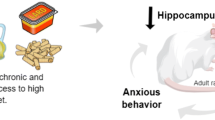
Abstract
Rationale
Obesity is considered one of the major global health problems and increases the risk of several medical complications, such as diabetes and mental illnesses.
Objective
The present study investigated the effect of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) on obesity parameters, behavioral and neurochemical alterations in hypothalamic obese rats. Methods: Male Wistar rats received subcutaneous neonatal injections of monosodium glutamate (MSG, 4g/kg) or saline. After the Lee Index evaluation, rats were divided into groups and treated with 4-PSQ (5 mg/kg, intragastric route) or canola oil once a day (post-natal days (PND) 60→76). Open-field, elevated plus-maze, forced swim task, object recognition/location memory, and stepdown inhibitory avoidance tasks were conducted from PND 66 to 74. On PND 76, rats were euthanized and epididymal fat, blood, cerebral cortex, andhippocampus were removed. Blood biochemical parameters and cortical/hippocampal acetylcholinesterase (AChE) and Na /K -ATPase activities were assessed.
Results
MSG increased the Lee Index characterizing the chemically induced hypothalamic obesity model. 4-PSQ reversed the increases of epididymal fat, blood glucose, and triglyceride levels caused by MSG exposure. 4-PSQ attenuated anxiety-like and depression-like behaviors induced by neonatal administrations of MSG. Memory deficits found in MSG-obese rats were reversed by treatment with 4-PSQ. Neurochemical alterations produced by MSG evidenced by stimulation ofNa+/K+-ATPase and AChE activities in the cerebral cortex and hippocampus of rats were normalized by 4-PSQ treatment.
Conclusions
In brief, 4-PSQ therapy improved hypothalamic obesity-related parameters, as well as psychiatric symptoms, cognitive impairment, and neurochemical alterations found in obese rats.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity can be defined as the accumulation of excessive amounts of body fat, and it is considered one of the major global health problems. Obese patients may develop several chronic complications, such as cardiovascular diseases, musculoskeletal disorders, and cancer (WHO 2016). Obesity also increases the risk of developing insulin resistance and diabetes mellitus. In this context, cerebral insulin resistance causes hyperphagia, anxiety-like and depression-like behaviors, and dopaminergic system impairment (Kleinridders and Pothos 2019). Indeed, Grigolon et al. (2019) supported a bidirectional relationship between diabetes mellitus and mood disorders. Moreover, obese subjects often have memory, learning, and executive function deficits (Agusti et al. 2018). Therefore, obesity is a significant risk factor and it contributes to increasing morbidity and mortality.
Obesity management usually involves changes in lifestyle, including appropriate diet and increased energy expenditure caused by physical exercises. Anti-obesity drugs are of great interest as an adjunctive support, especially when lifestyle modification has failed (Cheung et al. 2013). However, several drugs have been withdrawn from the market, and many individuals diagnosed with obesity have few treatment options due to the costs, adverse effects and minimal weight loss associated with the current anti-obesity drugs (Cheung et al. 2013; Rosa-Goncalves and Majerowicz 2019). Thus, experimental models with rodents sharing human obesity characteristics remain indispensable to discover, validate, and optimize novel therapeutics for their safe use (Kleinert et al. 2018).
Monosodium glutamate (MSG) is a sodium salt used as a flavor enhancer in processed food products (Wu et al. 2013) to boost their palatability. Neonatal administration of MSG is considered one of the most studied models for obesity induction in rodents and is widely used in pre-clinical studies for the discovery of new treatments against obesity and its complications (Quines et al. 2016; Rosa et al. 2015; Wu et al. 2013). In early life, MSG penetrates the central nervous system (CNS) due to the lack of a fully developed blood-brain barrier (Ribeiro 2009). The consequent excess of neuronal stimulation leads to oxidative stress and neuronal death in important hypothalamic nuclei, i.e., the arcuate nucleus and adjacent areas (Guimaraes et al. 2017). Since the hypothalamus is a pivotal structure for the control of body mass and energy metabolism (Ribeiro 2009), injuries may trigger an altered balance of the autonomic nervous system activity and hormonal dysfunction in adult life (Guimaraes et al. 2017). Several negative consequences are found in rats during adulthood following neonatal exposure to MSG, such as memory deficits and anxiety-like and depression-like behaviors (Quines et al. 2014), which fit with human obesity.
Selenium is an essential trace element and also deserves special attention for its protective role against obesity and related metabolic disorders. In this respect, a negative correlation between obesity and dietary selenium intake has been reported (Wang et al. 2016). Selenate (inorganic selenium) prevents adipogenesis through induction of selenoprotein S and attenuation of endoplasmic reticulum stress (Kim and Kim 2018). In addition, organoselenium compounds have shown promise for the treatment of obesity and its complications in rodents (Quines et al. 2016; Rosa et al. 2015). At the same time, quinoline derivatives have been described as potential anti-obesity candidates by acting as pancreatic lipase inhibitors (Arabiyat et al. 2017).
Here, we focused our attention on a selenium-functionalized quinoline, 7-chloro-4-(phenylselanyl) quinoline (4-PSQ), which has shown beneficial effects on experimental rodent models for diseases that affect the CNS (Barth et al. 2019; Reis et al. 2017). In view of the well-known effects of selenium-based compounds and quinoline derivatives, the objective of the present study was to investigate the potential of 4-PSQ as a new candidate for the treatment of obesity and its comorbidities—anxiety-like behavior, depression-like behavior, and cognitive deficits—by using the MSG model in rats.
Material and methods
Chemicals and reagents
4-PSQ (Fig. 1) was prepared and characterized by a previously described method (Duarte et al. 2017). The chemical purity of 4-PSQ (99.9%) was determined by gas chromatography-mass spectrometry (GC/MS). All other chemicals, including MSG, were of analytical grade and obtained from standard commercial suppliers.
Animals
The experiments were carried out using male newborn Wistar rats obtained from a local breeding colony. Rats were kept in a separate animal room, under controlled conditions at a constant temperature (22 ± 1 °C) and a 12-h light/dark cycle (with the light on at 6:00 a.m.). Rats had free access to water and food. At the end of the experimental protocol, the measures of body weight ranged from 250 to 350 g. The experiments were approved by the Committee on Care and Use of Experimental Animal Resources, Federal University of Pelotas, Brazil (CEEA 8358-2017), following the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Every effort was made to minimize animal suffering and reduce the number of rats used in the experiments.
Exposure protocol
The experimental design is illustrated in Fig. 1. As described by a previous MSG study (Dolnikoff et al. 2001) with a minor modification, the pups received daily administrations of MSG (4 g/kg, by subcutaneous (s.c) route) or 0.9% saline (1 ml/kg, the vehicle) (from the 5th to the 14th day after birth), totaling ten administrations (Balbo et al. 2000; Nardelli et al. 2011). Rats were weaned on post-natal day (PND) 21. On PND 59, the Lee index was recorded by measuring body weight and nasal-anal length. The Lee index was used to evaluate the growth performance of rats and obesity development. It was calculated using the formula: ∛(Body weight (g))/Naso-anal length (cm); and rats with a score of ≥ 0.3 were considered obese (Bernardis and Patterson 1968).
