Abstract
Ischemic stroke is caused by obstructed cerebral blood flow, which results in neurological injury and poor outcomes. Pro-inflammatory signaling from both residential and infiltrating immune cells potentiates cerebral injury and worsens patient outcomes after stroke. While the occurrence of a stroke exhibits a time-of-day-dependent pattern, it remains unclear whether disrupted circadian rhythms modulate post-stroke immunity. In this study, we hypothesized that stroke timing differentially affects immune activation in mice. Following middle cerebral artery occlusion (MCAO), circadian genes BMAL1, CLOCK, Cry1, and Cry2 elevated at ZT06, with a significant difference between ZT06 and ZT18. Conversely, expression of the negative limb circadian clock gene, Per1, decreased at ZT06 and ZT18 in stroke mice compared to sham. Paralleling these circadian gene expression changes, we observed a significant increase in TNF-α and a decrease in IL-10 expression at 48 h post-MCAO, when the procedure was performed at ZT06 (MCAO-ZT6), which corresponds to a deep sleep period, as compared to when the stroke was induced at ZT12 (MCAO-ZT12), ZT18 (MCAO-ZT18), or ZT0 (MCAO-ZT12). Similarly, increased pro-inflammatory, decreased anti-inflammatory monocytes, and increased NLRP3 were observed in blood, while changes in the expression of CD11b and Iba1 were noted within brain tissue at 48 h of MCAO-ZT06, as compared to MCAO-ZT18. Consistent with the increased immune response, infarct volume and sensorimotor deficits were greater in MCAO-ZT06 mice compared to MCAO-ZT18 mice at 48 h. Finally, we found reduced weight and length of the spleen while splenocytes showed significant time-dependent changes in Tregs, Bregs, and monocytes in MCAO-ZT06 mice. Taken together, this study demonstrates that circulating and splenic immune responses following ischemic stroke exhibit a circadian expression pattern which may contribute to time-of-day-dependent stroke outcomes.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second most common cause of disability and death worldwide [1, 2]. Ischemic stroke leads to brain injury, cell death, and debilitated functional outcomes via a multitude of pathological mechanisms [3]. Time of day correlates with stroke onset and functional outcomes [4]. In a study of 583 patients with anterior large-vessel occlusion stroke, the patients whose onset of stroke occurred at night (peaking at 11 PM) had larger ischemic core volumes compared with patients with onset during the day [5]. Further, patients with night-onset stroke suffered worsened clinical severity with heightened deterioration within the first 72 h following symptom onset [6]. Similarly, outcomes in experimental animal models are worse when the stroke occurs during sleep and inactivity [7, 8]. Thus, there is a circadian variation in ischemic stroke progression and severity.
Neuroinflammation is a major contributing factor toward poor stroke outcomes [9]. Microglia, residential innate immune cells within the CNS, initiate the response to tissue damage and mediate neuroinflammation to influence brain injury and ischemic stroke [10, 11]. The M1-like microglia subtypes are pro-inflammatory, while the alternative M2-like microglia promote neuroprotection [12, 13]. M1 microglia secrete cytokines and oxidative metabolites such as IL-1β, TNF-α, IL-6, IL-18, and nitric oxide [14], causing tissue damage that further activates microglia and worsens brain injury. Conversely, M2 microglia (anti-inflammatory) contribute to recovery after brain injury. Microglia communicate with neutrophils in an animal model of stroke, and microglial depletion increases the neutrophil population in ischemic brain tissue leading to brain injury [15, 16]. Neutrophils are the first blood-derived immune cells in response to brain injury that invade ischemic tissue, followed by monocytes. Neutrophil counts increase in the peripheral blood of stroke patients, and increased neutrophil numbers are an early indicator of stroke outcomes [17,18,19,20,21].
As ischemic stroke is known to have a circadian rhythm [5, 7, 22,23,24,25] (which is complex and may vary with individual and molecular rhythms), the cellular neuroinflammasome of the ischemic brain may have an essential interaction with the molecular components of circadian rhythm, the circadian clock. The circadian clock controls rhythms throughout the brain and body, orchestrated from the hypothalamic suprachiasmatic nucleus (SCN). Driving circadian rhythms are the circadian clock components that are transcription factors, including circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like protein 1 (BMAL1). There are also repressor circadian clock components, including period (Per) and cytochrome (Cry), which together form a transcription–translation feedback loop that is unique in its timing of expression at 24 h. The circadian clock genes oscillate and target other genes in the brain and body to control rhythm. The loss of this anticipatory mechanism or its miscommunication with its target genes can adversely affect health.
Circadian rhythms play a key reciprocal role in peripheral and circulatory inflammatory responses [26, 27]. Circadian rhythms also regulate the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) immune complex [28]. The NLRP3-inflammasome is a protein complex that mediates the activation of caspase-1, interleukin-1β (IL-1β), and interleukin-18 (IL-18), generating a feedback mechanism that perpetuates the inflammatory response [29]. IL-1β and IL-18 have been shown to play a role in various cellular processes, including cell apoptosis.
In this present study, we aimed to investigate the influence of the circadian time of stroke on inflammatory–immune response and functional outcomes. We found a strong correlation between the molecular oscillations of circadian rhythm and the immune response, functional outcomes, and stroke severity.
Material and Methods
Animals
Male, 6–7-month-old C57BL/6J (WT) mice underwent transient middle cerebral artery occlusion (tMCAO) to induce cerebral ischemia. The experimental protocol for stroke in mice was approved by the Augusta University Institutional Animal Care and Use Committee (IACUC). All the mice had free to access food and water during housing and experimentation. Mice were housed in a temperature- and humidity-controlled vivarium with alternating light-cycle environments (23.2 °C; 12-h light/dark cycle; lights on at 6 AM and off at 6 PM).
Middle Cerebral Artery Occlusion (MCAO)
Cerebral ischemia was induced by occlusion of the MCA for 60 min using 6–0 monofilament (Cat# 602112PK10Re, 602212PK10Re, 602312PK10Re; Doccol Corporation, Sharon, MA). The filament was used according to the body weight of mice as per our previous reports [7]. The MCAO surgical procedure was conducted under controlled anesthesia at a flow rate of 1.5% isoflurane. A regulated heating pad was used to keep optimal body temperature and control throughout the surgery to minimize any side effects of hypothermia. Surgery was performed over the circadian cycle, with lights on at 6 AM and off at 6 PM. Surgery time/hour is recorded in zeitgeber time (ZT). In the first set of experiments, MCAO surgery was performed at ZT0, ZT06, ZT12, and Z18 to estimate the immune response in the blood. A light cycle was not reversed to conduct this stroke and sham surgery. For the ZT06 surgery, mice were brought in the evening and surgery room light was off until the surgery started. During the surgery, only dim light was used to maintain light. Acute stroke surgery was done at ZT06 and ZT18 time points for the second set of experiments only to estimate circadian and inflammatory parameters. Relative cerebral blood flow (CBF) was monitored by laser speckle before and after MCAO to confirm the occlusion in mice. The monofilament’s withdrawal after 60 min of MCA occlusion restored blood flow. The sham-operated mice were subjected to the same anesthetic and surgical procedure except for the insertion of monofilament [7, 30, 31]. All surgery was done at actual ZT time ± 30 min. After surgery, post-operative care was given immediately, and mice were kept in a temperature-controlled recovery area.
