candas D, Li JJ (2014) MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. https://home.liebertpub.com/ars 20:1599–1617. https://doi.org/10.1089/ARS.2013.5305
Allen RG, Tresini M (2000) Oxidative stress and gene regulation. Free Radic Biol Med 28:463–499. https://doi.org/10.1016/S0891-5849(99)00242-7
Rodriguez C, Mayo JC, Sainz RM et al (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9. https://doi.org/10.1046/J.1600-079X.2003.00092.X
Maurya R, Namdeo M (2021) Superoxide dismutase: a key enzyme for the survival of intracellular pathogens in host.https://doi.org/10.5772/INTECHOPEN.100322
Kamei J, Hayashi S, Sakai A et al (2017) Rikkunshito prevents paclitaxel-induced peripheral neuropathy through the suppression of the nuclear factor kappa B (NFκB) phosphorylation in spinal cord of mice. PLoS One 12.https://doi.org/10.1371/JOURNAL.PONE.0171819
Grace PM, Gaudet AD, Staikopoulos V et al (2016) Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci 39:862. https://doi.org/10.1016/J.TINS.2016.10.003
Doyle T, Chen Z, Muscoli C et al (2012) Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci 32:6149–6160. https://doi.org/10.1523/JNEUROSCI.6343-11.2012
Cinelli MA, Do HT, Miley GP, Silverman RB (2019) Inducible nitric oxide synthase: regulation, structure, and inhibition. Med Res Rev 40:158–189. https://doi.org/10.1002/MED.21599
Boehmerle W, Splittgerber U, Lazarus MB et al (2006) Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc Natl Acad Sci U S A 103:18356. https://doi.org/10.1073/PNAS.0607240103
Kidd JF, Pilkington MF, Schell MJ et al (2002) Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore *. J Biol Chem 277:6504–6510. https://doi.org/10.1074/JBC.M106802200
Usachev YM, DeMarco SJ, Campbell C et al (2002) Bradykinin and ATP accelerate Ca2+ efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca2+ pump isoform 4. Neuron 33:113–122. https://doi.org/10.1016/S0896-6273(01)00557-8
Gangloff A, Hsueh W-A, Kesner AL et al (2005) Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with 18F-fluoropaclitaxel. Soc Nucl Med 46(11):1866–1871
Kemper EM, Van Zandbergen AE, Cleypool C et al (2003) Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res 9(7):2849–2855
Chang A, Chung NC, Lawther AJ et al (2020) The anti-inflammatory drug aspirin does not protect against chemotherapy-induced memory impairment by paclitaxel in mice. Front Oncol 10.https://doi.org/10.3389/FONC.2020.564965/FULL
Huehnchen P, Boehmerle W, Springer A et al (2017) A novel preventive therapy for paclitaxel-induced cognitive deficits: preclinical evidence from C57BL/6 mice. Transl Psychiatry 7:e1185. https://doi.org/10.1038/TP.2017.149
Flohé L, Brigelius-Flohé R, Saliou C et al (1997) Redox regulation of NF-kappa B activation. Free Radic Biol Med 22:1115–1126. https://doi.org/10.1016/S0891-5849(96)00501-1
Biswas SK (2016) Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev 2016.https://doi.org/10.1155/2016/5698931
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395:203–230. https://doi.org/10.1515/HSZ-2013-0241/ASSET/GRAPHIC/J_HSZ-2013-0241_FIG_012.JPG
Zaidi A (2010) Plasma membrane Ca-ATPases: targets of oxidative stress in brain aging and neurodegeneration. World J Biol Chem 1:271. https://doi.org/10.4331/WJBC.V1.I9.271
Miller RJ (1991) The control of neuronal Ca2+ homeostasis. Prog Neurobiol 37:255–285. https://doi.org/10.1016/0301-0082(91)90028-Y
Xiao W, Boroujerdi A, Bennett GJ, Luo ZD (2007) Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience 144:714–720. https://doi.org/10.1016/J.NEUROSCIENCE.2006.09.044
Bao T, Basal C, Seluzicki C et al (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159:327. https://doi.org/10.1007/S10549-016-3939-0
Matsos A, Johnston IN (2019) Chemotherapy-induced cognitive impairments: a systematic review of the animal literature. Neurosci Biobehav Rev 102:382–399. https://doi.org/10.1016/J.NEUBIOREV.2019.05.001
Flatters SJL, Dougherty PM, Colvin LA (2017) Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. BJA Br J Anaesth 119:737–749. https://doi.org/10.1093/BJA/AEX229
Funding
This study received financial support and scholarships from the following Brazilian agencies: National Council for Scientific and Technological Development (CNPq) (429859/2018–0, 312747/2020–9) and Research Support Foundation of Rio Grande do Sul (FAPERGS) (PqG 17/2551-0001013-2, 21/2551-0001943-3). This study was also financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES)—Finance Code 001. C.L., D.A., E.A.W., V.F.C, and R.F.S. are recipients of CNPq fellowship. This study also received financial assistance from L’ORÉAL-UNESCO-ABC for Women in Science.
Author information
Authors and Affiliations
Contributions
J.J.P. and A.S.R. performed the experiments and the analysis of data. J.J.P., A.S.R., C.L., and E.A.W. conceived and designed the study. A.W.S.M., E.B.B., E.N.D., and V.F.C. were responsible for perform qRT-PCR. L.K.C., R.F.S., and D.A. performed the 4-PSQ synthesis. J.J.P and E.A.W. wrote the manuscript. E.A.W and C.L. supervised the study. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Animal care and all experimental procedures were conducted in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80–23, revised in 1996) and in accordance with the Committee on Care and Use of Experimental Animal Resources, Federal University of Pelotas, Brazil (CEEA 4506–2017). All efforts were made to minimize the number of animals used and their suffering.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paltian, J.J., dos Reis, A.S., Martins, A.W.S. et al. 7-Chloro-4-(Phenylselanyl) Quinoline Is a Novel Multitarget Therapy to Combat Peripheral Neuropathy and Comorbidities Induced by Paclitaxel in Mice. Mol Neurobiol 59, 6567–6589 (2022). https://doi.org/10.1007/s12035-022-02991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02991-4