From PND 60 to 76, rats were treated with 4-PSQ (5 mg/kg, dissolved in canola oil) or canola oil (1 ml/kg, vehicle) by intragastric (i.g.) route, resulting in the following experimental groups: group I: Saline + oil; group II: saline + 4-PSQ; group III: MSG + oil; and group IV: MSG + 4-PSQ (6-7 rats/group). The 4-PSQ dose was chosen based on a previous study performed by our research group (Barth et al. 2019; Luchese et al. 2020). Within this period, 30 min after 4-PSQ treatment, behavioral tests were performed on alternate days: open-field, elevated plus-maze, forced swim, object recognition and location, and step-down inhibitory avoidance tasks. At the end of the experimental protocol (PND 76), rats were anesthetized with isoflurane. During the experimental protocol period, the animal`s weight, in grams, was also assessed Blood was collected by cardiac puncture for estimation of biochemical parameters. Afterwards, epididymal fat was removed and weighed to calculate its relative weight by the formula: Epididymal fat (g)/Body weight (g). Also, the cerebral cortex and hippocampus were removed and dissected for determination of acetylcholinesterase (AChE) and Na+/K+-ATPase activities.
Behavioral tests
All behavioral tests were scored by a blinded observer, when the researcher does not know which treatment a mouse is undergoing). The tests were performed in the following sequence: open-field, elevated plus-maze, forced swim, object recognition and location, and step-down inhibitory avoidance tasks.
Open-field test
The rats were submitted to an open-field test on PND 66. The open-field apparatus was made of plywood and surrounded by 30-cm-high walls. The floor of the apparatus (40 × 40 cm) was divided into 9 quadrants (3 × 3). Each animal was placed individually at the center of the open-field and observed for 6 min. The number of segments crossed with the four paws and the number of rearings on hind limbs were recorded to express locomotor and exploratory activities, respectively (Walsh and Cummins 1976).
Elevated plus-maze test
The elevated plus-maze test was also performed on PND 66, shortly after the open-field test. This test is widely validated to determine anxiety-like related behaviors in rodents (Pellow et al. 1985). The apparatus consists of four arms, two closed arms and other two opposed open arms (50 × 10 cm) mounted at an angle of 90°, all facing a central platform (10 × 10 cm) elevated 50 cm from the floor. Each rat was placed individually in the center of the apparatus facing one of the open arms. The number of entries into either open or closed arms and the time in each type of arm were measured for 5 min. The data were expressed as percentage of entries (with the four paws) into, and percentage time spent in the open arms.
Forced swim test
The forced swim test was carried out using the method described (Porsolt et al. 1979). On PND 67, a pre-training session was performed, where rats were individually placed for 15 min in open cylinders (45-cm height × 20-cm diameter) containing water (23 cm) at 25 ± 1 °C. The rats were then dried and warmed by a lamp and returned to their cages. Twenty-four hours later (PND 68), the test session was performed. Rats were placed again in the apparatus and the duration of immobility (s) was recorded for 6 min. Each rat was recorded as immobile when floating motionless or making only those movements necessary to keep its head above water.
Object recognition and object location tasks
The object recognition task was performed in the open-field apparatus (Stangherlin et al. 2009). This task is used to evaluate the short-term (STM) and long-term (LTM) memories in rodents. All objects were made of plastic material, with primary colors (blue, red, and yellow), in different shapes, and too heavy to be moved by rats. On PND 69, each animal was submitted to a habituation session and left to explore the apparatus freely for 5 min, in the absence of objects. Afterward, four objects were used: A1, A2, B, and C. During the training (PND 69), rats were placed in the apparatus containing two identical objects (A1 and A2) for 5 min. Exploration was defined when the animal directed its nose within 2 cm of the object while looking, sniffing, or touching it. The STM of rats was evaluated 1.5 h after the training session in the presence of a familiar object (A1) and a new object (B). The total time spent exploring each object was determined. The LTM was evaluated 24 h after the training session (PND 70), when rats were placed to explore a familiar object (A1) and a new object (C) for 5 min, and the total time spent in exploring each object was also determined. The objects were positioned in two adjacent corners, 9 cm from the walls of the apparatus.
The location memory (LM) was evaluated 4 h after the LTM. For this, object C was moved to a location that was diagonally opposite to object A1, and the rat was left in the apparatus for 5 min for exploration (Dix and Aggleton 1999). The time spent exploring new and familiar object locations was recorded. Exploratory preference was expressed as the total time spent exploring the objects in seconds (s).
Step-down inhibitory avoidance
The step-down inhibitory avoidance task investigates non-spatial long-term aversive memory in rodents. The procedure was carried out as described by a previous study (Sakaguchi et al. 2006), with modification in electric shock intensity and exposure time. During the training session (PND 73), each animal was placed on the platform and when it stepped down and placed its four legs on the grid, an electric stimulus (0.6 mA) was delivered for 2 s. The test session was performed 24 h (PND 74) after the training session. Each rat was placed again on the platform and the transfer latency time (s) (i.e., time taken to step down from the platform) was recorded, but the electric shock was not delivered. The cut-off for transfer latency time was 300 s.
Ex vivo assays
Blood glucose and triglyceride (TG) levels were measured by Bioclin® Kits. The ratio of variation in the animal’s weight during the experimental protocol was expressed in grams.
The cerebral cortex and hippocampus were dissected, washed with cold saline solution, and submitted to tissue homogenization for determination of AChE and Na+/K+-ATPase activities. Cerebral structures were separated into two hemispheres in order to submit each sample to all neurochemical determinations. The right hemispheres were homogenized in 0.25 M sucrose buffer (1:10, w/v) and centrifuged at 900×g for 10 min, and the supernatant fraction was used for the determination of AChE activity. The left hemispheres were homogenized in 50 mM Tris-HCl, pH 7.4 (1:10, w/v). The homogenate was centrifuged at 900×g for 10 min at 4 °C, and the supernatant fraction was used for the determination of Na+, K+ ATPase activity.
AChE activity
The AChE activity was measured in accordance with a method previously described in the literature (Ellman et al. 1961), with some modifications. Enzyme activity was measured spectrophotometrically at 412 nm, using acetylthiocholine (AcSCh) as a substrate. Results are expressed as μmol AcSCh/h/mg protein.
Na+/K+-ATPase activity
For Na+/K+-ATPase activity assay, a reaction mixture was used containing supernatant, 3 mM MgCl2, 125 mM NaCl, 20 mM KCl, and 50 mM Tris/HCl pH 7.4, at a final volume of 500 μl. The reaction was initiated by the addition of 3 mM adenosine triphosphate (ATP). Control samples were collected under the same conditions with the addition of 0.1 mM ouabain. The samples were incubated at 37 °C for 30 min and incubation was stopped by adding trichloroacetic acid solution (10%) with 10 mM HgCl2. Enzyme activity was calculated from the difference between amounts of inorganic phosphate (Pi) found after incubation in the absence and presence of ouabain. The color reaction was assayed spectrophotometrically at 650 nm (Fiske and Subbarow 1925). Results were expressed as nmol Pi/mg protein/min.
Protein determination
The protein concentration was measured by the Bradford method (Bradford 1976), using bovine serum albumin (1 mg/ml) as the standard.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). The normality of data was evaluated by the DʼAgostino and Pearson normality test. Statistical analysis was performed by GraphPad Prism® software using two-way analysis of variance (ANOVA) followed by Newman-Keuls. For the Lee index, an unpaired t-test was selected. Results were expressed as the mean ± standard error of the mean (S.E.M.). Values of p < 0.05 were considered statistically significant.
Results
Metabolic parameters
The Lee index is demonstrated in Fig. 2a. Rats that received neonatal injections of MSG had a Lee index higher than 0.3 at PND 59, being statistically different from the control group [unpaired t-test; df = 18, t = 9.21, p = 0.0001].