Experiment 1
A total of n = 10–12 mice at circadian time (zeitgeber) ZT0, ZT06, ZT12, and Z18 were subjected to stroke to confirm the association between circadian and immune responses. Blood was collected 48 h after the stroke for each time point for flow cytometry assay to analyze the immune response. Flow cytometry data group (blood sample) was blinded to the person who performed assay and analysis.
Experiment 2
A total of n = 86 mice (n = 24 sham and n = 46 stroke) had stroke and sham surgery to study the immune response in the brain at ZT06 and ZT18. The mice were used for different purposes (TTC, n = 8 sham and n = 12 stroke, histology: n = 8 sham and 10 strokes, WB and qRT-PCR: n = 8 sham and n = 16 stroke). Naïve mice were used for WB, qRT-PCR, and flow cytometry (ZT18, n = 8 and ZT06, n = 8). Both experiment 1 and 2 study timelines are shown in supplementary Fig. 1. In this experiment setup, the performer was blinded to the experiment behavioral analysis, TTC assay and analysis, WB, qPCR, and flow cytometry.
Mortality
Mortality percentages were calculated to confirm the death of mice at ZT06 and ZT18 time points after acute stroke and are presented in the “Results” section.
Neurobehavioral Function
Mice were transferred to the behavioral room 1 h before testing. Behavioral assays were executed under similar conditions after 48 h of tMCAO surgery. All surfaces were cleaned with 70% ethanol between each mouse before and after use.
Bederson Test
The Bederson score scale assessed neurological deficits. A grading scale of 0–3 was used to determine global neurological deficits after ischemic stroke. Scoring criteria include forelimb flexion, resistance to lateral push, and circling behavior. Mice scored at 24 h and 48 h after the stroke.
Beam Walk Test
Motor balance and coordination tests were performed within 48 h of the stroke using the balance beam walk test reported earlier by our laboratory, with a slight modification [32]. The beam apparatus consists of 1.25-m beams (scale marked) with a flat surface of 6 mm width) resting 20 cm above the tabletop on two poles. A4 size black box is placed at the end of the beam to reach mice at finish points. Mice were pretested for three trials to acclimatize and familiarize themselves with the object and condition. A timer counts the time to cross the beam (100 cm). A soft bed/cloth is spread below the beam above the tabletop to cushion any falls. Trials in which the animal stopped or turned around were repeated. The average time of the trials was calculated in seconds.
Four-Limb Wire Hanging Test
The mesh wire hanging test assesses motor functions and muscle strength in rodents and were performed after 48 h of stroke. Mice were hanging upside down for maximum 120-s limit to hang through the mesh, and latency of fall time was noted in seconds.
Open Field Activity Test
All the testing mice were given 1 h to acclimatize to the room condition. Mice were placed in a 40 × 40 × 40 cm white box, and activity was digitally recorded for 30 min. Each mouse was tested after 48 h of sham or stroke. Boxes were cleaned between each trial. Distance traveled, mean velocity, and time spent in the center zone were determined using EthoVision XT video tracking software (Noldus Information Technology).
TTC Staining and Infarction Volume Quantification
Infarct size was measured using 2,3,5-triphenynyltetrazolium chloride (TTC) staining. At the experimental endpoint, mice were decapitated under deep isoflurane anesthesia. Brains were carefully removed, washed with PBS, and then coronally sectioned into 2-mm slices, which were stained with 2% TTC and kept at 37 °C for 5 min. The infarct area (pale white) was quantified using the ImageJ free-hand selection tool (NIH ImageJ software) and summated to calculate the final infarct volume.
Blood and Spleen Preparation
At 48 h of acute ZT0, ZT06, ZT12, and ZT18 stroke, mice were euthanized with 5% isoflurane, and blood was collected by cardiac puncture and transferred to an Eppendorf tube containing blood cell fixing agents for flow cytometry. Blood samples were stored at – 80 °C until use. For the histological and immunohistochemical assay, mice were transcardially perfused with 20 mL of ice-cold phosphate-buffered saline (PBS) and 20 mL of 4% paraformaldehyde (PFA). Brains were removed and post-fixed in 4% PFA. Brain tissue collected for Western blot assay was not perfused with PFA and cryopreserved with frozen snap tissue using liquid nitrogen and stored at – 80 °C until use. We also collected spleens for measurements (e.g., weight, length). In a separate cohort of study, spleen cells were collected by macerating the spleen in 100-µm sieves and suspended in PBS. Later, cells were fixed with cell fixative agents and kept at − 80 °C for further analysis.
Effects of Circadian Time on Stroke-Induced Monocytes, Neutrophils, IL-10, TNFα, and NLRP3 in Blood
Whole blood was collected using heparinized microtubes, as described, with slight modification [33]. Blood samples were incubated with phenotypic markers for the identification of monocytes (CD45+CD11b+CD68+Ly-6C+Ly-6G−CD206+/−) and neutrophils (CD45+CD11b+Ly-6G+Ly-6C−CD68−CD206+/−) for 20 min on ice in the dark. After washing, blood samples were fixed and permeabilized using fix/perm concentrate (eBioscience, USA) before incubation with antibodies for intracellular staining of functional markers, including TNF-α (for M1 macrophages and N1 neutrophils) and IL-10 (for M2 macrophages and N2 neutrophils) as well as NLRP3 as activation marker (all antibodies are purchased from BioLegend unless otherwise noted). Samples were then washed and run through a NovoCyte Quanteon flow cytometer. Cells were gated based on forward and side scatter properties and marker combinations to select viable cells of interest. Single stains were used to set compensation, and isotype controls were used to determine the level of non-specific binding. Analysis was performed using FlowJo (version 11.0) analytical software. Cells expressing a specific marker were reported as a percentage of the number of gated events. A population was considered positive for a particular marker if the population exceeded a 2% isotypic control threshold.
Western Blot Analysis for the Inflammatory Marker in Ischemic Brain
Western blots were performed to quantify protein expression in the ipsilateral hemisphere of ischemic mouse brains. Briefly, proteins were extracted using RIPA buffer with added protease inhibitors. Protein concentrations were determined using the Pierce BCA Protein assay kit (Thermo Scientific- Cat#23,227). An equal amount (20–30 μg) of protein per sample was separated by SDS-PAGE using Mini-PROTEIN TGX Stain free gel (Bio-Rad, USA) and then transferred onto a PVDF membrane (Bio-Rad, Hercules, CA) by the wet transfer method. Non-specific binding was blocked with 3% non-fat dry milk in TBS-T (50 mM Tris–HCl, 150 mM NaCl, 0.1% Tween 20, pH 7.4) for 1 h at room temperature. Membranes were washed with washing buffer (pH 7.6, TBS, 0.1% Tween 20) 3 times, 5 min each. Blots were incubated overnight at 4 °C with the appropriate primary antibody in TBS-T solution. Antibodies were procured from Cell Signaling (caspase-3 antibody-9662), Santa Cruz Biotechnology (IL-10 (SC 8438), IL-6 (SC28343), TNF-α (SC-130349), β-actin (SC8432), NLRP3 (LS-B4321)) companies. The blots were washed with TBS-T and incubated with appropriate HRP-conjugated secondary antibody for 1.5 h at room temperature. After washing with TBS-T, Super Signal West Pico Chemiluminescent substrate (Thermo Scientific, USA) was applied to the blot for protein expression, and images were captured in a gel documentation system (Bio-Rad ChemiDoc-MP Imaging system, USA). The relative optical density of protein bands was analyzed using NIH ImageJ software. The same membranes were used to estimate β-actin, loading control for all the proteins of interest.
