Abstract
Dendritic spines, a special kind of structure in nerve cells, play a key role in performing cellular function. Structural abnormalities of the dendritic spine may contribute to synaptic dysfunction and have been implicated in memory formation. However, the molecular mechanisms that trigger dendritic spine loss remain unclear. Here, we show that the absence of dendritic cell factor 1 (Dcf1) appeared dendritic spines dysplasia, which in turn leads to the damage of learning and memory; in contrast, enhancing Dcf1 expression rescues dendritic spines morphology and function, indicating a pivotal role of Dcf1 in cellular function. Electrophysiological test indicates that there is a significant reduction in the frequency of miniature excitatory postsynaptic currents in Dcf1−/− knockout (KO) mice. Subsequent to optogenetic ignition, we observed a weaker neuronal activation in Dcf1 KO mice, explaining the neural circuit cause. On molecular mechanism, we demonstrated an unprecedented discovery that Dcf1 triggers the dendritic spine and synaptic function through the recruitment of Lcn2 and activation of PSD95-NMDAR signaling. Removing this brake leads to memory damage. Our results highlight an unexpected regulatory mechanism of dendritic spine development and formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendritic spines serve as storage sites for synaptic strength, help to transmit electrical signals to the neuronal cell body, and are shown to be involved in memory formation [1], [2]. As postsynaptic compartments of excitatory synapses, dendritic spines undergo dynamic changes during development, including rapid spinogenesis in early postnatal life and significant pruning during adolescence [3]. The dendritic spines on pyramidal cells represent the main postsynaptic elements of cortical excitatory synapses [4]. Several genes play pivotal roles in dendritic spine development and formation, and their dysfunction may be involved in the development of mental and behavioral disorders. For instance, Sema3F, a secreted semaphorin, promotes the loss of dendritic spines and excitatory synapses in dissociated neurons through a form of negative regulation [5]. The dysregulation of GSK-3β activity has dramatic consequences on dendritic spine morphology [6], and GRK5, a G protein-coupled receptor kinase, is critically involved in neurite outgrowth, dendrite branching, and spine morphogenesis [7]. Dopamine D1 receptors regulate dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons [8].
Dcf1, a dendritic cell factor also known as TMEM59, is a protein-coding gene including an N-terminal signal sequence, a mitochondrial targeting sequential motif, a transmembrane region, and a cytoplasmic tail. Dcf1 was first discovered to exhibit high expression in neural stem cells (NSCs) in our previous study [9]. However, its function was unknown at that time. Furthermore, we cloned, expressed, and silenced the Dcf1 gene and offered insight into its function in the differentiation of NSCs [10]. Regarding the regulatory mechanisms of Dcf1, we found that microRNA-351, a noncoding RNA, targets, binds, and negatively regulates Dcf1 expression, resulting in cellular morphological changes and decreases in cell volume [11]. Boada Romero et al. [12] reported that Dcf1 defines a novel ATG16L1 binding motif that promotes the local activation of LC3 and mediates autophagy in response to Staphylococcus aureus infection. All the previous findings suggested that Dcf1 plays a key role in neural development. Here, we address the function and mechanistic involvement of Dcf1 in dendritic spine development linked to memory behaviors.