Effects of 4-PSQ and/or MSG on a the Lee index and b relative epididymal fat (epididymal fat weight/body weight). Data are reported as mean ± standard error of the mean (SEM) of seven rats per group. †††p < 0.001 as compared with the control group before the beginning of oil or 4-PSQ treatments (unpaired t-test); ***p < 0.001 as compared with the control group; ###p < 0.001 as compared with the MSG group (two-way analysis of variance/Newman-Keuls test)
Absolute body weights at the beginning and end of the study are shown in Table 1. There was no significant difference between the groups on beginning the treatment [two-way ANOVA + Newman-Keuls test; main effect of 4-PSQ: F(1,24) = 1.356, p = 0.2557; main effect of MSG: F(1,24) = 0.09962, p = 0.7550]. Also, at the end of treatment there was no difference between the groups [two-way ANOVA + Newman-Keuls test; main effect of 4-PSQ: F(1,24) = 3.053, p = 0.0934; main effect of MSG: F(1,24) = 1.461, p = 0.2385].
The body lengths (cm) are shown in Table 1. Results demonstrated that the body length of rats treated with MSG and MSG associated 4-PSQ treatment decreased, when compared with the control group [two-way ANOVA + Newman-Keuls test; main effect of 4-PSQ: F(1,24) = 300.5, p = 0.0001; main effect of MSG: F(1,24) = 1.195, p = 0.02852].
Relative weights of epididymal fat are demonstrated in Fig. 2b. Results revealed that MSG-treated rats accumulated greater amounts of epididymal fat (around 67%), when compared with the control group. Treatment with 4-PSQ was effective against the increase in relative weight of epididymal fat in MSG-treated rats. No change in relative weight of the epididymal fat was observed in rats treated only with 4-PSQ [Two-way ANOVA + Newman-Keuls test; interaction: F(1,24) = 19.73, p = 0.0002].
Absolute weights of the epididymal fat pads are presented in Fig. 2c. MSG administration increased the absolute weights of the epididymal fat pads (43%), when compared with the control group. Treatment with 4-PSQ was effective against the increase in this parameter. No change in absolute weights of the epididymal fat was observed in rats treated only with 4-PSQ [two-way ANOVA + Newman-Keuls test; interaction: F(1,24) = 8.003, p = 0.0093].
The effects of treatments on blood glucose and TG levels in rats are shown in Table 1. The results showed that the induction with MSG increased glucose (around 61%) and TG levels (around 177%), when compared with the control group. 4-PSQ treatment reversed the levels of glucose and TG of rats exposed to MSG. No change in glucose and TG levels was observed in rats treated only with 4-PSQ. [For glucose levels—two-way ANOVA + Newman-Keuls test; main effect of 4-PSQ: F(1,24) = 5.526, p = 0.0273; main effect of MSG: F(1,24) = 11.11, p = 0.0028], [For TG levels—two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 11.87, p = 0.0021].
Open-field test
Table 1 demonstrates the number of crossings and rearings of rats in the open-field test. There were no differences in the number of crossings and rearings among groups [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 1.673, p = 0.2081 for number of crossings] and [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 2.076, p = 0.1626 for number of rearings].
Elevated plus-maze test
Figure 3 shows the effects of treatments on the behavioral parameters in the elevated plus-maze test in rats. Neonatal MSG administration reduced (around 95%) the time spent in the open arms, when compared with the control group (Fig. 3a). Treatment with 4-PSQ significantly reversed the decrease in time spent in the open arms induced by MSG (Fig. 3a). No change in time spent in the open arms was observed in rats treated only with 4-PSQ (Fig. 3) [two-way anova + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 4.706, p = 0.0402].
Effects of MSG and/or 4-PSQ treatments on a the time spent in the open arms and b frequency of open arm entries (%) in the elevated plus-maze test. Data are reported as mean ± SEM of seven rats per group. *p < 0.05 and ***p < 0.001 as compared with the control group; #p < 0.05 and ###p < 0.001 as compared with the MSG group (two-way analysis of variance/Newman-Keuls test)
MSG administration decreased the open arms entries (around 99%), when compared with the control group (Fig. 3b). Treatment with 4-PSQ was effective to attenuate the decrease in open arms entries caused by MSG (Fig. 3b). 4-PSQ per se did not show open arms entries (Fig. 3b) [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 12.33, p = 0.0018].
Forced swim test
Figure 4 demonstrates the effects of treatments on the time spent in an immobility posture of rats in the forced swim test. MSG administration increased the immobility time (around 338%), when compared with the control group. 4-PSQ treatment was able to repair the depression-like behavior induced by MSG in rats. No statistical difference was observed after treatment with 4-PSQ per se [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 59.09, p = 0.0001].
Object recognition and location tasks
The effects of treatments on STM, LTM, and LM are shown in Fig. 5. In the training phase of the object recognition task, there was no difference in the exploratory preference of objects among groups (Fig. 5a) [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 0.2975, p = 0.8280].
Effects of MSG and/or 4-PSQ treatments on object location and object recognition memory tasks. a Training, b the first trial (short-term memory in novel object recognition test—4 h after training), c second trial (long-term memory in novel object recognition test—24 h after training), and d third trial (new object location test—4h after the second trial). Data are reported as mean ± SEM of seven rats per group. *p < 0.05 and ****p < 0.0001 as compared the preference of the new object vs familiar object in the same group (two-way analysis of variance/Newman-Keuls test)
In the probe test, MSG did not change the exploratory preference of the new object vs familiar object on STM, LTM, and LM (Fig. b–d), indicating that the animals did not remember the familiar object. However, the treatment with 4-PSQ attenuated this behavior, given that rats treated with 4-PSQ had an increase (54% for STM, 72% for LTM, and 86% for LM) in the exploratory preference of the new object, when compared with familiar object (Fig. 5b–d, respectively). Moreover, exploratory preference on object recognition and location tasks of animals of the MSG+4-PSQ group was similar to the Sham group, indicating no cognitive damage. Also, 4-PSQ alone increased (105% for STM, 124% for LTM and 89% for LM) the exploratory preference of the new object, when compared with familiar object (Fig. 5b–d, respectively). Animals of Sham group increased (166% for STM, 88% for LTM and 113% for LM) the exploratory preference of the new object, when compared with familiar object. [Two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 20.430, p = 0.0001 for STM], [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 10.530, p = 0.0001 for LTM] and [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 33.83, p = 0.0001 for LM].
Step-down inhibitory avoidance task
Figure 6 illustrates the effects of treatments on the step-down inhibitory avoidance task in rats. In the training session, there was no difference in the transfer latency time among the experimental groups [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 0.07377, p = 0.7882].
Effects of MSG and/or 4-PSQ treatments on transfer latency time (non-spatial memory) in the step-down inhibitory avoidance. Data are reported as mean ± SEM of seven rats per group. **p < 0.01 as compared with the control group; ###p < 0.001 as compared with the MSG group (two-way analysis of variance/Newman-Keuls test)
In the test session, MSG decreased (around 93%) the transfer latency time and treatment with 4-PSQ significantly restored this reduction, when compared with the control group. Treatment with 4-PSQ alone did not alter the transfer latency time [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 9.082, p = 0.0062].
AChE activity
Figure 7a and b show the effects of treatments on AChE activity in cerebral cortex and hippocampus of rats, respectively. Results demonstrated that neonatal MSG injections increased the AChE activity in the cerebral cortex (around 523%) and hippocampus (around 1134%) of rats, when compared with the control group (Fig. 7a and b, respectively). 4-PSQ treatment significantly restored the increase in AChE activity in the cerebral structures caused by MSG (Fig. 7a for the cerebral cortex and Fig. 7b for the hipoccampus). No change in cerebral AChE activity was seen after the treatment with 4-PSQ alone (Fig. 7a for the cerebral cortex and Fig. 7b for the hippocampus) [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 360.7, p = 0.0001 for the cerebral cortex] and [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 73.64, p = 0.0001 for the hippocampus].
