Abstract
Occurrence of stroke cases displays a time-of-day variation in human. However, the mechanism linking circadian rhythm to the internal response mechanisms against pathophysiological events after ischemic stroke remained largely unknown. To this end, temporal changes in the susceptibility to ischemia/reperfusion (I/R) injury were investigated in mice in which the ischemic stroke induced at four different Zeitgeber time points with 6-h intervals (ZT0, ZT6, ZT12, and ZT18). Besides infarct volume and brain swelling, neuronal survival, apoptosis, ischemia, and circadian rhythm related proteins were examined using immunohistochemistry, Western blot, planar surface immune assay, and liquid chromatography–mass spectrometry tools. Here, we present evidence that midnight (ZT18; 24:00) I/R injury in mice resulted in significantly improved infarct volume, brain swelling, neurological deficit score, neuronal survival, and decreased apoptotic cell death compared with ischemia induced at other time points, which were associated with increased expressions of circadian proteins Bmal1, PerI, and Clock proteins and survival kinases AKT and Erk-1/2. Moreover, ribosomal protein S6, mTOR, and Bad were also significantly increased, while the levels of PRAS40, negative regulator of AKT and mTOR, and phosphorylated p53 were decreased at this time point compared to ZT0 (06:00). Furthermore, detailed proteomic analysis revealed significantly decreased CSKP, HBB-1/2, and HBA levels, while increased GNAZ, NEGR1, IMPCT, and PDE1B at midnight as compared with early morning. Our results indicate that nighttime I/R injury results in less severe neuronal damage, with increased neuronal survival, increased levels of survival kinases and circadian clock proteins, and also alters the circadian-related proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclic environmental changes led to the evolution of circadian systems which synchronize behavioral and physiological processes in organisms. Indeed, all eukaryotes and even a number of prokaryotic organisms have circadian clock mechanisms [1, 2]. These mechanisms differ in complexity and provide an innate adaptive capacity to the organisms against the cyclic environmental changes. These circadian systems result in differential response to the exact same impact according to the time-of-day it occurs [2, 3].
On the other hand, the circadian rhythm is able to adjust to the changing environmental conditions by resetting its own timing [4]. Although the ability to adjust circadian rhythms according to new environmental situations has its own advantages, its chronic disruption causes negative effects on brain, physiology, and behavior. In severe cases, these disruptions may turn into cardiac and metabolic disorders which eventually result in cerebrovascular events, including stroke [5]. Moreover, deficiencies of circadian clock proteins also lead to increased level of reactive oxygen species playing critical roles in the pathological conditions such as ischemic stroke [6]. It was revealed that deficiency of the circadian clock transcriptional factor Aryl hydrocarbon receptor nuclear translocator-like protein 1(Bmal1) results in premature aging in mice, and overexpression of Bmal1 and Circadian Locomotor Output Cycles Kaput (Clock) proteins may lead to directly or indirectly activation of hypoxia inducible factor-1alpha (HIF-1α), which plays critical roles in the regulation of innate neuroprotective molecules [6].
In fact, occurrence of human stroke cases exhibits time-of-day variation. Even though patients may suffer from an ischemic attack throughout the day, it is well known that ischemic stroke cases peak in the early morning hours, especially in the first 2 h after the patient is awake [7, 8]. It was also reported that the incidence of stroke is the lowest between midnight and 6 am [7]. However, the molecular or cellular signaling linking circadian rhythm and the development of pathophysiological events after ischemic stroke remained largely unknown [9].
In addition to the diurnal variability in the incidence of ischemic stroke, it was hypothesized that the stroke severity and hence, mortality, also displays a circadian rhythm [10]. Here, we hypothesized that the tolerability after an ischemic stroke depends on the time-of-day the injury occurred and explored the molecular mechanisms behind this phenomenon. We investigated the expression levels of circadian clock machinery proteins and survival kinases in mice subjected to 30 min middle cerebral artery occlusion (MCAo) at four different Zeitgeber time points (ZT0, ZT6, ZT12, and ZT18). With the use of proteomic analyses, we identified eight proteins that were differentially regulated in the ischemic striatum. Together, our data suggest that molecular clock mechanisms, AKT and ERK signaling pathways, and an array of intracellular proteins with the possible contribution of physiological factors contribute to the increased neuronal survival following a nighttime ischemia/reperfusion injury compared with an early morning injury.
Materials and Methods
Ethics Statement
This study has been conducted in accordance with the ethical standards and according to the Declaration of Helsinki and according to national and international guidelines and has been approved by the Ethics Committee of Istanbul Medipol University.
Experimental Groups
Experiments were performed using 10–12 weeks, male Balb/c mice (22–25 g). All animals were maintained under a constant 12:12-h light-darkness regimen with ad libitum access to food and water. A total 70 animals were divided into three main groups (i) for proteomic experiments; (ii) histological, Western blot, and planar surface immunohistochemistry experiments; and iii) for the analysis of infarct volume, brain swelling, and neurological deficit scores. In the first two sets, mice were exposed to 30 min of focal cerebral ischemia followed by 72 h reperfusion. These animals were further divided into four different time points based on their Zeitgeber time; ZT0 (06:00), ZT6 (12:00), ZT12 (18:00), and ZT18 (24:00) (ZT0 is lights on, and ZT12 is lights off) (n = 7 for each group). The third set was designed according to the results of 30 min of ischemia and exposed to 90 min of focal cerebral ischemia followed by 24 h reperfusion. This set was performed for ZT0 and ZT18 groups (n = 7 for each group).