Quantitative (qRT-PCR) Analysis for Circadian Gene Expression
Total RNA from brain tissues was isolated using TRIzol® reagent (Invitrogen, Grand Island, NY, USA) per the manufacturer’s protocol. The RNA’s purity and quantity were confirmed using NanoDrop-1000 (Thermo Scientific, Waltham, MA, USA). Complimentary DNA (cDNA) was prepared from RNA using a cDNA Synthesis Kit (iScript cDNA Synthesis Kit, Bio-Rad Laboratory, USA) following the manufacturer’s protocol. Mouse brain cDNA samples were amplified for the given genes by gene-specific primers (BMAL1, CLOCK, Per1, Per2, Cry1, Cry2, IL-18, NLRP3, CD11b, IBA1, 18s rRNA) and iQ SYBER Green Supermix (Bio-Rad Laboratory, USA) using MyiQ Real-time PCR system (Bio-Rad Laboratory, USA). After baseline and threshold adjustments, CT (cycle threshold) values were determined. The values were normalized to 18S rRNA expression levels as an endogenous control gene using the ∆∆CT method. Fold change (2∆∆CT-value) was used for the statistical analysis. A list of primers and sequences used for qRT-PCR is summarized in Table 1.
Cresyl Violet Stain
Cresyl Violet Staining (CV): Neuronal cell loss was assessed by using cresyl violet staining. Paraffin-embedded sections were stained using standard cresyl violet. Staining slides were mounted using mounting media (PROTOCOL™, Fisher Scientific, USA) and were scanned using HP Scanjet G4050. Neuronal loss was defined as regions with hypodense cresyl violet staining reflecting areas of dead or dying nuclei.
Statistical Analysis
All values are expressed as mean ± SEM. Interaction between groups was determined by a one-way analysis of variance (ANOVA) test followed by an appropriate post hoc test. A p-value equal to or less than 0.05 was considered statistically significant. A Pearson correlation two-tailed test was used for correlation analysis.
Results
The Inflammatory Response Exhibits a Blunted Circadian Pattern During Rest After MCA Occlusion in Mice
Effects of Circadian Time on Status of Monocytes (M1 and M2) and Neutrophil (N1 and N2) in Mouse Blood at ZT0, ZT06, ZT12, and ZT18 Stroke
Figure 1a shows the full two dimensions of forward scatter (FSC)/side scatter (SSC) of whole blood at ZT0, ZT6, ZT12, and ZT18 analyzed using live gating (G1) on whole blood cells except RBCs and platelets (based on size). Figure 1b shows the functional N2 neutrophils based on their expression of IL-10 versus the rest of the neutrophils (N1) (LY-6G+CD206+IL-10) at four ZT times. In Fig. 1g, CD68 and CD206 phenotypic antigens were used to identify the monocyte cells as CD68+CD206+ double-positive cells. Ischemic mice showed increased (p < 0.001) expression of N1 neutrophils at ZT06 in comparison to ZT18 and ZT12 of stroke as indicated by the high expression [F (3, 28) = 10.18, p < 0.0001] of IL10, CD206, and Ly-6G (Fig. 1d). ZT06 and ZT12 stroke also showed significant differences (p < 0.05). However, no differences were observed between ZT0 and ZT06 strokes. Similarly, we found a significant decrease (p < 0.001) in N2 level [F (3, 28) = 10.81, p < 0.0001] at ZT06 when compared to ZT18 and ZT12 stroke (Fig. 1e). ZT0 neutrophils were significant from ZT12 and ZT18 stroke. Contrary to that, we also confirm the time-of-day-dependent changes in the N1 and N2 expression in blood. We measured the ratio of N1 to N2 at different ZT time points of stroke and found the higher ratio at ZT06 (midnight/sleep period) compared to other ZT time points (Fig. 1f). ANOVA followed by Tukey test analysis and multiple comparison tests was used to confirm M1 and M2 status in the whole blood of stroke mice.
Circadian ZT time points affect monocytic and neutrophil polarization. Flow cytometric analysis of whole blood: the middle panel displays two dimensional dot plots based on side scatter/forward scatter and gating strategy for monocytes and neutrophils. The right panels show identification of M2-type macrophages based on two phenotypic markers of CD68 and CD206 at different circadian ZT time points, and the histogram confirms the M2 functional features by demonstrating IL-10 expression. The lower panels on the right display the quantifications and ratio of M1-type to M2-type macrophages. The left panels show identification of N2-type neutrophils, for the first time, based on two phenotypic markers of LY-6G and CD206, and the histogram confirms the N2 functional features by demonstrating IL-10 expression. The lower panels on the right display the quantifications and ration of N1-type to N2-type macrophages. Quantifications of flow analysis represent immune response and their level after stroke (n = 8–10 mice/group). A value of p < 0.05 is considered statistically significant
Blood samples collected from mice at 48 h of stroke show increased (p < 0.005) expression of M1 monocytes [F (3, 28) = 7.856, p < 0.0006] at ZT06 in comparison to ZT0, ZT18, and ZT12 of stroke as confirmed by expression of IL-10, CD206, and CD68 (Fig. 1h). There was decreased (p < 0.005) M2 expression [F (3, 28) = 7.856, p < 0.0006] at ZT06 (Fig. 1j) when compared to other ZT stroke. We also observed the M1 and M2 ratio at different ZT time points of stroke and found the higher ratio (Fig. 1k) at ZT06 (midnight/sleep period) stroke compared to other ZT time points of stroke.
Time Dimension Effects of TNF-α and IL-10 Expression in Stroke-Induced Mice at ZT0, ZT06, ZT12, and ZT18
Pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine IL-10 were estimated in blood collected at 48 h of ZT0, ZT06, ZT12, and ZT18 stroke mice (Fig. 2a). We found that there was comparatively higher (p < 0.05) TNF-α (Fig. 2d) at ZT06 stroke mice in comparison [F (3, 28) = 5.946, p < 0.005] to other ZT time points of stroke. Interestingly, ZT06 stroke mice showed comparatively (p < 0.001) low expression [F (3, 28) = 11.86, p < 0.0001] of IL-10 in comparison to ZT18 stroke mice (Fig. 2c, d). Other time points did not show significant differences in IL-10 from the ZT06 stroke. The results suggest that the cytokine expression in stroke mice depends on the circadian time and shows the time dimensional effects. ANOVA followed by Tukey and multiple comparison tests was used for TNF-α and IL-10 analysis in whole blood samples.