Effects of 4-PSQ and/or on AChE activity in a cerebral cortex and b hippocampus of rats. The values of the enzymatic activity are expressed in μmol AcSCh/h/mg protein. Data are reported as mean ± SEM of six rats per group. ***p < 0.001 as compared with the control group; ###p < 0.001 as compared with the MSG group (two-way analysis of variance/Newman-Keuls test)
Na+/K+-ATPase activity
Figure 8a and b demonstrate the effects of treatments on Na+/K+-ATPase activity in the cerebral cortex and hippocampus of rats, respectively. Neonatal MSG administration increased the activity of the Na+/K+-ATPase in the cerebral cortex (around 198%) and hippocampus (around 124%) of rats, when compared with the control group (Fig. 8a and b, respectively). Results showed that treatment with 4-PSQ restored the enzyme activity to normal levels in the cerebral cortex and hippocampus of rats (Fig. 8a and b, respectively). Treatment with 4-PSQ per se did not change the Na+/K+-ATPase activity in the cerebral structures (Fig. 8a for the cerebral cortex and Fig. 8b for the hippocampus) [two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 18.09, p = 0.0003 for the cerebral cortex] and [Two-way ANOVA + Newman-Keuls test; MSG X 4-PSQ interaction: F(1,24) = 11.72, p = 0.0022 for the hippocampus].
Effects of MSG and/or 4-PSQ treatments on Na+/K+-ATPase activity in a cerebral cortex and b hippocampus of rats. Data are reported as mean ± SEM of six rats per group. *p < 0.05 and **p < 0.01 as compared with the control group. #p < 0.05, ##p < 0.01 as compared with the MSG group (two-way analysis of variance/Newman-Keuls test)
Discussion
The high prevalence rates of obesity and its (neuro)comorbidities have encouraged us to seek a new a new anti-obesity and neuroprotective candidate. The present study demonstrated, for the first time, the action of 4-PSQ, a quinoline derivative containing selenium, in an obesity model created by neonatal MSG exposure in adult rats. Additionally, 4-PSQ attenuated anxiety-like and depression-like symptoms, as well as cognitive impairments in adult rats. In addition, our findings demonstrated that 4-PSQ normalized the AChE and Na+/K+-ATPase activities in the cerebral cortex and hippocampus of rats.
Different species of rodents exposed to MSG develop the hypothalamic obesity syndrome which is associated with a dysfunction of the hypothalamic-pituitary axis culminating in reduced growth, hypogonadism, fat accumulation, and high serum levels of corticosteroids (Bray and York 1979). In accordance with several studies, we demonstrated that MSG administration in neonatal rats caused significant increases in obesity parameters during adult life, as evidenced by a higher Lee Index and large amounts of accumulated epididymal fat (Guimaraes et al. 2017; Quines et al. 2016; Quines et al. 2014; Rosa et al. 2015). In the current study, rats exposed to MSG also exhibited blood biochemistry changes, such as elevated TG and glucose levels. Indeed, MSG can cause metabolic alterations such as dyslipidemia and/or glucose metabolism impairment (Madhavadas and Subramanian 2015; Quines et al. 2016; Quines et al. 2018; Sasaki-Hamada et al. 2015).
Our data revealed that repeated treatment with 4-PSQ was effective in reducing fat deposits as well as normalizing blood glucose and TG levels in rats induced by MSG. These pioneering findings demonstrate the beneficial effects of 4-PSQ against metabolic profile disturbances in obese rats. Importantly, this is the first study that demonstrates the effect of 4-PSQ on an obesity model. Previous studies have proposed quinoline-based compounds as selective antagonists of the melanin-concentrating hormone (MCH) receptor (Warshakoon et al. 2006; Wu et al. 2014) and as in vitro adipogenesis supressant (Zou et al. 2014). It is also important to note that an organic (phenyl) portion containing selenium has been introduced into the chemical structure of the compound. Interestingly, an inverse association of body mass index with serum selenium concentrations has been found in men and women (Zhong et al. 2018). Also, p-choro-diphenyl diselenide, a synthetic organoselenium compound, is known to reduce body weight and modulate hypothalamic neuropeptides in lean rats (Bortolatto et al. 2017), as well as stabilizing metabolic function in MSG-obese rats (Quines et al. 2016; Quines et al. 2018).
A reciprocal link has been established between psychiatric disorders and obesity. These conditions are marked by shared structural and functional abnormalities in brain regions related to cognitive and/or affective processing, as well as alterations in several interacting biological networks (Soczynska et al. 2011). As previously demonstrated (Onaolapo et al. 2017; Quines et al. 2014; Rosa et al. 2016), our results showed that MSG caused an anxiety-like behavior in rats. This higher level of anxiety-like behavior can be related to different factors resulting from MSG neurotoxicity, including alterations in GABAergic and serotoninergic systems (Rosa et al. 2016). Anxiety-like behavior in MSG obese rats has also been associated with increased levels of plasma corticosterone, suggesting sensitization of the hypothalamus-pituitary-adrenal axis activity (Guimaraes et al. 2017).
Of particular importance, our data demonstrated that the repeated treatment of obese MSG rats with 4-PSQ reversed the anxiety-related behavior. This finding is reinforced by a study (Pinz et al. 2018) in which 4-PSQ (via antioxidant mechanisms) protected against anxiety-like symptoms in mice submitted to an amyloid beta (Aβ) fragment-induced neurodegenerative disorder model. Furthermore, it is known that 4-PSQ per se elicits anxiolytic-like action probably mediated by the glutamatergic system (Reis et al. 2017). Thus, anxiety-like behavior in mice exposed to MSG is related to an increase in brain glutamate levels (Onaolapo et al. 2017), which, in turn, could result in high levels of reactive oxygen species (ROS) and mitochondrial dysfunctions. Considering an existing relationship between anxiety-like behavior and redox imbalance (Salim et al. 2010), and the well-documented contribution of oxidative stress in MSG-induced toxicity (Rosa et al. 2018; Sadek et al. 2016; Villagarcia et al. 2018), we suggest that the antioxidant action of 4-PSQ could contribute to reducing the glutamatergic excitotoxicity triggered by MSG, and thus the subsequent impairments in behavioral and emotional disorders.
Exposure to MSG has also been linked to the emergence of depression-like behavior in rodents. As could be seen from experimental data collected in the forced swim task, MSG caused depression-like behavior in obese rats, represented by a longer time spent in a typical posture of immobility. In agreement with this finding, Quines et al. (2014) demonstrated that MSG-injected rats are more susceptible to developing depression-like behavior and present increased [3H]5-HT uptake in the cerebral cortex. Mental health can be compromised in obese people, since overweight causes atrophy in the frontal lobes (Raji et al. 2010), and may modify many social conditions. It is certain that depressive disorder is among the mood disorders related to obesity (Soczynska et al. 2011). SSRIs may be used to manage certain subgroups of persons with comorbidities, such as depression, associated with obesity, binge eating disorder and type 2 diabetes mellitus (Appolinario et al. 2004). Experimentally, there are reports about the influence of antidepressants on body weight and/or depression-like symptoms in obese rodents. For example, body weight in female mice treated with MSG was decreased by fluoxetine (Kaur and Kulkarni 2001). At the same time, depression-like behavior and biochemical alterations in obese mice subjected to chronic unpredictable mild stress were reversed by escitalopram (Kurhe et al. 2014). Further, depression-like symptoms (e.g. increased immobility time) and reduced hippocampal 5-HT concentration in high-fat diet-induced obese mice were also prevented by escitalopram (Kurhe et al. 2017).
The present study demonstrated that 4-PSQ therapy was effective in reversing depression-like related behavior in MSG rats. Obesity and mood disorders are chronic low-grade pro-inflammatory states with abnormalities in key effector proteins of the pro-inflammatory cascade (Soczynska et al. 2011). In addition to its role as an antioxidant, 4-PSQ also has antinociceptive and anti-inflammatory effects in mice and has been found to modulate serotonergic, nitrergic, and glutamatergic systems (Silva et al. 2017).