Animal Surgery
All MCAo experiments were done with respect to ZT. Mice were anesthetized with 1% isofluorane (30% O2, reminder N2O), and rectal temperature was controlled between 36.5 and 37.0 °C using a feedback-controlled heating system. During the experiments, cerebral blood flow (CBF) was monitored via laser Doppler flowmetry (LDF) using a flexible 0.5 mm fiber optic probe (Perimed, Sweden) which was attached with tissue adhesive to the intact skull overlying the MCA territory (2 mm posterior and 6 mm lateral from the bregma). Focal cerebral ischemia was induced using an intraluminal filament technique [11]. Briefly, after a midline neck incision, the left common and external carotid arteries were isolated and ligated. A microvascular clip (FE691; Aesculap) was temporarily placed on the internal carotid artery. A 7-0 silicon-coated nylon monofilament (701934PK5Re, Doccol; USA) was inserted through a small incision into the common carotid artery and advanced 9 mm distal to the carotid bifurcation for MCAo. Reperfusion was initiated 30 or 90 min after onset of ischemia by gentle monofilament removal. Thereafter, mice were placed back into their home cages.
In mice exposed to 90 min MCAo, neurological deficits were evaluated 24 h after MCAo using the following 5-point score: 0 = normal function, 1 = flexion of torso and of the contralateral forelimb upon lifting of the animal by the tail, 2 = circling to the contralateral side but normal posture at rest, 3 = reclination to the contralateral side at rest, and 4 = absence of spontaneous motor activity. At 72 h (for 30 min MCAo) or 24 h (for 90 min MCAo) after reperfusion, mice were sacrificed under deep anesthesia (4% isofluorane with 30% O2, remainder N2O). Brains were removed, frozen on dry ice, and cut on a cryostat into coronal 18-μm sections, which were subsequently used for the analysis of infarct volume and brain swelling. For 30 min MCAo, tissue samples obtained from the ipsilateral to the stroke were collected for proteomics, planar surface immunoassay (PSI), and Western blots.
Analysis of Neuronal Survival
Adjacent brain sections of the same animals were fixed in 4% paraformaldehyde (PFA)/0.1 mol/l phosphate-buffered saline (PBS) and washed and immersed for 1 h in 0.1 mol/l PBS containing 0.3% Triton X-100 (PBS-T)/10% normal goat serum. Sections were incubated overnight at 4 °C with Alexa Fluor 488-conjugated monoclonal mouse anti-NeuN (Mab377X; Chemicon). Next day, sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI). Sections were analyzed using a confocal Zeiss LSM 780 microscope (Carl Zeiss, Jena, Germany). Nine different region of interest (ROI) in the striatum, each measuring 62,500 μm2, were evaluated. Mean numbers of NeuN cells were analyzed in the ischemic and contralesional striatum. By dividing results obtained in both hemispheres, the percentage of surviving neurons in the ischemic striatum was determined.
Analysis of DNA Fragmentation
For the evaluation of neuronal injury, coronal brain sections at the level of the bregma from mice exposed to 30 min MCAo were fixed with 4% PFA/0.1 M PBS and were labeled using a terminal transferase dUTP nick end labeling (TUNEL) kit (In Situ Cell Death Detection Kit; Roche, Switzerland). Sections were counterstained with DAPI. Stainings were analyzed by tifying DNA-fragmented cells (which in 30 min MCAo are equivalent to neurons) in 12 adjacent ROI in the striatum, each measuring 62,500 μm2, under a confocal Zeiss LSM 780 microscope.
Western Blot and Planar Surface Immunoassay
For Western blot analysis, brain tissue samples were harvested from the ischemic striatum of mice exposed to 30 min MCAo. Tissue samples belonging to the same group were pooled, homogenized, sonicated, and treated with protease inhibitor cocktail and phosphatase inhibitor cocktail. Total protein content was evaluated using Qubit 2.0 Fluorometer according to the manufacturer’s protocol (Invitrogen, Life Technologies Corporation, Carlsbad, CA, USA). Equal amounts of protein (20 μg) were size-fractionated using any-kD Mini-Protean TGX gel electrophoresis and then transferred to a nitrocellulose membrane using the Trans-Blot TurboTransfer System (Bio-Rad, Life Sciences Research). Thereafter, membranes were blocked in 5% nonfat milk in 50 mMol Tris-buffered saline (TBS) containing 0.1% Tween (TBS-T; blocking solution) for 1 h at room temperature, washed in 50 mMol TBS-T, and incubated overnight with monoclonal rabbit Bmal1 (14020; Cell Signaling), monoclonal rabbit Clock (5157; Cell Signaling), polyclonal rabbit anti-PerI (Ab3443; Abcam), polyclonal rabbit anti-PerII (Ab180655; Abcam), monoclonal mouse phosphorylated p53 (2524; Cell Signaling), and monoclonal mouse PDE10A (sc-515023; Santa Cruz Biotechnology). The next day, membranes were washed with 50 mM TBS-T and incubated with horseradish peroxidase-conjugated goat anti-rabbit (sc-2004; Santa Cruz Biotechnology) or goat anti-mouse (sc-2005; Santa Cruz Biotechnology) antibody (diluted 1:2500) for 1 h at room temperature. Blots were performed at least three times. Protein loading was controlled by stripping and reprobing with polyclonal rabbit anti-β-actin antibody (4967; Cell Signaling Technology). Blots were developed using Clarity Western ECL Substrate kit (Bio-Rad; Life Sciences Research) and visualized using the ChemiDoc MP System (Bio-Rad; Life Sciences Research). Protein levels were analyzed densitometrically using an image analysis system (Image J; National Institute of Health, Bethesda, MD, USA), corrected with values determined on β-actin blots and expressed as relative values compared with ZT0 mice.