Impact of circadian ZT time points on cytokine production in peripheral blood. Two cytokines of TNFα (pro-inflammatory) and IL-10 (anti-inflammatory) were measured to represent the two spectrums of Immunologic function. The histograms on the right demonstrate the quantified measures of cytokine production (p-value < 0.0029) and anti-inflammatory IL-10 (p-value < 0.0001) (n = 8–10 mice/group)
Worsened Pathological and Behavioral Stroke Outcomes in Mice Undergoing MCAO at Night
Effects of Circadian Time on Neurological Deficit Score, Hanging Wire Test, Motor Function, Mortality, and Infarction Volume
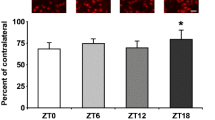
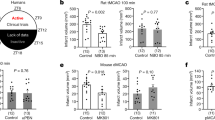
Neurological deficit was scored and no significant difference was found in NDS between ZT06 and ZT18 stroke groups though a significant difference was observed at ZT06 (p < 0.001) and ZT18 (p < 0.001) when compared to sham group mice after 24 h and 48 h (Fig. 3b). Motor function: The motor function was observed by beam walk test at 48 h of stroke and sham group. ANOVA test showed a significant [F (3, 39) = 8.476; p < 0.0005] difference at circadian time points for motor function in mice (Fig. 3c). There was a significant (p < 0.001) difference in latency time to reach the dark box at ZT06, though no significance was observed at ZT18 compared to the sham. We found that stroke at ZT06 takes a significant (p < 0.05) more extended time to reach the dark box than ZT18. Also, there was a higher time to reach in both stroke groups when compared to sham. Hanging wire test: Hanging wire test was performed to test the grip and muscular strength of the mice after stroke. ANOVA test showed a significant [F (3, 16) = 21.49; p < 0.0001] difference between circadian time points. Stroke mice showed reduced latency of fall from hanging wire at both ZT18 (p < 0.001) and ZT06 (p < 0.0001) in comparison to sham. However, no significant difference was observed between ZT06 and ZT18 stroke mice (Fig. 3d). Mortality: We used n = 86 mice to conduct the study for experiment 2. We found 9% mortality at ZT18 and 24% mortality in ZT06 stroke mice. However, no mortality was observed in the sham group (Fig. 3e). Stroke volume: Infarction volume was calculated by using NIH ImageJ software. There was a significant difference in stroke volume compared to sham at ZT18 and ZT06. Intrestingly, there was a significant increase in stroke volume in ZT06 stroke mice compared to (p < 0.005) to ZT18 stroke mice (Fig. 3f, g). The results of differential stroke volume at different ZT of stroke indicate that time dimension on stroke has significant effects on stroke outcomes.
Neurobehavioral function and cerebral infarction. Bederson test in stroke mice showed no difference in NDS between ZT06 and ZT18 time points while there was a significant difference at ZT18 and ZT06 in comparison to sham (a). In the beam walk test, mice showed a significant change to cross bean at ZT06 in comparison to sham but non-significant at ZT18 (c). Additionally, no differences were observed in the hanging wire test in between ZT06 and ZT18 at 48 h of stroke (d) (n = 10–11 mice/group). Mortality showed in percentage at ZT18 and ZT06. The infarct volume was observed by TTC staining of brain slices (f). Quantitative evaluation of percent infarction volume in different cohorts of mice was observed at 48 h after MCAO (g) (n = 5–6 mice/group)
Effects of Circadian on Open Field Activity in ZT06 and ZT18 Stroke Mice
Open field activity test was used to assess mice’s general locomotor activity levels after stroke and look for circadian effects. A heat plot shows the activity in an open field square box (Fig. 4a). ANOVA test showed significant differences among the ZT06 and ZT18 circadian stroke for distance traveled [F (3, 16) = 12.71; p < 0.0005], for frequency in the center zone [F (3, 16) = 9.225; p < 0.0005], and for velocity [F (3, 16) = 12.87; p < 0.0005]. We found that there was a significant difference in distance traveled at ZT18 (p < 0.001) and ZT06 (p < 0.005) in comparison to a respective sham group (Fig. 4b). That indicates circadian time of the stroke affects the mice activity. Overall, stroke mice also tended to spend less time in the central zone compared to sham, indicating reduced exploratory behavior in stroke mice. When compared to sham, stroke mice spent significantly low time in the central zone at ZT18 (p < 0.005) and ZT06 (p < 0.05) zone (Fig. 4c). Additionally, there was reduced velocity in ZT18 (p < 0.005) and ZT06 (p < 0.005) stroke mice in comparison to sham (Fig. 4d). However, no significant difference was observed in ZT18 vs. ZT06 for distance traveled, frequency of spent time in the central zone, and velocity of mice.
Rhythmic Immune Cell and Circadian Signaling in the Spleen, Blood, and Brains of MCAO Mice
Circadian Effects on Spleen Weight, Length, and Immune Response
Because the spleen is a key source of a heterogeneous population of inflammatory cells and has also been shown to be important in stroke. We collected spleen from ZT06 and ZT18 stroke mice after 48 h of stroke. We also collected spleen from naïve mice at ZT18 and ZT06. Interestingly, there was significantly reduced weight (p < 0.005) of spleen compared to naïve mice in ZT06 stroke mice though no significance was observed at ZT18. Additionally, we also found reduced spleen length (p < 0.05) at ZT18 and at ZT06 (p < 0.05) stroke compared to naïve mice. Interestingly, the spleen length was also significantly different between ZT18 and ZT06 strokes mice (Fig. 5a–c). Further, we collected spleen from sham and stroke mice in separate experiments and analyzed the M1, M1, myeloid cells, T cells, and B cells. The macrophage’s M1 level was elevated [F = (3, 16) 20.91, p < 0.0001] in stroke mice splenocytes at ZT06 (p < 0.01) and ZT18 (p < 0.0001) compared to sham; however, M2 level in splenocytes was decreased [F = (3, 16) 20.91, p < 0.0001] at ZT06 (p < 0.01) and ZT18 stroke (p < 0.0001). There was a significant decrease [F = (3, 16) 26.36, p < 0.0001] in T cells at ZT06 (p < 0.01) and ZT18 stroke (p < 0.0001). Interestingly, B cells were decreased [F = (3, 16) 8.58, p < 0.001] only at ZT18 (p < 0.005) (Fig. 5d–h).