There are data indicating that obesity and metabolic dysfunction are both correlated with increased rates of cognitive decline (Agusti et al. 2018; Farruggia and Small 2019). Besides, individuals diagnosed with obesity have an increased risk of developing neurodegenerative conditions, such as Alzheimer disease (AD). In this context, anti-inflammatory and anti-obesity drugs could be useful for the management of cognitive decline (Solas et al. 2017). Researchers have demonstrated that MSG-treated Wistar rats exhibit marked cognitive malfunctions and hippocampal synaptic plasticity impairments, which are partially related to deficits in glutamatergic pre- and post-synapses (Sasaki-Hamada et al. 2015). Here, rats had impaired performance in memory tasks as a consequence of neonatal MSG exposure. Indeed, memory deficits were found in obese rats during the evaluation of LTM (object recognition test), spatial memory (object location test), and aversive memory (step-down inhibitory avoidance task). Our results are supported by other experiments designed to estimate memory impairment in rodents exposed to MSG (Madhavadas and Subramanian 2015). It should also be noted that memory deficits found in the present study were accompanied by a stimulation of both cortical and hippocampal AChE activities in obese MSG rats. In view of this, overstimulation of hippocampal AChE activity has already been described for MSG-treated rodents (Madhavadas and Subramanian 2015), which predicts a reduction of acetylcholine availability in the synaptic cleft, a neurotransmitter of particular importance in cognition.
Interestingly, the treatment with 4-PSQ reversed the emergence of cognitive deficits induced by MSG in rats and also prevented the stimulation of AChE activity in the cerebral cortex and hippocampus. Thus, the neuroprotective effects of this selenium-based quinoline can be partially explained by its ability to modulate the brain AChE activity as already demonstrated in previous studies (Barth et al. 2019; Pinz et al. 2018). In fact, 4-PSQ has demonstrated restoring effects on the cognitive impairments caused by aging by modulating synaptic plasticity, cholinergic system and cholesterol levels in rats (Barth et al. 2019). Moreover, the anticholinesterase and antioxidant effects of 4-PSQ seem to be protective factors against learning and memory impairments in an AD model (Pinz et al. 2018). We also believe that the well-described anti-inflammatory actions of 4-PSQ and its capacity of modulate the glutamatergic system (Silva et al. 2017) could reinforce its memory-enhancing action, a hypothesis to be further examined.
In the present study, not only AChE but also cortical and hippocampal Na+/K+-ATPase activities suffered the impact of MSG exposure. The activity of these enzymes has been normalized by the total protein levels of the analyzed tissues. Despite this, it is worth recalling that possibly, increased enzyme activity could be the result of upregulation of the expression of AChE and Na+/K+-ATPase protein levels, not assessed here. Na+/K+-ATPase is shown to be affected by MSG in different tissues of rodents such as the liver, pancreas, and brain (de Oliveira et al. 2011; Liu et al. 2008; Quines et al. 2016). A stimulation of hippocampal Na+/K+-ATPase activity in MSG rats was also demonstrated previously (Rosa et al. 2015). Excess glutamate can be excitotoxic to neurons (Kinoshita et al. 2016), an event which is accompanied by high levels of ROS. The increase in Na+/K+-ATPase activity observed in this study could represent a compensatory mechanism in an attempt to control brain excitability. In this regard, there is a strong correlation between abnormal glutamatergic signaling and neurodegenerative/psychiatric diseases (Kinoshita et al. 2016). We demonstrated that 4-PSQ normalized the Na+/K+-ATPase activity in the cerebral cortex and hippocampus of rats exposed to MSG. The effects of 4-PSQ in normalizing this enzyme activity in cerebral structures of rats exposed to MSG could be related to the improvements observed in their behavioral profile, including reversed action against depression-like and anxiety-like behaviors, as well as memory deficits.
Additionally, compounds were commonly administered in experimental design tests using rodents to investigate and discover a new drug for the treatment of different diseases. A route commonly chosen for investigating the effect of new compounds is peroral or intragastric, using gavage. This is the most often used route to deliver precisely the oral dose desired. In this sense, it is very safe, reliable, precise, economical, and convenient. In other ways, additives to the food or voluntary consumption were an ideal route that may not be completely reliable regarding the dose administered to each animal, due to individual taste preferences, palatability problems and behavioral changes over time. In this manner, the oral route dominates contemporary drug therapy, considered to be easily accessible with minimal discomfort compared with other routes, especially and more importantly it can ensure the exact dose to be administered to each animal, which is important to express the most faithful result possible compared with other routes (Lennernas 2007; Turner et al. 2011). Also, the transport properties and permeability for different compounds were highly correlated between rats and humans when using rat intestinal specimens, establishing high experimental quality when using oral administration on in vivo models.
Although several animal models have been used to perform basic research on obesity, none of them reproduce all the characteristics of human obesity, a complex and multifactorial disorder. Genetic and non-genetic (surgical, chemical, dietetic) pre-clinical models of obesity have been employed over the years but they have only provided clues to the causes, aftermaths, and potential therapies for human adiposity (Suleiman et al., 2019). These models, including MSG-induced hypothalamic obesity, present some drawbacks. It is worth mentioning that systemic MSG treatment also injures neurons in the circumventricular organs due to their open blood-brain barrier; MSG lesions are therefore not restricted to the ARC (Lutz and Woods, 2012). Besides, MSG rats are not hyperphagic (Bunyan et al. 1976) and present relative obesity (increase in body fat without any change in body weight) (Djazayery et al., 1979). Based on these considerations, studies directed at testing the impact of 4-PSQ treatments on complementary obesity models are crucial to characterize the anti-obesity efficacy of this compound and provide additional information about mechanisms implicated in its pharmacological profile; these studies will be the focus of continuous 4-PSQ research.
We assume as potential limitations of this study the fact that (1) a single dose of GMS and 4-PSQ were used, and not a dose-response curve and (2) the effects of 4-PSQ were tested only in an obesity model using MSG in rats. A dose-response curve would help demonstrate, at which maximum and minimum dose, the inducer (MSG) and treatment (4-PSQ) would have the most significant effect, demonstrating the feature of the hypothalamic obesity model and treatment of disorder generated by the model.
Experimental models are important to evaluate specific cause and effects of obesity, but study and extrapolation to humans is limited, since in most cases of human obesity it is the result of specific characteristics of individuals and interaction with the environment. Among the models, an alternative to induction was based on a lesion of the ventromedial hypothalamic nucleus, using a 1.2-mA current lasting 4 s, repeated 3 times at 30-s intervals after positioning the electrodes to cause destruction of the hypothalamic nucleus, leading to obesity (Yoshimatsu et al. 1985). Paradoxically for a model of MSG, this electrical ablation causes obesity by hyperphagia and studies demonstrated involvement of levels of leptin, insulin and neuropeptide Y resulting in weight gain after lesion. If we had used this model, we could have seen the effect of the compound in the face of an increased leptin level, reduction of the total neuropeptide Y, maintenance of fluctuations in circadian rhythm, and there seems to be loss of the feedback mechanism between insulin and leptin (Marks and Brown, 2013; Sun et al., 2018). However, our research group considered a more aggressive model and that MSG induction would be would initially be more appropriate.
Another obesity induction model with rats used oophorectomy, on the contrary of the previous ones. This model is used in order to achieve a better understanding of these modifications in women after the end of their fertile age and study interventions that could alter the impact of hormone reduction in women (Mccarthy et al., 2012). Thus, compared with the MSG induction, this model has very specific characteristics. Considering that the compound had not been tested against metabolic parameters previously, this model would not be useful at this time.