In order to analyze Akt signaling pathways, planar surface immunoassay (PSI) (PathScan Akt Signaling Antibody Array Kit, 9474; Cell Signaling) was used according to the manufacturer’s instructions. Briefly, PSI allows for the simultaneous detection of 16 phosphorylated proteins predominantly belonging to the Akt signaling pathways. Each well in the glass slide was blocked by array blocking buffer for 15 min at room temperature. Thereafter, equal amount of protein (75 μg) was loaded and incubated overnight at 4 °C on an orbital shaker. The next day, wells were washed with array wash buffer. Detection Antibody Cocktail was added to each well and incubated for 1 h at room temperature. Afterwards, horseradish peroxidase-linked streptavidin was added to each well and the slide was incubated for 30 min at room temperature. The plates were then covered with LumiGLO/peroxide reagent and visualized using the ChemiDoc MP System (Bio-Rad; Life Sciences Research). Protein levels were analyzed densitometrically using the Image J program, corrected with values determined with respect to positive and negative controls on array slides.
Sample Preparation for Liquid Chromatography-Mass Spectrometry Analysis
Tryptic peptides were generated according to the Filter Aided Sample Preparation Protocol (FASP) [12]. The brain tissues taken from the ischemic striatum and were homogenized in 50 mM ammonium bicarbonate and lysed by heating at 95 °C in UPX buffer (Expedeon). After incubation at 4 °C for an hour, samples were centrifuged at 14,000×g for 10 min and the supernatants were transferred to a clean 1.5 ml microcentrifuge tube. The total protein concentration was measured with Qubit assay. Tryptic peptides were generated by the FASP kit (Expedeon). Briefly, 50 μg protein was filtered with 6 M urea in a 30-kDa cut-off spin column, alkylated with 10 mM iodooacetamide in the dark for 20 min at room temperature and incubated with trypsin (1:100 trypsin to protein ratio, Pierce) overnight at 37 °C. The tryptic peptides were eluted from the columns and lyophilized. The peptides were redissolved in 0.1% formic acid (FA, Sigma-Fluka) and diluted to 100 ng/μl before injecting to the liquid chromatography-mass spectrometry (LC-MS/MS) system.
LC-MS/MS Analysis and Data Processing
The LC-MS/MS analysis and the subsequent protein identifications were done according to an earlier published protocol [13, 14]. The tryptic peptides were loaded onto the ACQUITY UPLC M-Class coupled to a SYNAPT G2-Si high-definition mass spectrometer (Waters). The columns were equilibrated with 97% mobile phase A (0.1% FA in UHPLC grade water, Merck) and temperature was set to 55 °C. Peptides were separated by a 90-min gradient elution from the trap column (Symmetry C18, 5 μm, 180 μm i.d. × 20 mm, Waters) to the analytic column (CSH C18, 1.7 μm, 75 μm i.d. × 250 mm, Waters) at 0.400 μl/min flow rate with a gradient from 4 to 40% ACN containing 0.1% FA (v/v). Positive ion modes of MS and MS/MS scans with 0.7 s cycle time were performed sequentially. Ten volts was set as low collision energy and 30 V as high CE. The ions were separated by ion mobility separation (IMS). A wave velocity was ramped from 1000 to 55 m/s over the full IMS cycle. The release time for mobility trapping was set as 500 μs, trap height was set to 15 V. IMS wave delay was 1000 μs for the mobility separation after trap release [15]. Without any precursor ion preselection, all the ions within the 50–1900 m/z range were fragmented in resolution mode. Additionally, 100 fmol/μl Glu-1-fibrinopeptide B was infused as lockmass reference with a 60-s interval. To identify and quantify the peptides, Progenesis-QI for proteomics software (Waters) was used. All proteins were identified by at least three unique peptide sequences and then, p values and expression ratio of proteins were calculated.
Analysis of Infarct Volume and Brain Swelling
For the evaluation of infarct volume and brain swelling, coronal brain sections were collected at four equidistant brain levels, 2 mm apart, from mice exposed to 90 min MCAo, which were stained with cresyl violet according to a standard protocol. Within the sections, the border between infarcted and non-infarcted tissues was outlined using an image analysis system (Image J; National Institute of Health, Bethesda, MD, USA), and the infarct area was assessed by subtracting the area of the non-infarcted ipsilateral hemisphere from that of the contralateral side. Infarct volume was calculated by integration of these infarct areas. Edema was calculated as the volume difference between the ischemic and the non-ischemic hemisphere and expressed as percentage of the volume of the non-ischemic hemisphere.
Statistics
Disseminate neuronal injury, Western blots, and PSI data were evaluated by one-way ANOVA followed by LSD tests. LC-MS/MS data were evaluated by independent samples t test and 1.4-fold change was considered as significant between ZT0 and ZT18. Data from 90 min MCAo followed by 24 h reperfusion were evaluated by independent samples t test. Data are presented as mean ± S.D. values. Throughout the study, p values <0.05 were considered significant.