Stroke affects the splenic morphology and immunologic profile at different circadian ZT times. a Whole spleen was harvested and a size/morphologic analytic comparison was performed as shown at different ZT circadian times with and without stroke. b, c Spleens showed different weights and size at different ZT times with/without stroke, as demonstrated by quantifying graphs. d Immunologic analysis of the spleen was performed by using a flow cytometric assay. First, the heterogeneous milieu of splenocytes was prepared in single cell suspension followed by analytical flow cytometry assay. Spleen resident monocytes (macrophages), as well as lymphoid (B and T cells), were measured and analyzed at several ZT time points with/without stroke. Phenotypic identifications of splenic macrophages and lymphocytes were followed by using IL-10 expression to analyze and measure the regulatory subpopulation of those cells as shown in histograms at the far right (M2 macrophages, T, and Bregs). e–h Quantifications of flow analysis
Circadian Effects of Stroke on M1 and M2 Monocytes in Blood and Microglial Expression in the Brain
Macrophage responses are important for functional recovery after stroke. Therefore, a whole blood flowcytometric assay was performed for estimation of monocytes M1 and M2 (Fig. 6a). We found that stroke mice showed increased expression of M1 (Fig. 6d) monocytes [F (3, 28) = 17.60, p < 0.0001] and decreased expression of M2 (Fig. 6e) monocytes [F (3, 28) = 17.60, p < 0.0001] at ZT06 and ZT18 in comparison to sham. ANOVA followed by Tukey test analysis and multiple comparison tests was used to confirm M1 and M2 status in stroke mouse blood. Macrophage alteration leads to stimulation of the mcroglial function. Thus, to confirm microglial expression in stroke mouse brains at ZT06 and ZT18, we have estimated the mRNA expression of CD11b and IBA1. ANOVA test was performed for both CD11b and IBA1 and showed a significant difference among groups of CD11b [F = 8.54 (3, 20), p < 0.0001] and IBA1 [F = 12.2 (3, 20), p < 0.0001]. As expected, we found increased expression of CD11b at ZT06 (p < 0.05) and ZT18 (p < 0.01) compared to sham (Fig. 6e). Similarly, increased IBA1 expression at ZT06 (p < 0.005) and ZT18 (p < 0.01) as compared to sham (Fig. 6f). We found a significant difference between ZT06 and ZT18 in mRNA expression of CD11b and IBA1.
Stroke affects the peripheral monocytic polarization in a circadian ZT time-dependent fashion in blood and their comparison to brain tissue microglial mRNA. Whole blood (WB) analysis was analyzed at different circadian ZT time points with/without stroke using a flow cytometry assay. a Full two dimension of forward scatter (FSC)/side scatter (SSC) of WB at ZT0, ZT6, ZT12, and ZT18, sham versus stroke, analyzed using live gating (G1) on WB cells except for RBCs and platelets (based on size). b Myeloid cells were identified using CD11b phenotypic antigen followed by further identification of myeloid cells (c) using LY-6G (for neutrophils, CD11b+LY-6G+) and LY-6C (for macrophages, CD11b+LY-6C+LY-6G−). d, e Quantifications of flow analysis. A p-value < 0.05 is considered significant n = 8–10 mice/group; ANOVA followed by Tukey multiple comparison tests was performed for data analysis. f, g The brain tissue microglia mRNA expression to compare with blood microglial expression. An expression of CD11b and IBA1 expression was higher at ZT06 stroke in comparison to sham. However, no significant differences were observed at ZT18 stroke in comparison to sham. Interestingly, there was a time-dependent significant difference in CD11b and IBA1 expression in stroke mice
Time Dimension of Stroke Affects TNFα, IL-6, and IL-10 in Stroke Mice
Later, we estimated the cytokines to find out how circadian time of stroke affects its level. We measured the inflammatory markers IL-6, IL-10, and TNF-α in the ipsilateral cortex of ischemic mouse brain at ZT18 and ZT06 (Fig. 7a). There were significant differences in TNF-α [F (3, 16) = 89.36, p < 0.0001], IL-6 [F (3, 16) = 5.96, p < 0.006], IL-1β [F (3, 16) = 9.474, p < 0.0008] group, IL-10 [F (3, 16) = 79.16, p < 0.0001]. We found that there was increased expression of TNF-α (p < 0.001) (Fig. 7b), IL-6 (p < 0.005) (Fig. 7c), and IL-10 (p < 0.0001) (Fig. 7d) at ZT06 in stroke mouse brain in comparison to sham. There was also a significant difference between IL10 (p < 0.05) at ZT18. However, no difference was observed in TNF-α, IL-6, and IL-1β. While comparing ZT18 vs. ZT06 for inflammatory marker protein expression, we found a significant difference in TNF-α (p < 0.001) and IL-10 (p < 0.0001). We also measured the TNF-α (Fig. 7e) and IL-10 (Fig. 7f) in blood monocytes. We found that there was a significant increase [F (3, 28) = 16.19, p < 0.0001] in TNF-α in M1 monocytes at ZT18 (p < 0.01) and ZT06 (p < 0.0001) in comparison to sham (Fig. 7g). Interestingly, TNF-α levels were also significant (p < 0.05) in between ZT18 and ZT06 stroke mice. A whole blood assay was performed to measure the IL-10 in M2 monocytes. We found that IL-10 level [F (3, 28) = 8.398, p = 0.0004] was decreased at ZT18 (p < 0.05) and ZT06 (p < 0.05) stroke mice in comparison to sham (Fig. 7h). Interestingly, there was also a significant difference in IL-10 (p < 0.05) from ZT18 to ZT06. ANOVA followed by the Tukey test was used to analyze data.
Stroke affects cytokine production in peripheral blood in a circadian ZT time-dependent fashion and their comparison with brain tissue cytokine expression. a–d WB assay was performed to estimate cytokine levels at ZT18 and ZT06 in ischemic mouse brains, and there was significantly higher expression of TNFα, IL-6, IL-10, and IL-1β (a–e) at ZT06 in comparison to ZT18 (n = 4 sham-6 stroke mice/group). Additionally, in whole blood assay, TNF-α was increased, and IL-10 levels were decreased at ZT06 (e, f). A p-value of < 0.05 is considered statistically significant. f, g Whole blood was analyzed using a flow cytometry assay. As displayed by histographs, two cytokines of TNFα (pro-inflammatory) and IL-10 (anti-inflammatory) were measured to represent the two spectrums of immunologic function. g, h Quantified measures of cytokine production (ANOVA: p-value < 0.0029) and anti-inflammatory IL-10 (ANOVA: p-value < 0.0001) cytokines as the functional manifestation of the immune system (n = 8–10 mice/group)
Time Dimension of Stroke Affects NLRP3 Protein and mRNA Expression in the Brain and Blood (Neutrophils and Monocytes) in Mice
We next investigated the NLRP3 inflammasome within neutrophils and monocytes at ZT06 and ZT18. In the whole blood flow cytometric assay, we found significant differences in neutrophilic (p < 0.001) (Fig. 8a) and monocytic (p < 0.0001) (Fig. 8c) NLRP3 expression at ZT06 stroke in comparison to sham. No significant difference was found at ZT18 stroke in comparison to sham. Interestingly, both neutrophilic (p < 0.005) and monocytic NLRP3 (p < 0.0001) were significantly different between ZT06 and ZT18. Additionally, we found increased NLRP3 mRNA expression [F (3, 19) = 3.39, p < 0.039] (Fig. 8e) in the brain of stroke mice at ZT06 in comparison to sham, though no significant differences were observed at ZT18. Similarly, a significant difference [F (3, 20) = 4.99, p < 0.009] in NLRP3 protein expression was observed at ZT06 and ZT18 stroke, in comparison to sham (Fig. 8f, g).