Results
The Deletion of Dcf1 Induces the Reduction of Spine Motility and Spine Density in the Hippocampal
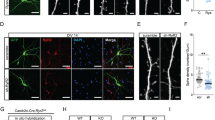
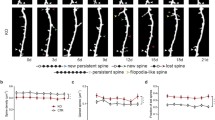
In view of the important role of Dcf1 in neural stem cell differentiation in our previous study, we want to know the effects of Dcf1 on brain development at the level of the animal. To this end, we created a Dcf1 gene knockout mouse (Dcf1−/−) (Fig. 1a and Supplementary Fig. 1a, b) and aimed to research the role that Dcf1 plays on nervous system. Firstly, we focused on the cell function and dynamic process in the hippocampal primary neurons. The results suggested a simpler dendritic complexity at DIV (days in vitro) 9 in KO hippocampal neurons (KO-EGFP), which can be rescued by restoring Dcf1 (KO-DCF1) (Fig. 1b, c). The dynamic process was traced by fast time-lapse imaging to calculate the spine motility, which describes any movements of spines, including growth, retraction, and changes in size, shape, and position [13]. The results powerfully identified a 32.6% reduction of spine motility in the KO, whereas Dcf1 could rescue the defective process (Fig. 1d, e). We then examined the neural dendritic spine change in the hippocampus using Golgi-Cox staining way. Results demonstrated that dendritic spine density in areas CA1 and CA3 was significantly reduced in Dcf1−/− mice (Fig. 2a, b), with decreases of 6.3% and 18.4% in areas CA1 and CA3, respectively; therefore, area CA3 was used for further observations. Dendritic spines are the site of synaptic contacts; often, reductions in the density of dendritic spines are a consequence of defects in the establishment and/or stabilization of the synapse [14]. Transmission electron microscopy (TEM) further revealed that synapse density was reduced by 15.9% in Dcf1−/− mice (Fig. 2c, d), consistent with the results of Golgi-Cox staining. We also examined dendritic spine morphology in these micrographs by measuring the head area of dendritic spines and the postsynaptic density (PSD) length in wild type (WT) and Dcf1−/− mice. We found that the average length of the PSD was shortened by 16.1% (Fig. 2e) and the spine head area was reduced by 20% in Dcf1−/− mice (Fig. 2f, Supplementary Fig. 2). These structural changes triggered our further consideration of its electrophysiology.
Dcf1 controls spine density and morphology in the hippocampus neurons. a Strategy for the construction of the Dcf1-floxed mouse line. Wild-type Dcf1 genomic DNA (top). Targeting vector in which a loxP site was inserted in the upstream regulatory sequence of Dcf1, selecting elements of the floxed-neo gene and PGK-TK gene were inserted in intron 1 (middle). Floxed Dcf1 genome (bottom). b, c Dcf1 promotes dendritic complexity. Dendrites are significantly fewer in Dcf1−/− neurons (KO-EGFP) than in their wild type controls (WT-EGFP); such a loss could be rescued by Dcf1 expression (KO-DCF1) at DIV9. Scale bar 20 μm. d, e Dcf1 promotes spine motility by fast time-lapse imaging (d) and the motility index is reduced by 68% (e) at DIV9. Scale bar 1 μm. One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001; all data are presented as mean ± SEM, 6 mice per group.
Dcf1 knockout reduces spine density and dendritic complexity. a, b Golgi staining of neurons in areas CA1 and CA3 of Dcf1−/− mice (a) indicates a significant reduction in spine density in areas CA1 and CA3 (b). Scale bar 1 μm. c, d TEM ultrastructural analysis of hippocampal CA3 synapse shows a decrease in synapse density in Dcf1−/− mice compared with that of WT mice. Scale bar 500 nm. e, f Mean PSD length (e) and dendritic spine head area (f) in Dcf1−/− mice were also significantly reduced. n = 4 mice per group. g In layer II/III, losing of Dcf1 (KO-P90) leads to a decrease in dendrite complexity compared with that of controls (WT-P90). Scale bar 50 μm. Total dendrite length (h) and branch numbers (i) were both significantly reduced in KO mice. j Representative confocal z-stack reconstructions of apical dendrite segments of GFP-expressing neurons in layer II/III from P90 WT and KO mice. Scale bar 1 μm. k Statistical analysis of the results exemplified in (j). l Representative confocal z-stack reconstructions of apical dendrite segments of GFP-expressing neurons in layer V/VI of WT and KO mice. Scale bar 1 μm. m Statistical analysis of the results exemplified in (l), showing a significant reduction of spine density in KO mice. Two tailed t test. *P < 0.05, **P < 0.01, ***P < 0.001; all data presented as mean ± SEM; 4 or 5 mice per group