Results
Neuronal Survival Following Ischemia/Reperfusion in Mice Exhibits Time-of-Day Variability
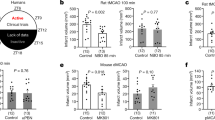
To investigate whether the tolerability to ischemia/reperfusion (I/R) injury varied with the time-of-day in which the insult occurred, we induced 30 min MCAo followed by 72 h reperfusion at four different time points (ZT0, ZT6, ZT12, and ZT18) in mice. LDF was used to evaluate the changes in CBF during and after the onset of MCAo (Fig. 1a). Approximately 85% decrease in LDF values during the occlusion with a subsequent and rapid increase with the onset of reperfusion is routinely observed in our model of ischemic stroke [11]. CBF values were similar in all the time points evaluated. Neuronal survival was assessed by NeuN staining of coronal sections (Fig. 1b). The number of NeuN-positive cells was calculated from nine different regions in the striatums of ischemic (ipsilateral) and non-ischemic hemispheres (contralateral). Neuronal survival, given as the percent of NeuN-positive cells in the contralateral hemisphere, was significantly higher in the Mid-Dark group (ZT18) when compared with the Light (ZT0) group. DNA fragmentation, which was evaluated with TUNEL staining of coronal brain sections, was also significantly lower in the Mid-Dark group relative to the Light group (Fig. 1c). Mid-Light (ZT6) or Dark (ZT12) groups did not demonstrate any significant change in either of the two parameters when compared with ZT0 or ZT18.
a Laser Doppler flow (LDF) recordings during ischemia and initial reperfusion onset. b Neuronal survival was assessed using NeuN-stained brain sections c Disseminate neuronal injury in the striatum assessed by terminal transferase dUTP nick end labeling (TUNEL) in mice submitted to 30 min of intraluminal middle cerebral artery occlusion (MCAo). Data are mean ± S.D. (n = 7 mice/group). *p < 0.05 compared with ZT0, § p < 0.05 compared with ZT6 group
Circadian Clock Proteins PerI, Clock, and Bmal1 are Significantly Increased at ZT18
To determine the underlying mechanisms responsible for the increase in neuronal survival following MCAo during the Mid-Dark period, we evaluated the expressions of circadian rhythm proteins PerI, PerII, Bmal1, and Clock. Levels of PerI, Clock, and Bmal1 proteins were significantly increased in the Mid-Dark (ZT18) group relative to ZT0 (Fig. 2). Oscillations of the expressions of PerI and Bmal1 were similar in the striatums of ischemic animals, while PerII and Bmal1 expressions were in antiphase. Interestingly, PerII was significantly increased and peaked at ZT12, but not at ZT18 (Fig. 2b). The highest variation was observed in the molecular clock gene, Clock, whose protein levels doubled in the ischemic striatum of the ZT18 group compared with ZT0 (Fig. 2c).
Circadian clock protein expressions in the ischemic striatum .Western Blots for a Period-I, b Period-II, c Clock, and d Bmal1 in ischemic tissue samples. On top of the figure, a representative β-actin blot is shown. Data are mean ± S.D. values (n = 3 blots/protein). **p < 0.01/*p < 0.05 compared with ZT0, §§ p < 0.01/compared with ZT6, and && p < 0.01/& p < 0.05 compared with the ZT12 group
Circadian Core Proteins Regulate Akt Phosphorylation
To further determine the underlying mechanisms, we examined the expression levels of AKT-mTOR-S6K pathway proteins, which are known to be involved in the regulation of the circadian rhythm [16]. By using planar surface immunoassay for AKT signaling (Fig. 3), we demonstrated that ischemia/reperfusion injury which was induced at ZT18 (Mid-Dark) resulted in a significantly higher p-AKT (Thr308) level (Fig. 3a), which was also previously ascribed to circadian rhythm in chick retinae [17]. We have also previously reported that AKT pathway mediates neuronal survival following ischemic stroke [18]. Consistently, these results suggest that increased p-AKT at the Mid-Dark period could be responsible for the increase in neuronal survival observed at this time point. In addition, levels of phosphorylated ERK1/2 (Fig. 3b), p-mTOR (Fig. 3c), phosphorylated ribosomal protein S6 (rpS6) (Fig. 3d), and p-Bad (Fig. 3e) were also significantly increased at ZT18, compared with the ZT0 group. However, p-PRAS40, a negative regulator of AKT and mTOR, was significantly decreased at ZT18 as well as ZT6 and ZT12 groups when compared with ZT0 (Fig. 3f). Moreover, p-PTEN, a negative regulator of AKT, was slightly, but not significantly, reduced at ZT18 compared with ZT0 (Fig. 3g). Relative to ZT0, phosphorylated PDK1, RSK1, AMPKα, or GSK3α/β were not significantly altered at ZT18 (Fig. 3h–l); however, with the exception of slightly reduced phosphorylated PDK1 levels, these proteins were significantly decreased at ZT12 (Fig. 3h–l). Further analysis of the levels of phosphorylated p53 displayed significant decrease at ZT18 compared with ZT0 (Fig. 3n), suggesting a decrease in the pro-apoptotic signals at this time point.
Expression levels of Akt signaling pathway proteins after 30 min of MCA occlusion. AKT signaling was evaluated using a planar surface immunoassay (PSI) tool from tissue samples collected 72 h after 30 min MCA occlusion. a p-Akt (Thr308), b p-ERK-1/−2 (Thr202/ Tyr204), c p-mTOR (Ser2481), d p-rpS6 (Ser235/ 236), e p-Bad (Ser112), f p-PRAS40 (Thr246), g p-PTEN (Ser380), h p-PDK1 (Ser241), i p-RSK1 (Thr421/ Ser424), j p-AMPKα (Thr172), k p-GSK-3α (Ser21), l p-GSK-3β (Ser9). m Representative examples of PSI’s and n protein expression level of phosphorylated p53 protein which was corrected with β-Actin. Data are mean ± S.D. (n = 7–8 mice/group). **p < 0.01/*p < 0.05 compared with ZT0, §§ p < 0.01/§ p < 0.05 compared with ZT6, and && p < 0.01/& p < 0.05 compared with the ZT12 group
Proteomic (LC-MS/MS) Analyses of Temporally Regulated Striatal Proteins
Due to the increase in the levels of circadian clock proteins which can act as transcription factors and promote the expression of other proteins, and the increase in the activities of AKT, mTOR, and ERK signaling pathways in the ZT18 group, we wanted to identify the other proteins whose expressions were also altered during the Mid-Dark period compared to the Light, and which could be responsible for better tolerability to ischemic injury. To this end, protein expressions that exhibited significant fluctuations in the ischemic striatum were analyzed in different time points using the LC-MS/MS method. Our analyses revealed that eight proteins for ischemic striatum (Fig. 4) exhibited statistically significant variations (p < 0.05 and 1.4-fold change). These proteins include calcium/calmodulin-dependent serine protein kinase (CSKP), guanine nucleotide-binding protein z subunit alpha (GNAZ), neuronal growth regulator 1 (NEGR1), imprinted and ancient protein (IMPACT), calcium/calmodulin-dependent 3′,5′-cyclic nucleotide phosphodiesterase 1B (PDE1B), and hemoglobin subunit beta-1, beta-2, and alpha (HBB1, HBB2, HBA) for ischemic striatum.