Stroke affects neutrophilic and monocytic NLRP3 in a circadian ZT time-dependent fashion. Whole blood was analyzed using a flow cytometry assay to measure NLRP3 in neutrophils (a) as well as macrophages (b) at different ZT circadian times. c, d Quantified measures of NLRP3 expression in neutrophils (c) and macrophages (d). qRT-PCR assay of NLRP3 was performed to see changes in mRNA expression (e), and we found a significantly increased (p < 0.05) mRNA at ZT06 time points compared to sham. However, we have found an increase in NLRP3 at ZT18 but it was not significant. Additionally, we also performed WB for NLRP3 (f, g), and interestingly, there was a significant increase in NLRP3 at ZT06. On the other hand, we also found a significant (p < 0.05) decrease in Rev-erbα mRNA level at ZT06 and ZT18 time points (h)
Circadian Gene Expression at ZT06 and ZT18 Stroke Mouse Brain
The circadian gene BMAL1 mRNA expression was significantly increased (p < 0.005) in mouse brains at ZT06 in comparison to sham. However, no significance difference was observed at ZT18 (Fig. 9a). A significant increase (p < 0.05) in BMAL1 was observed at ZT06 in comparison to ZT18. ANOVA test for the CLOCK gene showed significant [F (3, 20) = 11.92, p < 0.0001] changes between groups which were significantly increased (p < 0.05) at ZT06, and there was an increase but not significant (p < 0.0899) in clock gene at ZT18. Interestingly, there was also a significant increase in CLOCK mRNA (p < 0.01) at ZT06, in comparison to ZT18 (Fig. 9b). CLOCK (p < 0.05), Per1 (Fig. 9c), Per2 (p < 0.05) (Fig. 9d), Cry1 (p < 0.01) (Fig. 9e), and Cry2 (p < 0.05) (Fig. 9f) were highly expressed at ZT06 in comparison to the sham. However, no significant difference was observed in BMAL1, Cry1, and CLOCK at ZT18 compared to sham, though increased expression can be seen but not significant. There was a significant decrease in Per1 gene expression at ZT18 and ZT06.
Circadian gene expression in the brain. Brain samples were run to quantify the mRNA expression of various circadian genes. We found that BMAL1 was significantly (p < 001) increased at ZT06 compared to sham (a). There was also a significant difference between ZT06 and ZT18, though no significant difference was observed sham of ZT06 and ZT18 (a). Similarly, we observed increased (p < 0.001) expression of CLOCK (b) at ZT06 compared to sham but no significant difference at ZT18. However, a significant (p < 0.0001) difference between ZT06 and ZT18 was observed. Per1 mRNA expression decreased at ZT18 and ZT06 compared to sham (c), though no difference was observed between sham. We also measured the Per2 mRNA, but differences were non-significant among group and time points. Similarly, we also measured the Cry1 and Cry2 mRNA levels (f), and we found that at ZT06, Cry1 (p < 0.05) and Cry2 (p < 0.005) were significantly higher in comparison to sham (e). Interestingly, their level also showed significant differences in Cry1 (p < 0.05) and Cry2 (p < 0.005) between ZT18 and ZT06. Cry1 and Cry2 expressions were not significant at ZT18 compared to sham. ANOVA followed by Tukey multiple comparison test was used to analyze the circadian genes in ipsilateral ischemic mouse brains
Inflammatory and Circadian Marker Status at ZT18 and ZT06 in Naïve Mouse Brain at ZT06 and ZT18
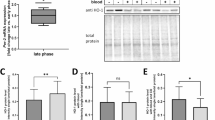
We next assessed the circadian profile of the clock and inflammasome components by using naïve mice and isolating cortex hemispheres at two time points that were 12 h apart: ZT18 (awake) and ZT06 (sleep). RNA expression by qRT-PCR showed that there was a significant variation in the expression of TNF-α (p < 0.005) (Fig. 10b), NLRP3 (p < 0.05) (Fig. 10d), and Rev-erbα (p < 0.0005) (Fig. 10e) at ZT18 versus ZT06. In contrast, there was no significant difference in mRNA expression of CD11b, IL-10, and BMAL1 (Fig. 10a, c, f) expression between the time points. Western blot analysis of proteins also showed evidence of a significant difference in expression in NLRP3 (p < 0.05) (Fig. 10 h) and Rev-erbα (p < 0.001) (Fig. 10i) and protein expression in the sleep period (ZT06) in comparison to awake period, while no significant changes were observed in IL-10 expression.
Inflammatory and circadian status in naïve mice. WB and qRT-PCR assay was used to confirm the key inflammatory markers and circadian genes in naïve mice. There was higher mRNA expression of TNF-α (p < 0.005) (b), NLRP3 (p < 0.05) (d), and Rev-erbα (p < 0.05) expression (e) in nighttime (ZT06). BMAL1 (f) and IL-10 (c) expressions showed no significant difference. Similarly, the WB assay showed higher protein expression of TNF-α (p < 0.005) (j), NLRP3 (p < 0.05) (h), and Rev-erbα (p < 0.05) (i) at ZT06 (nighttime). Again, IL-10 protein (k) expression was unchanged when compared between ZT18 and ZT06. A T-test was used to analyze mouse brains’ circadian genes and inflammatory markers
Correlation Analysis of NLRP3, Rev-erbα, and Monocyte for Circadian Time ZT18 and ZT06
We tested the association of major factors with circadian and inflammation. We tested the correlation between blood monocytes M1 and monocytic NLRP3. We found a significant (p < 0.0445, R2 = 0.1870) correlation in between monocyte M1 and monocytic NLRP3 (Fig. 2a). Interestingly, there was also significant (p < 0.0491, R2 = 0.2660) correlation in between circadian gene Rev-erbα and inflammasome NLRP3 (Fig. 2b).
Time Dimension Effects on Neuronal Cells in Stroke Mouse Brain
To measure the neuronal cell viability, we stain the brain slices with cresyl violet stain. We observed that there was a significant number of dying cell in the cortical region at ZT18 (p < 0.05) and ZT06 (p < 0.005) vs sham mouse brain (Supplementary Fig. 3).
Discussion
In the present study, we induced MCAO in mice at different times of day such as ZT06 (inactive period) and ZT18 (active period) and found differential effects on outcomes such as infarct size, neurobehavioral outcomes, circadian remodeling, and immune response. The brains were harvested at 48 h of stroke. We found larger infarct sizes at ZT6 compared to ZT18. Mortality also trended to be higher when MCAO was induced at ZT6. With the exception of the beam walk which showed worse function at ZT6, neurobehavioral outcomes were not different between these time points.
As reported by [34], monocyte numbers increase in patients with stroke or associated severe stroke. We found increased level of M1 monocytes and decreased level of M2 at ZT06 stroke compared to other time points. M1 to M2 ratio was higher at ZT06 stroke and lower at ZT18 stroke. Cytokines contribute to the progression of ischemic brain injury [35]. There was significant increase in TNF-α level at ZT06 and significant decrease in IL-10 number in monocytes. Neutrophils are the first immune cells in the blood to immediately respond after ischemic stroke brain injury [36, 37]. We found that N1 were higher at ZT06 stroke and N2 were lower at ZT06 stroke in comparison to other ZT times of stroke. And the ratio of N1 to N2 was also higher at ZT06 and lower at ZT18. These immune responses at all four ZT stroke mice led us to conclude that immune responses are higher at ZT6, the inactive, sleep period for mice, than stroke at ZT18, the active periods. Circadian differences in immune responses at ZT06 (sleep/rest period) and ZT18 (awake/active period) mirrored differences in neurological outcomes.