The simplest obesity-induction model used hypercaloric diets. There are several types of diets, like adding carbohydrates or eating fats to attain hypercaloric values. These models are very interesting because they directly represent the population’s diet (Arbo et al., 2017; Rodrigues-Razon et al. 2020); however, our research group is not specialized in obesity studies, and therefore, it does not have the structure to produce and store diets high in animal calories) and cannot be used in our current situation. Another example of the experimental study is a genetic model to investigate obesity. It is also a good choice, because the cloning and identification of the genes causing obesity was precise, using knockout animals (Hansen, 2019; Souza et al., 2020). But, again, our research group does not have the structure to employ this model.
However, the administration of MSG to newborn rats, used in this study, destroys the ventromedial hypothalamic and arcuate nuclei, leading the rats to develop obesity due to the lack of control between absorption and energy expenditure (Tordoff et al., 2012; Brosnan et al., 2014; Ferreira, 2015; Rojas-Castañeda et al. 2016And, further, it is important to know that the obesity effect is not due to increased food intake, but rather to greater accumulation of fat in the tissue. Administration during the neonatal period caused a drop in the hypothalamic levels of dopamine, changing the control of the hypothalamic-pituitary axis, loss of the inhibitory regulatory level of leptin on the adrenal gland. In conclusion, this model has some relevant advantages, such as easy administration, directly impacting on metabolic and oxidative stress, effects similar to those found in patients with obesity classifying this model with MSG as an inducer of hypothalamic obesity.
Conclusion
To sum up, 4-PSQ (a quinoline derivative containing selenium) reversed the experimental obesity and the consequent blood biochemical dysfunctions in rats exposed to neonatal MSG injections. 4-PSQ also protected against anxiety-like and depression-like behaviors, as well as memory deficits in hypothalamic obese rats. The normalization of cortical and hippocampal Na+/K+-ATPase and AChE activities is suggested as possible neurological mechanisms of 4-PSQ in behavioral, psychiatric, and cognitive complications related to hypothalamic obesity. Thus, the bioactivity of selenium-based quinolines emerges as a new important avenue for drug development targeting the management of obesity and its comorbidities.
Abbreviations
- 4-PSQ:
-
7-Chloro-4-(phenylselanyl) quinoline
- MSG:
-
Monosodium glutamate
- PND:
-
Post-natal days
- AChE:
-
Acetylcholinesterase
- CNS:
-
Central nervous system
- LTM:
-
Long-term memories
- STM:
-
Short-term memories
- LM:
-
Location memory
- TG:
-
Triglyceride
- AcSCh:
-
Acetylthiocholine
- ATP:
-
Adenosine triphosphate
- Pi:
-
Inorganic phosphate
- MCH:
-
Melanin-concentrating hormone
- ROS:
-
Oxygen species
References
Agusti A, Garcia-Pardo MP, Lopez-Almela I, Campillo I, Maes M, Romani-Perez M, Sanz Y (2018) Interplay between the gut-brain axis, obesity and cognitive function. Front Neurosci 12:155. https://doi.org/10.3389/fnins.2018.00155
Agusti A, Moya-Perez A, Campillo I, Montserrat-de la Paz S, Cerrudo V, Perez-Villalba A, Sanz Y (2018) Bifidobacterium pseudocatenulatum CECT 7765 ameliorates neuroendocrine alterations associated with an exaggerated stress response and anhedonia in obese mice. Mol Neurobiol 55:5337–5352. https://doi.org/10.1007/s12035-017-0768-z
Appolinario JC, Bueno JR, Coutinho W (2004) Psychotropic drugs in the treatment of obesity: what promise? Cent Nerv Syst Drugs 18(10):629–651. https://doi.org/10.2165/00023210-200418100-00002
Arabiyat S, Kasabri V, Al-Hiari Y, Bustanji YK, Albashiti R, Almasri IM, Sabbah DA (2017) Antilipase and antiproliferative activities of novel fluoroquinolones and triazolofluoroquinolones. Chem Biol Drug Des 90:1282–1294. https://doi.org/10.1111/cbdd.13049
Arbo B, Niches G, Zanini P, Bassuino DM, Driemeier D (2017) Aging affects the response of female rats to a hypercaloric diet. https://doi.org/10.1016/j.exger.2017.11.008
Balbo SL, Mathias PC, Bonfleur ML, Alves HF, Siroti FJ, Monteiro OG, Ribeiro FB, Souza AC (2000) Vagotomy re- duces obesity in MSG-treated rats. Res Commun Mol Pathol Pharmacol 108:291–296
Barth A, Vogt AG, dos Reis AS, Pinz MP, Krüger R, Domingues WB, Alves D, Campos VF, Pinton S, Paroul N, Wilhelm EA, Luchese C (2019) 7-Chloro-4-(Phenylselanyl) Quinoline with memory enhancer action in aging rats: modulation of neuroplasticity, acetylcholinesterase activity, and cholesterol levels. Mol Neurobiol 56:6398–6408. https://doi.org/10.1007/s12035-019-1530-5
Bernardis LL, Patterson BD (1968) Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol 40:527–528
Bortolatto CF, Nogueira CW, Porteiro B, Imbernon M, Nogueiras R (2017) Hypothalamic pathways regulate the anorectic action of p-chloro-diphenyl diselenide in rats. Eur J Pharmacol 815:241–250. https://doi.org/10.1016/j.ejphar.2017.09.032
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Bray GA, York DA (1979) Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev 59:719–809. https://doi.org/10.1152/physrev.1979.59.3.719
Brosnan JT, Drewnowski A, Friedman MI (2014) Is there a relationship between dietary MSG obesity in animals or humans? Amino Acids, London 46(9):2075–2087. https://doi.org/10.1007/s00726-014-1799-7
Bunyan J, Murrell EA, Shah PP (1976 Jan) The induction of obesity in rodents by means of monosodium glutamate. Br J Nutr 35(1):25–39. https://doi.org/10.1079/bjn19760005
Cheung BM, Cheung TT, Samaranayake NR (2013) Safety of antiobesity drugs. Ther Adv Drug Saf 4:171–181. https://doi.org/10.1177/2042098613489721
de Oliveira MC, Torrezan R, da Costa CE, Ambiel CR, Constantin RP, Ishii-Iwamoto EL, Salgueiro-Pagadigorria CL (2011) Changes in calcium fluxes in mitochondria, microsomes, and plasma membrane vesicles of livers from monosodium L-glutamate-obese rats. Metabolism 60:1433–1441. https://doi.org/10.1016/j.metabol.2011.02.011
Dix SL, Aggleton JP (1999) Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res 99:191–200
Djazayery A, Miller DS, Stock MJ (1979) Energy balances in obese mice. Nutr Metab 23(5):357–367. https://doi.org/10.1159/000176281
Dolnikoff M, Martin-Hidalgo A, Machado UF, Lima FB, Herrera E (2001) Decreased lipolysis and enhanced glycerol and glucose utilization by adipose tissue prior to development of obesity in monosodium glutamate (MSG) treated-rats. Int J Obes Relat Metab Disord 25:426–433. https://doi.org/10.1038/sj.ijo.0801517
Duarte LFD et al (2017) A simple method for the synthesis of 4-arylselanyl-7-chloroquinolines used as in vitro acetylcholinesterase inhibitors and in vivo memory improvement. Tetrahedron Lett 58:3319–3322. https://doi.org/10.1016/j.tetlet.2017.07.039