Protein identification with respect to ischemic ZT0 and ZT18 via LC-MS/MS analysis. a CSKP (gene ID: 12,361; calcium/calmodulin-dependent serine protein kinase), b GNAZ (gene ID: 14,687; guanine nucleotide-binding protein z subunit alpha), c NEGR1 (gene ID: 320,840; neuronal growth regulator 1), d IMPCT (gene ID: 16,210; imprinted and ancient protein), e PDE1B (gene ID:18,574; calcium/calmodulin-dependent 3′,5′-cyclic nucleotide phosphodiesterase), f HBA (gene ID: 15,122; hemoglobin subunit alpha), g HBB1 (gene ID: 15,129; hemoglobin subunit beta-1), and h HBB2 (gene ID: 15,130; hemoglobin subunit beta-2). Data are mean ± S.D. (p < 0.05 and >1.4-fold change between ZT0 and ZT18)
Infarct Volume, Brain Swelling, and Neurological Deficits are Regulated by Circadian Rhythm After Focal Cerebral Ischemia
In order to investigate brain injury, edema formation, and neurological deficits, mice were exposed to 90 min MCAo, followed by 24 h reperfusion which induces brain infarction of the striatum and overlying cortex [19]. LDF was used to evaluate the changes in CBF during and after the onset of MCAo (Fig. 5a). Mid-Dark decreased development of tissue injury (Fig. 5b) and edema formation (Fig. 5c) with respect to ZT0. In addition, neurological deficit scores (Fig. 5d) were also significantly decreased in the Mid-Dark group.
Effects of circadian rhythm on neurological deficits, infarct volume and brain swelling. a Laser Doppler flow (LDF) above the core of the MCA territory, b infarct volume, c brain swelling, and d neurological deficits in mice submitted to 90 min of intraluminal MCAo which induces brain infarcts of the striatum [19]. Note that the infarct volume and brain swelling were significantly decreased and neurological function was improved in the ZT18 group. Data are mean ± S.D. (n = 7 mice/group). *p < 0.05 compared with the ZT0group.
Discussion
It was documented that ischemic stroke cases peak in the early morning hours [7, 8], and fatality rates of morning strokes are higher even when adjusted for age, gender, and severity [20]. This circadian variability was linked to differential body temperature, heart rate, blood pressure, blood viscosity, or platelet aggregation [21]. In addition, sleep is fundamental to restore brain functions. A direct relationship between sleep disorders and neurodegenerative disorders, including Alzheimer disease, traumatic brain injury, and ischemic stroke, was noted [22, 23]. It was also shown that recovered sleep-wake cycle and consolidated nighttime occurred simultaneously after acute traumatic brain injury in humans, suggesting the involvement of improved common motor responses [24]. On the other hand, sleep disorders impede the efficiency of post-stroke recovery by interfering with neuroplasticity processes [23]. Apart from the physiological changes suggested, to date, molecular mechanisms behind this circadian variation were largely unknown. Given the higher incidence of ischemic stroke cases in morning hours compared with nighttime in humans, we surmised that the tolerance to ischemic stroke could also exhibit day-to-night fluctuations. However, fairly little is known about the temporal fluctuations in ischemic stroke sensitivity in mice as well as in humans. The MCAo is used to mimic ischemic stroke in mice, as ischemic stroke occurs more frequently than hemorrhagic strokes in humans and hence, more frequently targeted in experimental stroke studies [25]. Here, the MCAo model chosen results in selective neuronal injury in the striatum and mainly affects small- and medium-sized neurons [26, 27].
Here, we provide evidence for the first time that in a mouse model of ischemic stroke, tolerance to ischemic injury varies significantly based on the time-of-day in which the injury occurred and the ischemic damage is the most severe during the Early-Light (ZT0) period, whereas the least severe during the Mid-Dark (ZT18) period. This is in line with a previous study which reported that rats subjected to ischemic stroke in the early morning hours displayed greater cerebral infarction compared to afternoon hours, suggesting a temporal ischemic sensitivity [28]. On the other hand, it was also reported in another study that global ischemia resulted in the highest levels of ischemic damage markers in the hippocampus of rats when performed during the evening (ZT14) period [29]. This inconsistency may be due to the use of a different animal species or a different model to mimic ischemic insult or the analysis of different markers in different brain regions. However, the molecular mechanisms were not studied in depth in these studies. We hypothesized that the temporal tolerability to ischemic stroke could be at least partially explained by the circadian changes in the general protein expression profile in neurons. It has been reported that expression of circadian genes oscillate throughout the day in almost all cell types [30] and in the case of the brain, these robust oscillations are region-specific and highly unique [31].