In the ischemic brain, microglia release inflammatory cytokines to orchestrate the peripheral immune response, which is characterized by the infiltration of macrophages, lymphocytes, neutrophils, and dendritic cells to the ischemic parenchyma (Gelderloos 2009) [38,39,40]. Microglia and infiltrating monocyte-derived macrophages (MDMs) are activated after stroke [14, 41, 42] and secrete predominantly toxic factors, such as pro-inflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), in the very early stage of cerebral stroke suggesting M1-like phenotype activation [43]. In addition, IL-6 is associated with poor functional outcomes after cerebral ischemia [44]. In the second set of experiments (ZT06 and ZT18 stroke), increased M1 macrophages and increased TNF-α were observed at ZT06, whereas M2 macrophages and IL-10 were reduced, as compared to ZT18. Similarly, the myeloid markers, CD11b and IBA1, were elevated at ZT06, as compared to sham. Consistent with these findings, elevated TNF-α, IL-1β, and IL-6 and reduced IL-10 expressions were observed in the brain and blood at ZT06. As immune responses correlated with stroke timing and larger infarct volumes, circadian changes in pro-inflammatory macrophages/microglia may contribute to the worse tissue injury of stroke at ZT6. Further, the relationship and any potential association between macrophage polarization and circadian rhythm are complex as well as bidirectional [45]. Phenotypic and functional alteration of macrophages during polarization is the result of highly regulated and complicated mechanisms with local and systemic consequences. Such shift in the macrophages are initiated and developed through a series of cellular and molecular changes and could be directly controlled and affected by circadian clock totally or for the most part. Therefore, circadian rhythm could affect the onset and progression of inflammatory responses, tissue remodeling, and homeostasis, which warrants further investigations.
Circadian rhythm dysfunction correlates with inflammation in the periphery, but the role of the core clock in neuroinflammation remains poorly understood. At ZT06 stroke, we found the increased expression of circadian genes such as BMAL1, CLOCK, and Cry1, Cry2, and these genes also showed time-dependent differences in expression. Since microglial and macrophages are involved in neuroinflammation during stroke, we studied a circadian gene, Rev-erbα, a nuclear receptor, and circadian clock component, which mediates microglial/macrophage activation and neuroinflammation [46,47,48,49]. Rev-erbα deletion caused spontaneous microglial activation in the hippocampus and increased expression of pro-inflammatory transcripts, as well as secondary astrogliosis [47]. We found decreased Rev-erbα mRNA expression in mouse brains at ZT06 and ZT18 after stroke, consistent with increased neuroinflammation. The NLRP3 inflammasome, a multiprotein complex that regulates innate immunity, was elevated in monocytes and neutrophils and in brain tissue at ZT06 after stroke. IL-18, a product of the NLRP3 inflammation that is associated with stroke severity, was elevated within the ischemic mouse brain at ZT06 but not ZT18 (Supplementary data 2). Consistent with this inflammatory response and circadian remodeling, infarct volume and sensorimotor deficits were greater in the sleep period of stroke. Neuronal cell death was also higher at ZT06, as evidenced by cresyl violet stain (Supplementary data 2). Thus, NLRP3, downstream from Rev-erbα, may be critical for circadian gene remodeling and neuroinflammation after stroke, potentially explaining the more severe short neurological outcome and larger infarct volume in mouse sleep period stroke (period of inactivity).
The spleen plays an important role in innate and adaptive immunity and the spleen is affected during stroke [50,51,52]. Therefore, here, we studied spleen size and splenocytes to correlate the brain and blood immune system to spleen immune function during stroke. We found significant changes in M1, M2, T cells (Tregs), and B cells (Bregs) in a time-dependent manner in stroke splenocytes. There were increased M1 and T cells and reduced M2 at ZT18 and ZT06, whereas B cells were only reduced at ZT18. Spleen weight decreased at ZT06 stroke compared to naïve mice though no changes were found at ZT18. Spleen length was lower at ZT18 and ZT06 stroke compared to naïve mice and the lengths were also significantly different between ZT18 and ZT06 strokes.
We found nearly 9% mortality at ZT18 stroke while 24% mortality was observed at ZT06 stroke, paralleling the circadian differences in infarct area. In a combined analysis of 583 patients who underwent computer tomography, perfusion showed that infarct cores were larger at night, with peak core infarct volume associated with an 11 pm onset, and infarct growth was faster [5]. In addition, the administration of a neuroprotective drug was effective in rodents during ZT03–09, the sleep time for rodents, but was ineffective if administered at ZT15–21, during the active period in mice [25]. Given that most laboratory studies occur during the rodent sleep period, while most human strokes occur during the awake/active period, circadian differences in the occurrence and treatment of stroke may explain the lack of translation of rodent preclinical work to the clinical arena.
In conclusion, our study suggests that infarct size, inflammation, neuronal injury, and short-term outcomes differ based on whether the stroke occurs during the sleep or awake period. Our study also suggests inflammation may aggravate stroke pathology downstream from circadian gene remodeling. The timing of stroke treatments may impact outcomes, and our findings require independent replication. Nonetheless, our results may help to explain the inconsistent success of acute cerebroprotective drugs after experimental and clinical stroke.
Limitations of the Study
Limitations of our study include the use of only young male mice and the measurement of only short-term outcomes (48 h). Future studies will seek to study the effects of long-term outcomes and if sex and age condition the circadian response in stroke. Also, while we investigated the effect of the time of day under normal conditions, we did not examine conditions that might mimic sleep disturbance, such as light cycle reversal.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMAL1:
-
Brain and muscle ARNT-Like 1
- Cry:
-
Cryptochrome
- CNS:
-
Central nervous system
- CLOCK:
-
Clock circadian regulator
- IL-1β, IL-10, IL-18, and IL-6:
-
Interleukin 1β, 10, 18, and 6
- M1 and M2:
-
Macrophages
- N1 and N2:
-
Neutrophil
- NLRP3:
-
NOD-like receptor proteins
- Rev-erbα:
-
Reverse erythroblastosis virus α
- TNF-α:
-
Tumor necrosis factor α
- ZT:
-
Zeitgeber time
References
Collaborators GBDS. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–58.
Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
Mao R, Zong N, Hu Y, Chen Y, Xu Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull. 2022;38(10):1229–47.
Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–6.
Reidler P, Brehm A, Sporns PB, Burbano VG, Stueckelschweiger L, Broocks G, Liebig T, Psychogios MN, Ricke J, Dimitriadis K, et al. Circadian rhythm of ischaemic core progression in human stroke. J Neurol Neurosur. 2023;94(1):70–3.
Ryu WS, Hong KS, Jeong SW, Park JE, Kim BJ, Kim JT, Lee KB, Park TH, Park SS, Park JM, et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. Plos Med. 2022;19(2):e1003910.