Dcf1 Knockout Causes the Dendritic Spine and Dendritic Complexity Deficit in Cerebral Cortex
To genetically fate-map Dcf1 neurons, we crossed Dcf1−/− mice with Thy1-EGFP reporter animals to generate Dcf1 KO Thy1-EGFP mice in which green fluorescent protein (GFP) is permanently expressed in the dendrites and spine (Supplementary Fig. 1c). EGFP-labeled neurons were observed in layer II/III of the cerebral cortex at P90, but very few were observed at P21 and P42 (Supplementary Fig. 3a); therefore, we examined how Dcf1 affects dendrite arbor length and branching pattern in layer II/III at P90 only. Using confocal microscopy, we found that dendritic arborizations were much simpler in layer II/III of Dcf1−/− mice than in WT mice (Fig. 2g). Total dendritic length in Dcf1−/− mice was decreased by 27.8% (Fig. 2h) and second and tertiary branching levels were significantly reduced (Fig. 2i) compared with those of WT littermates. Moreover, dendritic spine density on the apical dendrites was reduced by 15.6% (Fig. 2j, k). Similarly, dendritic spine density in layer V/VI of the cortex was significantly reduced at P42 (Supplementary Fig. 3b, c), and P90 (Fig. 2l, m; Supplementary Fig. 3b, c) in Dcf1−/− mice when compared with that of WT littermates. In addition, statistical analysis demonstrated a significant increase in dendritic spine density in layer V/VI neurons across development in WT mice, whereas spine density was constant from P21 to P42 to P90 in Dcf1−/− mice (Supplementary Fig. 3c).
Dcf1 Triggers Action in Dendritic Spines through Recruitment of Lcn2
In light of all of the above results, the mechanisms underlying dendritic spine formation can be further addressed. By microarray analysis and chromatin immunoprecipitation (CHIP) verification, we identified 29 genes were closely related to the synaptic changes in Dcf1−/− mice (Fig. 3a). The expression of PSD95 (DLG4), which is known to both bind to NMDA receptor subunits and be involved in synaptic plasticity [15], was significantly reduced (Fig. 3b, c). Lcn2 is one of the most significantly upregulated genes (Fig. 3b, c) in the hippocampus of KO mice that may play an important role in the dendritic spine formation, suggesting that Dcf1 may affect the density of the spine by suppressing the level of Lcn2. The interaction between Dcf1 and Lcn2 was further investigated in co-immunoprecipitation (co-IP) experiments (Fig. 3d), which lent support to the proposed role of these two proteins in the molecular mechanism of dendritic spine formation. To determine the in vivo mechanism, the intracellular localization of Dcf1 and Lcn2 was explored. Lcn2 presented a uniform distribution around the nucleus (Fig. 3e), whereas N1-Dcf1 cotransfected with Lcn2 colocalized in an aggregate pattern (Fig. 3f). This result strongly indicates the role of Dcf1 recruitment of Lcn2 in the mechanism of dendritic spine formation. In view of the important role of Rac1 in promoting the growth of dendritic spines, we next assessed the relationships among Dcf1, Lcn2, and Rac1 in detail. The expression of Lcn2 was significantly reduced in the presence of Dcf1, whereas Rac1 expression was activated, in turn, overexpression of Lcn2 did not affect Dcf1 or Rac1 expression (Fig. 3g–i). Even in the case of overexpression of both Dcf1 and Lcn2, the Lcn2 protein level was significantly lower than in the control (Fig. 3g, h).