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Farruggia MC, Small DM (2019) Effects of adiposity and metabolic dysfunction on cognition: A review. Physiol Behav 208:112578. https://doi.org/10.1016/j.physbeh.2019.112578
Ferreira FS (2015) Aditivos alimentares e suas reações adversas no consumo infantil. Rev Univ Vale Rio Verde, Três Corações 13(1):397–407. https://doi.org/10.5892/ruvrd.v13i1.1845
Fiske CH, Subbarow YJ (1925) The colorimetric determination of phosphorus. Biol Chem 66:375–400
Grigolon RB, Brietzke E, Mansur RB, Idzikowski MA, Gerchman F, De Felice FG, McIntyre RS (2019) Association between diabetes and mood disorders and the potential use of anti-hyperglycemic agents as antidepressants. Prog Neuro-Psychopharmacol Biol Psychiatry 95:109720. https://doi.org/10.1016/j.pnpbp.2019.109720
Guimaraes ED et al (2017) Altered behavior of adult obese rats by monosodium l-glutamate neonatal treatment is related to hypercorticosteronemia and activation of hypothalamic ERK1 and ERK2. Nutr Neurosci 20:153–160. https://doi.org/10.1179/1476830515Y.0000000004
Hansen CT. (2019) The use of rodents for the study of atherogenisis and obesity: the development of potential genetic models. https://doi.org/10.1201/9780367812782-7
Kaur G, Kulkarni SK (2001) Differential effect of polyherbal, antiobesity preparation, OB-200G in male and female mice and monosodium glutamate-treated rats. Indian J Exp Biol 39(6):551–557
Kim CY, Kim KH (2018) Selenate prevents adipogenesis through induction of selenoprotein s and attenuation of endoplasmic reticulum stress. Molecules 23. https://doi.org/10.3390/molecules23112882
Kinoshita PF, Leite JA, Orellana AM, Vasconcelos AR, Quintas LE, Kawamoto EM, Scavone C (2016) The influence of Na(+), K(+)-ATPase on glutamate signaling in neurodegenerative diseases and senescence. Front Physiol 7:195. https://doi.org/10.3389/fphys.2016.00195
Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Müller TD, Tschöp MH (2018) Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14:140–162. https://doi.org/10.1038/nrendo.2017.161
Kleinridders A, Pothos EN (2019) Impact of brain insulin signaling on dopamine function, food intake, reward, and emotional behavior. Curr Nutr Rep 8:83–91. https://doi.org/10.1007/s13668-019-0276-z
Kurhe Y, Mahesh R, Devadoss T (2017) Novel 5-HT3 receptor antagonist QCM-4 attenuates depressive-like phenotype associated with obesity in high-fat-diet-fed mice. Psychopharmacology 234(7):1165–1179. https://doi.org/10.1007/s00213-017-4558-0
Kurhe Y, Mahesh R, Gupta D, Devadoss T (2014) QCM-4, a serotonergic type 3 receptor modulator attenuates depression co-morbid with obesity in mice: an approach based on behavioral and biochemical investigations. Eur J Pharmacol 740:611–618. https://doi.org/10.1016/j.ejphar.2014.06.020
Lennernas H (2007) Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 37:1015–1051. https://doi.org/10.1080/00498250701704819
Liu SN, Liu Q, Shen ZF (2008) A preliminary study on the mechanism of impaired beta cell function in monosodium glutamate obese rat with insulin resistance. Yao Xue Xue Bao 43:1106–1111
Luchese C, Barth A, Da Costa GP, Alves D, Novo DR, Mesko MF, Wilhelm EA (2020) Role of 7-chloro-4-(phenylselanyl) quinoline as an anti-aging drug fighting oxidative damage in different tissues of aged rats. Exp Gerontol 130:110804
Lutz TA, Woods SC (2012) Overview of animal models of obesity. Curr Protoc Pharmacol Chapter 5:Unit5.61. https://doi.org/10.1002/0471141755.ph0561s58
Madhavadas S, Subramanian S (2015) Combination of Spirulina with glycyrrhizin prevents cognitive dysfunction in aged obese rats. Indian J Pharm 47:39–44. https://doi.org/10.4103/0253-7613.150327
Marks HE, Brown GE (2013) The effects of VMH lesions in Charles River rats. Psychon Sci 23(1):117–119. https://doi.org/10.3758/BF03336035
McCarthy AM, Menke A, Ouyang P, Visvanathan K. (2012) Bilateral oophorectomy, body mass index, and mortality in US women aged 40 years and older. https://doi.org/10.1158/1940-6207.CAPR-11-0430
Nardelli TR, Ribeiro RA, Balbo SL, Vanzela EC, Carneiro EM, Boschero AC, Bonfleur ML (2011) Taurine prevents fat deposition and ameliorates plasma lipid profile in monosodium glutamate obese rats. Amino Acids 41:901–908
Onaolapo OJ, Aremu OS, Onaolapo AY (2017) Monosodium glutamate-associated alterations in open field, anxiety-related and conditioned place preference behaviours in mice. Naunyn Schmiedeberg's Arch Pharmacol 390:677–689. https://doi.org/10.1007/s00210-017-1371-6
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Pinz MP et al (2018) Current advances of pharmacological properties of 7-chloro-4-(phenylselanyl) quinoline: Prevention of cognitive deficit and anxiety in Alzheimer’s disease model. Biomed Pharmacother 105:1006–1014. https://doi.org/10.1016/j.biopha.2018.06.049
Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M (1979) Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol 57:201–210
Quines CB, Rosa SG, Chagas PM, da Rocha JT, Dobrachinski F, Carvalho NR, Soares FA, da Luz SCA, Nogueira CW (2016) Homeostatic effect of p-chloro-diphenyl diselenide on glucose metabolism and mitochondrial function alterations induced by monosodium glutamate administration to rats. Amino Acids 48:137–148. https://doi.org/10.1007/s00726-015-2073-3
Quines CB, Rosa SG, Da Rocha JT, Gai BM, Bortolatto CF, Duarte MM, Nogueira CW (2014) Monosodium glutamate, a food additive, induces depressive-like and anxiogenic-like behaviors in young rats. Life Sci 107:27–31. https://doi.org/10.1016/j.lfs.2014.04.032
Quines CB, Rosa SG, Velasquez D, Da Rocha JT, Neto JS, Nogueira CW (2016) Diphenyl diselenide elicits antidepressant-like activity in rats exposed to monosodium glutamate: a contribution of serotonin uptake and Na(+), K(+)-ATPase activity. Behav Brain Res 301:161–167. https://doi.org/10.1016/j.bbr.2015.12.038
Quines CB, Rosa SG, Velasquez D, Prado VC, Neto JSS, Nogueira CW (2018) (p-ClPhSe)2 stabilizes metabolic function in a rat model of neuroendocrine obesity induced by monosodium glutamate. Food Chem Toxicol 118:168–180. https://doi.org/10.1016/j.fct.2018.05.010
Raji CA et al (2010) Brain structure and obesity. Hum Brain Mapp 31:353–364. https://doi.org/10.1002/hbm.20870
Reis AS, Pinz M, Duarte LFB, Roehrs JA, Alves D, Luchese C, Wilhelm EA (2017) 4-phenylselenyl-7-chloroquinoline, a novel multitarget compound with anxiolytic activity: contribution of the glutamatergic system. J Psychiatr Res 84:191–199. https://doi.org/10.1016/j.jpsychires.2016.10.007
Ribeiro EB (2009) Studying the central control of food intake and obesity in rats. Rev Nutr 22:163–171. https://doi.org/10.1590/S1415-52732009000100015
Rodrigues-Razon CM, Cobain TAG, Orozco AKR, Gonzalez AEA, Santillan VR (2020) Inflammatory, hypoglycemic and antioxidant effect of Azadirachta Indica infusion on obesity rats induced by hypercaloric diet. https://doi.org/10.1016/j.metabol.2019.12.079
Rojas-Castañeda JC, Vigueras-Villaseñor RM, Chavez-Saldaña M, Rojas P, Guitérrez-Pérez O, Rojas C (2016) Arteaga-Silva M (2016) Neonatal exposure to monosodium glutamate induces morphological alterations in suprachiasmatic nucleus of adult rat. Int J Exp Pathol 97(1):18–26. https://doi.org/10.1111/iep.12157