Kamat PK, Khan MB, Wood K, Siddiqui S, Rudic DR, Dhandapani K, Waller J, Hess DC. Preclinical evaluation of circadian rhythm in ischemic stroke outcomes. Cond Med. 2021;4(6):280–4.
Esposito E, Li W, Mandeville TE, Park JH, Sencan I, Guo S, Shi J, Lan J, Lee J, Hayakawa K, et al. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582(7812):395–8.
Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–98.
Lian L, Zhang Y, Liu L, Yang L, Cai Y, Zhang J, Xu S. Neuroinflammation in ischemic stroke: focus on microRNA-mediated polarization of microglia. Front Mol Neurosci. 2020;13:612439.
Planas AM. Role of immune cells migrating to the ischemic brain. Stroke. 2018;49(9):2261–7.
Pedragosa J, Salas-Perdomo A, Gallizioli M, Cugota R, Miro-Mur F, Brianso F, Justicia C, Perez-Asensio F, Marquez-Kisinousky L, Urra X, et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol Commun. 2018;6(1):76.
Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17(1):2–8.
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70.
Otxoa-de-Amezaga A, Miro-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruiz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019;137(2):321–41.
Perez-de-Puig I, Miro-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129(2):239–57.
Datsi A, Piotrowski L, Markou M, Koster T, Kohtz I, Lang K, Plottner S, Kafferlein HU, Pleger B, Martinez R, et al. Stroke-derived neutrophils demonstrate higher formation potential and impaired resolution of CD66b + driven neutrophil extracellular traps. BMC Neurol. 2022;22(1):186.
Jickling GC, Dziedzic T. Neutrophil count is related to stroke outcome following endovascular therapy. Neurology. 2019;93(5):194–5.
Jickling GC, Russo TL. Predicting stroke outcome: role of a biomarker panel. Neurology. 2019;92(4):157–8.
Weisenburger-Lile D, Dong Y, Yger M, Weisenburger G, Polara GF, Chaigneau T, Ochoa RZ, Marro B, Lapergue B, Alamowitch S, et al. Harmful neutrophil subsets in patients with ischemic stroke: association with disease severity. Neurol Neuroimmunol Neuroinflamm. 2019;6(4): e571.
Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, Hu X, Colello J, Zheng SG, Lu Z. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. 2020;11(1):108–21.
Fodor DM, Marta MM, Perju-Dumbrava L. Implications of circadian rhythm in stroke occurrence: certainties and possibilities. Brain Sci 2021;11(7):865.
Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke - a hospital-based study. Stroke. 1998;29(9):1873–5.
Lo EH, Albers GW, Dichgans M, Donnan G, Esposito E, Foster R, Howells DW, Huang YG, Ji XM, Klerman EB, et al. Circadian biology and stroke. Stroke. 2021;52(6):2180–90.
Esposito E, Li WL, Mandeville ET, Park JH, Sencan I, Guo SZ, Shi JF, Lan J, Lee J, Hayakawa K, et al. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582(7812):395.
Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM, Angiari S, Ryan DG, Corcoran SE, et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A. 2018;115(36):E8460–8.
Timmons GA, Carroll RG, O’Siorain JR, Cervantes-Silva MP, Fagan LE, Cox SL, Palsson-McDermott E, Finlay DK, Vincent EE, Jones N, et al. The circadian clock protein BMAL1 acts as a metabolic sensor in macrophages to control the production of pro IL-1beta. Front Immunol. 2021;12:700431.
Pourcet B, Duez H. Circadian control of inflammasome pathways: implications for circadian medicine. Front Immunol. 2020;11:1630.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–86.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral-artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91.
Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation in blood-pressure. Lancet. 1978;1(8075):1210–1.
Khan MB, Hafez S, Hoda MN, Baban B, Wagner J, Awad ME, Sangabathula H, Haigh S, Elsalanty M, Waller JL, et al. Chronic remote ischemic conditioning is cerebroprotective and induces vascular remodeling in a VCID model. Transl Stroke Res. 2018;9(1):51–63.
Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type IIFN signaling following B7 ligation. Int Immunol. 2005;17(7):909–19.
Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40(4):1262–8.
Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31(10):2325–9.
Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, Ali LK, Kim D, Villablanca JP, Salamon N, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39(2):355–60.
Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46(10):2916–25.
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179(2):292–311.
Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis. 2015;30(2):381–92.
Weinstein JR, Koerner IP, Moller T. Microglia in ischemic brain injury. Future Neurol. 2010;5(2):227–46.
Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci. 2017;18(10):2135.
Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247–72.
Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81.
Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke. 2002;33(4):1041–7.
Lellupitiyage Don SS, Mas-Rosario JA, Lin HH, Nguyen EM, Taylor SR, Farkas ME. Macrophage circadian rhythms are differentially affected based on stimuli. Integr Biol (Camb). 2022;14(3):62–75.
Griffin CD, Foster A, Soanes D, Plessis A, Hanley ME, Talbot NJ, Littlejohn G. The role of circadian entrainment in the outcome of rice blast infection. Mol Plant Microbe In. 2019;32(10):45–45.
Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeno MR, Nadarajah CJ, Ezerskiy LA, et al. Circadian clock protein Rev-erbalpha regulates neuroinflammation. Proc Natl Acad Sci U S A. 2019;116(11):5102–7.
Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeno MR, Nadarajah CJ, Ezerskiy LA, et al. Circadian clock protein Rev-erba regulates neuroinflammation. P Natl Acad Sci USA. 2019;116(11):5102–7.
Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485(7396):123–7.
Liu Q, Sorooshyari SK. Quantitative and correlational analysis of brain and spleen immune cellular responses following cerebral ischemia. Front Immunol. 2021;12:617032.
Pennypacker KR, Offner H. The role of the spleen in ischemic stroke. J Cereb Blood Flow Metab. 2015;35(2):186–7.
Seifert HA, Offner H. The splenic response to stroke: from rodents to stroke subjects. J Neuroinflammation. 2018;15(1):195.
Funding
The authors greatly acknowledge the source of funding from the National Institutes of Health (NIH) (1U01NS130590-01, R01RF1NS122863, and R01 NS112511) to David C. Hess to support this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: DCH (Clinical neuroscientist), PKK (Neuroscientist), MBK (neuroscientist) KD (Neuroscientist), RD (Circadian expert) and BB and AA (Immunology expert) provided concepts for this project.
Funding acquisition: DCH acquired the funding to support the study.
Investigation: DCH, PKK and MBK.
Flow cytometry assay and analysis: ESLS and SEN.
Histology analysis: DW.
Methodology: PKK and MBK.
Project administration: DCH.
Supervision: DCH, KD, AA and BB supervised this study.
Manuscript: Writing review and editing by PKK, MBK, SS, AA, RD, KD, BB and DCH.
Corresponding author
Ethics declarations
Ethical Approval
The experimental protocol for stroke in mice was approved by the Augusta University, Augusta Institutional Animal Care and Use Committee (IACUC).
Competing Interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamat, P.K., Khan, M.B., Siddiqui, S. et al. Time Dimension Influences Severity of Stroke and Heightened Immune Response in Mice. Transl. Stroke Res. (2023). https://doi.org/10.1007/s12975-023-01226-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-023-01226-5