Presently, we provide evidence on the protein level that circadian genes oscillate in the striatum of mice and upon ischemic injury; levels of these proteins are altered. It has been proposed that expressions of molecular clock genes are regulated on the transcriptional and translational level through interacting positive and negative feedback loops which provide functional oscillations [32]. It was also reported that expressions of canonical circadian clock genes, PerI, PerII, and Bmal1 are in antiphase, thus result in opposing effects to maintain the functional oscillations on the molecular level [33]. In agreement with these reports, our data suggest that levels of PerII and Bmal1 proteins are in antiphase in the ischemic mouse striatum, while PerI and Bmal1 levels seem to coincide, rather than being antiphase. Furthermore, it was reported that circadian rhythm proteins are involved in tissue homeostasis by controlling reactive oxygen species (ROS) production and oxidative stress mechanisms [6]. Bmal1 and its dimerization partner Clock manage the regulation of ROS levels [9, 34]. In the ischemic brain, ROS levels increase, which in turn result in HIF-1α protein stabilization and activation [35]. Overexpression of Bmal1 and Clock may lead to directly or indirectly activation of HIF-1α at ZT18. In addition, activation of the Akt signaling pathway also modulates HIF-1α expression through the mammalian target of rapamycin (mTOR) independent pathway [36].
It has been well established that neuronal injury results in the disruption of circadian rhythms [37] and alters the expression profiles of clock genes [38]. Indeed, we demonstrated that expressions of circadian proteins were altered in response to ischemic injury. Accordingly, in the Mid-Dark period in which the highest rate of neuronal survival was observed, in addition to the circadian clock proteins, phosphorylation-activated AKT was also increased. It is known that increase in the active AKT following ischemia has protective effects against neuronal death [18, 39]. AKT-mTOR and S6K signaling pathways were reported to regulate nutrient influx, endocrine signaling, and cellular energy balance and that nutrient and energy metabolisms affect circadian rhythm [16]. Thus, it is safe to assume that when the energy metabolisms are disrupted due to an ischemic insult, AKT signaling pathways as well as circadian clock proteins are all affected from this disruption. It is tempting to speculate that increased expressions of Clock, PerI, and Bmal1 during the Mid-Dark period may contribute to increased neuronal survival by regulating the expressions of an array of other genes. In fact, it was reported that these genes not only play roles in circadian rhythm regulation, but are also involved in other metabolic activities [40, 41] and thereby, may have other unknown functions. However, it should be noted that the physiological factors, such as blood pressure, heart rate, or body temperature, may have also contributed to the increased neuronal survival during the Mid-Dark period. In addition, phosphorylation of the Bad protein at Ser112 reverses its pro-apoptotic effect, by inhibiting its activity [42]. In accordance with this report, our finding showed that Bad phosphorylation level was highest at ZT18 after ischemic stroke. Moreover, expression of several proteins at ZT12 such as p-Akt, p-ERK-1/2, p-mTOR, and p-rpS6 were regulated in contrast with the ZT18 group. We surmise that this differential regulation could be related to the expression of the Bmal1 protein.
In addition, proteome-wide analysis revealed that a number of proteins have altered levels in the Mid-Dark period of ischemic mice. These proteins include regulators of ion currents, neural development, and neuritogenesis, as well as mitochondrial outer membrane protein assembly and formation. Of the significantly altered proteins, CSKP is a calcium/calmodulin-dependent serine protein kinase enriched in brain tissue [43] and was reported to have roles in regulation of L-type calcium current [44], and ATP efflux through association with P2X3 receptors [45] and also in the regulation of metabolic proteins [46]. This GNAZ was reported to interact with neurotransmitter and neuropeptide receptors, such as adenosine A1, alpha2-adrenergic, or dopamine D2 receptors [47], participating to neuronal development, axonal growth as well as ion channel regulation, and its expression was suggested to be temporally and spatially regulated in neurons [48, 49]. It was reported that IMPCT, a highly abundant neuronal protein in the brain, promotes neurite outgrowth [50, 51]. PDE1B was demonstrated to be highly and selectively expressed in the mouse and human striatum compared to other brain regions, and it was suggested that their expressions are regulated by cyclic AMP and cyclic GMP-dependent mechanisms [52]. NEGR1 is a GPI-anchored protein that was suggested to have a role in cell-cell adhesion in cancer cells as well as cholesterol trafficking in neurons [53]. Expression of hemoglobin subunits beta (HBB1, HBB2) and alpha (HBA) were reported primarily in neurons of the rat brain, and it was demonstrated that upon ischemic injury, levels of neuronal hemoglobin and hemoglobin released from erythrocytes were significantly increased [54]. Increased neuronal hemoglobin may worsen the brain injury after trauma [55] Likewise, our findings indicate that at the time point in which the highest neuronal survival is observed, levels of hemoglobin subunits were also significantly reduced. By and large, the involvement of these proteins in the regulation of circadian rhythm and in the protection of neurons against ischemic damage should be addressed in further studies.
In order to determine the effect of circadian rhythm on tissue injury and edema formation after cerebral ischemia, Light and Mid-Dark time points were chosen. For this, mice were submitted to 90 min MCAo followed by 24 h reperfusion. The ZT18 group had less infarct area and brain swelling and improved neurological function compared to ZT0. Results showed that there is a strong relationship between development of tissue injury and circadian rhythm.