Rosa SG, Chagas PM, Pesarico AP, Nogueira CW (2018) Monosodium glutamate induced nociception and oxidative stress dependent on time of administration, age of rats and susceptibility of spinal cord and brain regions. Toxicol Appl Pharmacol 351:64–73. https://doi.org/10.1016/j.taap.2018.05.019
Rosa SG, Quines CB, da Rocha JT, Bortolatto CF, Duarte T, Nogueira CW (2015) Antinociceptive action of diphenyl diselenide in the nociception induced by neonatal administration of monosodium glutamate in rats. Eur J Pharmacol 758:64–71. https://doi.org/10.1016/j.ejphar.2015.03.060
Rosa SG, Quines CB, Stangherlin EC, Nogueira CW (2016) Diphenyl diselenide ameliorates monosodium glutamate induced anxiety-like behavior in rats by modulating hippocampal BDNF-Akt pathway and uptake of GABA and serotonin neurotransmitters. Physiol Behav 155:1–8. https://doi.org/10.1016/j.physbeh.2015.11.038
Rosa-Goncalves P, Majerowicz D (2019) Pharmacotherapy of obesity: limits and perspectives. Am J Cardiovasc Drugs 19:349–364. https://doi.org/10.1007/s40256-019-00328-6
Sadek K, Abouzed T, Nasr S (2016) Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model. Can J Physiol Pharmacol 94:394–401. https://doi.org/10.1139/cjpp-2015-0388
Sakaguchi M, Koseki M, Wakamatsu M, Matsumura E (2006) Effects of systemic administration of beta-casomorphin-5 on learning and memory in mice. Eur J Pharmacol 530:81–87. https://doi.org/10.1016/j.ejphar.2005.11.014
Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G (2010) Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res 208:545–552. https://doi.org/10.1016/j.bbr.2009.12.039
Sasaki-Hamada S, Hojo Y, Koyama H, Otsuka H, Oka J (2015) Changes in hippocampal synaptic functions and protein expression in monosodium glutamate-treated obese mice during development of glucose intolerance. Eur J Neurosci 41:1393–1401. https://doi.org/10.1111/ejn.12891
Silva VDG, Reis AS, Pinz MP, da Fonseca CAR, Duarte LFB, Roehrs JA, Alves D, Luchese C, Wilhelm EA (2017) Further analysis of acute antinociceptive and anti-inflammatory actions of 4-phenylselenyl-7-chloroquinoline in mice. Fundam Clin Pharmacol 31:513–525. https://doi.org/10.1111/fcp.12295
Soczynska JK, Kennedy SH, Woldeyohannes HO, Liauw SS, Alsuwaidan M, Yim CY, McIntyre RS (2011) Mood disorders and obesity: understanding inflammation as a pathophysiological nexus. NeuroMolecular Med 13:93–116. https://doi.org/10.1007/s12017-010-8140-8
Solas M, Milagro FI, Ramirez MJ, Martinez JA (2017) Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol 37:87–92. https://doi.org/10.1016/j.coph.2017.10.005
Souza TA, Souza DW, Siqueira BS, Rentz T, Emilio HRO (2020) Splenic participation in glycemic homeostasis in obese and non-obese male rats. DOI 14:479–486. https://doi.org/10.1016/j.orcp.2020.07.009
Stangherlin EC, Rocha JB, Nogueira CW (2009) Diphenyl ditelluride impairs short-term memory and alters neurochemical parameters in young rats. Pharmacol Biochem Behav 91:430–435. https://doi.org/10.1016/j.pbb.2008.08.020
Suleiman JB, Mohamed M, Bakar ABA (2019) A systematic review on different models of inducing obesity in animals: advantages and limitations. J Adv Vet Anim Res 7(1):103–114. https://doi.org/10.5455/javar.2020.g399
Sun H, Zhao P, Liu W, Li L, Ai H (2018) Ventromedial hypothalamic nucleus in regulation of stress-induced gastric mucosal injury in rats. https://doi.org/10.1038/s41598-018-28456-0
Tordoff MG, Aleman TR, Murphy MC (2012) No effects of monosodium glutamate consumption on the body weight or composition of adult rats and mice. Physiol Behav, Amsterdam 107(3):338–345. https://doi.org/10.1016/j.physbeh.2012.07.006
Turner PV, Brabb T, Pekow C, Vasbinder MA (2011) Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50(5):600–613
Villagarcia HG, Castro MC, Arbelaez LG, Schinella G, Massa ML, Spinedi E, Francini F (2018) N-Acetyl-l-cysteine treatment efficiently prevented pre-diabetes and inflamed-dysmetabolic liver development in hypothalamic obese rats. Life Sci 199:88–95. https://doi.org/10.1016/j.lfs.2018.03.008
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
Wang Y, Gao X, Pedram P, Shahidi M, du J, Yi Y, Gulliver W, Zhang H, Sun G (2016) Significant beneficial association of high dietary selenium intake with reduced body fat in the CODING study. Nutrients 8. https://doi.org/10.3390/nu8010024
Warshakoon NC, Sheville J, Bhatt RT, Ji W, Mendez-Andino JL, Meyers KM, Kim N, Wos JA, Mitchell C, Paris JL, Pinney BB, Reizes O, Hu XE (2006) Design and synthesis of substituted quinolines as novel and selective melanin concentrating hormone antagonists as anti-obesity agents. Bioorg Med Chem Lett 16:5207–5211. https://doi.org/10.1016/j.bmcl.2006.07.006
WHO (2016) Obesity and overweight. World Health Organization
Wu M, Li Y, Fu X, Wang J, Zhang S, Yang L (2014) Profiling the interaction mechanism of quinoline/quinazoline derivatives as MCHR1 antagonists: an in silico method. Int J Mol Sci 15:15475–15502. https://doi.org/10.3390/ijms150915475
Wu X, Xie CY, Yin Y, Deng ZY (2013) The results of some studies involving animal models of obesity induced by monosodium glutamate are not conclusive. Eur J Clin Nutr 67:228. https://doi.org/10.1038/ejcn.2012.211
Yoshimatsu H, Oomura Y, Katafuchi T, Niijima A, Sato A (1985) Lesions of the ventromedial hypothalamic nucleus enhance sympatho-adrenal function. Brain Research, 339:390–392. https://doi.org/10.1016/0006-8993(85)90112-X
Zhong Q, Lin R, Nong Q (2018) Adiposity and serum selenium in U.S. adults Nutrients 10. https://doi.org/10.3390/nu10060727
Zou P et al (2014) Targeting FoxO1 with AS1842856 suppresses adipogenesis. Cell Cycle 13:3759–3767. https://doi.org/10.4161/15384101.2014.965977
Acknowledgements
We are grateful to UFPel, CNPq (408874/2016-3, 429859/2018-0), FAPERGS (16/2551-0000526-5, 17/2551-0001013-2) for financial support. This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível superior - Brasil (CAPES) - Finance Code 001. K.C.R. is the recipient of a FAPERGS fellowship. D.A., E.A.W., and C.L. are recipients of CNPq fellowship.
Author information
Authors and Affiliations
Contributions
K.C.R. and C.F.B. performed the experiments and the analysis of data and wrote the manuscript. K.C.R., K.P.M., R.L.O, and J.J.P performed the experiments. K.C.R., E.A.W., and C.L. designed the project. R.K. and D.A. synthesized the compound 4-PSQ. C.L. and E.A.W. supervised the experiments. All authors critically reviewed the content and approved the final version for publication
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodrigues, K.C., Bortolatto, C.F., da Motta, K.P. et al. The neurotherapeutic role of a selenium-functionalized quinoline in hypothalamic obese rats. Psychopharmacology 238, 1937–1951 (2021). https://doi.org/10.1007/s00213-021-05821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05821-y