In conclusion, we provide evidence that a differential response to ischemic stroke occurs according to the time of the injury. We also demonstrated that nighttime injury resulted in less severe neuronal damage, infarct volume, brain swelling, and neurological scores with increased neuronal survival, increased levels of AKT and ERK survival kinases as well as PerI, Bmal1, and Clock proteins. Furthermore, alterations in the proteome suggested the interplay of a number of other proteins; however, the functional importance of these remains to be clarified in future studies.
References
Roenneberg T, Merrow M (2002) "What watch?...such much!" Complexity and evolution of circadian clocks. Cell Tissue Res 309(1):3–9. doi:10.1007/s00441-002-0568-1
Paranjpe DA, Sharma VK (2005) Evolution of temporal order in living organisms. J Circadian Rhythms 3(1):7. doi:10.1186/1740-3391-3-7
Fodor DM, Babiciu I, Perju-Dumbrava L (2014) Circadian variation of stroke onset: a hospital-based study. Clujul medical 87(4):242–249. doi:10.15386/cjmed-328
Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE et al (2012) Human responses to bright light of different durations. J Physiol 590(13):3103–3112. doi:10.1113/jphysiol.2011.226555
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 108(4):1657–1662. doi:10.1073/pnas.1018375108
Khapre RV, Kondratova AA, Susova O, Kondratov RV (2011) Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 10(23):4162–4169. doi:10.4161/cc.10.23.18381
Pardiwalla FK, Yeolekar ME, Bakshi SK (1993) Circadian rhythm in acute stroke. J Assoc Physicians India 41(4):203–204
Elliott WJ (1998) Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 29(5):992–996
Razorenova OV (2012) Brain and muscle ARNT-like protein BMAL1 regulates ROS homeostasis and senescence: a possible link to hypoxia-inducible factor-mediated pathway. Cell Cycle 11(2):213–214. doi:10.4161/cc.11.2.18786
Manev H, Uz T (1998) The role of the light-dark cycle and melatonin in stroke outcome. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 7(3):165–167
Beker MC, Caglayan AB, Kelestemur T, Caglayan B, Yalcin E, Yulug B, Kilic U, Hermann DM et al (2015) Effects of normobaric oxygen and melatonin on reperfusion injury: role of cerebral microcirculation. Oncotarget 6(31):30604–30614. doi:10.18632/oncotarget.5773
Wisniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362. doi:10.1038/nmeth.1322
Hacariz O, Baykal AT, Akgun M, Kavak P, Sagiroglu MS, Sayers GP (2014) Generating a detailed protein profile of Fasciola hepatica during the chronic stage of infection in cattle. Proteomics 14(12):1519–1530. doi:10.1002/pmic.201400012
Serhatli M, Baysal K, Acilan C, Tuncer E, Bekpinar S, Baykal AT (2014) Proteomic study of the microdissected aortic media in human thoracic aortic aneurysms. J Proteome Res 13(11):5071–5080. doi:10.1021/pr5006586
Acioglu C, Mirabelli E, Baykal AT, Ni L, Ratnayake A, Heary RF, Elkabes S (2016) Toll like receptor 9 antagonism modulates spinal cord neuronal function and survival: direct versus astrocyte-mediated mechanisms. Brain Behav Immun 56:310–324. doi:10.1016/j.bbi.2016.03.027
Zheng X, Sehgal A (2010) AKT and TOR signaling set the pace of the circadian pacemaker. Current biology : CB 20(13):1203–1208. doi:10.1016/j.cub.2010.05.027
Ko ML, Jian K, Shi L, Ko GY (2009) Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J Neurochem 108(6):1607–1620. doi:10.1111/j.1471-4159.2009.05931.x
Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM (2006) The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF’s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20(8):1185–1187. doi:10.1096/fj.05-4829fje
Spudich A, Kilic E, Xing H, Kilic U, Rentsch KM, Wunderli-Allenspach H, Bassetti CL, Hermann DM (2006) Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat Neurosci 9(4):487–488
Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y et al (2012) Is there any circadian variation consequence on acute case fatality of stroke? Takashima Stroke Registry, Japan (1990-2003). Acta Neurol Scand 125(3):206–212. doi:10.1111/j.1600-0404.2011.01522.x
Kubota K, Sakurai T, Tamura J, Shirakura T (1987) Is the circadian change in hematocrit and blood viscosity a factor triggering cerebral and myocardial infarction? Stroke 18(4):812–813
Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ et al (2015) Beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18(7):1051–1057. doi:10.1038/nn.4035
Zunzunegui C, Gao B, Cam E, Hodor A, Bassetti CL (2011) Sleep disturbance impairs stroke recovery in the rat. Sleep 34(9):1261–1269. doi:10.5665/SLEEP.1252
Soddu A, Bassetti CL (2017) A good sleep for a fresh mind in patients with acute traumatic brain injury. Neurology 88(3):226–227. doi:10.1212/WNL.0000000000003529
Kunz A, Dirnagl U, Mergenthaler P (2010) Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pract Res Clin Anaesthesiol 24(4):495–509. doi:10.1016/j.bpa.2010.10.001
Hermann DM, Kilic E, Hata R, Hossmann KA, Mies G (2001) Relationship between metabolic dysfunctions, gene responses and delayed cell death after mild focal cerebral ischemia in mice. Neuroscience 104(4):947–955
Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ et al (2009) Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain : a journal of neurology 132(Pt 8):2239–2251. doi:10.1093/brain/awp174
Vinall PE, Kramer MS, Heinel LA, Rosenwasser RH (2000) Temporal changes in sensitivity of rats to cerebral ischemic insult. J Neurosurg 93(1):82–89. doi:10.3171/jns.2000.93.1.0082
Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM (2007) Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol 208(2):314–322. doi:10.1016/j.expneurol.2007.09.003
Okamura H, Yamaguchi S, Yagita K (2002) Molecular machinery of the circadian clock in mammals. Cell Tissue Res 309(1):47–56. doi:10.1007/s00441-002-0572-5
Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD (2002) Circadian rhythms in isolated brain regions. The Journal of neuroscience : the official journal of the Society for Neuroscience 22(1):350–356
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T (2004) Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5:18. doi:10.1186/1471-2199-5-18
Fahrenkrug J, Hannibal J, Georg B (2008) Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J Neuroendocrinol 20(3):323–329. doi:10.1111/j.1365-2826.2008.01651.x
Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP (2009) Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging 1(12):979–987. doi:10.18632/aging.100113
Sharp FR, Bergeron M, Bernaudin M (2001) Hypoxia-inducible factor in brain. Adv Exp Med Biol 502:273–291
Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A (2006) Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Molecular cancer research : MCR 4(7):471–479. doi:10.1158/1541-7786.MCR-05-0234
Meng H, Liu T, Borjigin J, Wang MM (2008) Ischemic stroke destabilizes circadian rhythms. J Circadian Rhythms 6:9. doi:10.1186/1740-3391-6-9
Boone DR, Sell SL, Micci MA, Crookshanks JM, Parsley M, Uchida T, Prough DS, DeWitt DS et al (2012) Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One 7(10):e46204. doi:10.1371/journal.pone.0046204
Kilic E, ElAli A, Kilic U, Guo Z, Ugur M, Uslu U, Bassetti CL, Schwab ME et al (2010) Role of Nogo-A in neuronal survival in the reperfused ischemic brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30(5):969–984. doi:10.1038/jcbfm.2009.268
Yan J, Wang H, Liu Y, Shao C (2008) Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol 4(10):e1000193. doi:10.1371/journal.pcbi.1000193
Kohsaka A, Bass J (2007) A sense of time: how molecular clocks organize metabolism. Trends in endocrinology and metabolism: TEM 18(1):4–11. doi:10.1016/j.tem.2006.11.005
Koh PO (2012) Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett 507(2):156–160. doi:10.1016/j.neulet.2011.12.012
Hata Y, Butz S, Sudhof TC (1996) CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. The Journal of neuroscience : the official journal of the Society for Neuroscience 16(8):2488–2494
Nafzger S, Rougier JS (2016) Calcium/calmodulin-dependent serine protein kinase CASK modulates the L-type calcium current. Cell Calcium. doi:10.1016/j.ceca.2016.10.001
Bele T, Fabbretti E (2016) The scaffold protein calcium/calmodulin-dependent serine protein kinase controls ATP release in sensory ganglia upon P2X3 receptor activation and is part of an ATP keeper complex. J Neurochem 138(4):587–597. doi:10.1111/jnc.13680
Srivastava S, McMillan R, Willis J, Clark H, Chavan V, Liang C, Zhang H, Hulver M et al (2016) X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta neuropathologica communications 4:30. doi:10.1186/s40478-016-0295-6
Wong YH, Conklin BR, Bourne HR (1992) Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science 255(5042):339–342
Ho MK, Wong YH (2001) G(z) signaling: emerging divergence from G(i) signaling. Oncogene 20(13):1615–1625. doi:10.1038/sj.onc.1204190
Hultman R, Kumari U, Michel N, Casey PJ (2014) Galphaz regulates BDNF-induction of axon growth in cortical neurons. Mol Cell Neurosci 58:53–61. doi:10.1016/j.mcn.2013.12.004
Roffe M, Hajj GN, Azevedo HF, Alves VS, Castilho BA (2013) IMPACT is a developmentally regulated protein in neurons that opposes the eukaryotic initiation factor 2alpha kinase GCN2 in the modulation of neurite outgrowth. J Biol Chem 288(15):10860–10869. doi:10.1074/jbc.M113.461970
Sattlegger E, Barbosa JA, Moraes MC, Martins RM, Hinnebusch AG, Castilho BA (2011) Gcn1 and actin binding to Yih1: implications for activation of the eIF2 kinase GCN2. J Biol Chem 286(12):10341–10355. doi:10.1074/jbc.M110.171587
Dlaboga D, Hajjhussein H, O'Donnell JM (2008) Chronic haloperidol and clozapine produce different patterns of effects on phosphodiesterase-1B, -4B, and -10A expression in rat striatum. Neuropharmacology 54(4):745–754. doi:10.1016/j.neuropharm.2007.12.002
Kim H, Chun Y, Che L, Kim J, Lee S, Lee S (2017) The new obesity-associated protein, neuronal growth regulator 1 (NEGR1), is implicated in Niemann-Pick disease Type C (NPC2)-mediated cholesterol trafficking. Biochem Biophys Res Commun 482(4):1367–1374. doi:10.1016/j.bbrc.2016.12.043
He Y, Hua Y, Lee JY, Liu W, Keep RF, Wang MM, Xi G (2010) Brain alpha- and beta-globin expression after intracerebral hemorrhage. Translational stroke research 1(1):48–56. doi:10.1007/s12975-009-0004-x
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. The Lancet Neurology 5(1):53–63. doi:10.1016/S1474-4422(05)70283-0
Acknowledgements
This study was supported by The Turkish Academy of Sciences (TUBA) and Necmettin Erbakan University (Scientific Research Project: 161330001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been conducted in accordance with the ethical standards and according to the Declaration of Helsinki and according to national and international guidelines and has been approved by the Ethics Committee of Istanbul Medipol University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Beker, M.C., Caglayan, B., Yalcin, E. et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol 55, 2565–2576 (2018). https://doi.org/10.1007/s12035-017-0524-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0524-4