Dcf1 affects Rac1 by suppressing the level of Lcn2. a David functional annotation bioinformatics microarray analysis showed that 29 gene involved in the synaptic change in Dcf1−/− mice, including a postsynaptic protein PSD95 (DLG4). b, c PSD95 showed a significant decrease but Lcn2 showed a significant increase in the hippocampus of KO mice. Two tailed t test. d Dcf1 interacts with Lcn2. The lanes in each panel are in the following order: lane 1, Lcn2 extracted with 1D4 antibody and detected using Lcn2 antibody and Dcf1 antibody; lane 2, Dcf1 extracted with His antibody and detected using Dcf1 and Lcn2 antibody; lane 3, beads with no antibody; lane 4, blank. e Neuro-2A cell transfected with N2-Lcn2 only, showing a uniform distribution of Lcn2 around the nucleus. f In cells cotransfected with N2-Lcn2 and N1-Dcf1, Lcn2 displayed aggregation and colocalization with Dcf1. g The level of Lcn2 was significantly reduced in cells cotransfected with Dcf1 and Lcn2 compared with cells transfected with Lcn2 only, and the level of Rac1 was significantly reduced when cells were transfected with Lcn2 only or cotransfected with Lcn2 and Dcf1 compared with cells that were transfected with Dcf1 alone. h, i Statistical analysis of Lcn2 and Rac1 levels. One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001; all data presented as mean ± SEM; 3 mice per group.
We then constructed three shRNA plasmids in an attempt to silence Lcn2 (Fig. 4a, b). These results indicated an increase in Rac1 activity relative to the control (Fig. 4a, c). Importantly, silencing of Lcn2 by shRNA-Lcn2–3 was able to rescue spine loss in Dcf1 KO mice (Fig. 4d, e), consistent with previous results that Rac1 could rescue spine loss in KO mice (Fig. 4f, g). These results provided evidence that Dcf1 regulates the formation of dendritic spine through Rac1 by suppressing the level of Lcn2.
Decreasing Lcn2 and increasing Rac1 could all rescue the dendritic spine lose causing by Dcf1 deletion. a, b When cells were transfected separately with each of three Lcn2 shRNA plasmids in combination with Lcn2 to test the ability of Lcn2 shRNAs to reduce the Lcn2 level, shRNA- Lcn2–3 demonstrated the best knockdown effect. c All three Lcn2 shRNAs resulted in increased levels of Rac1, and shRNA-Lcn2–3 resulted in a significant change. d, e shRNA-Lcn2–3 can rescue spine loss in KO mice. Scale bar 1 μm. f, g Decreased spine density (KO-EGFP) is rescued by Dcf1 expression (KO-DCF1) and Rac1 expression (KO-Rac1) compared with that of controls (WT-EGFP). Scale bar 1 μm. One-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001; all data presented as mean ± SEM; 4 or 5 mice per group
Dcf1 Knockout Leads to PSD95-NMDAR Signaling Suppression
To further research the aforementioned Dcf1-Lcn2-Rac1 signal, we detected the relative expression of Rho GTPases (Rac1 and Cdc42). Results showed that Rac1 and Cdc42 and their downstream effector, Pak3, were all reduced in the hippocampus (Fig. 5a, b) of KO mice. These results strongly supported that Rac1 could rescue the dendritic spine lose in the KO mice (Fig. 4f, g). As the balance of the activity of c-Jun N-terminal/stress-activated kinases (JNKs) and extracellular signal-regulated kinases (ERKs) appears to play major role in mediating neural cells [16], the active JNK (p-JNK / JNK) and active ERK1/2 (p-ERK1/2/ERK1/2) were detected in the hippocampus. Active JNK showed a significant decrease but active ERK showed a notable increase (Fig. 5c, d). The increased phosphorylation of JNK and c-Jun was observed after PAK3 overexpression [17], also Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons [18]. It revealed that PAK3 affects the phosphorylation of JNK, and the phosphorylation of JNK can affect the level of PSD95. Further, we compared the expression of three glutamate receptor subunits (GluR1, GluR2, and NR1) binding to PSD95 which involve in synaptic plasticity [15] and cellular substrates of learning and memory [19]. We found that GluR1 and NR1 are significantly downregulated in Dcf1−/− mice (Fig. 5e, f).
Dcf1 knockout leads to PSD95-NMDAR signal suppression. a, b Western blot analysis (a) and statistical analysis (b) demonstrate a downregulation of Rac1, Cdc42, and Pak3 expression in the hippocampus of Dcf1−/− mice. c, d The level of JNK and ERK1/2 all showed an increase in the hippocampus of KO mice compared to WT, and the active JNK (p-JNK / JNK) also showed a significant increase, but the active ERK (p-ERK1/2 / ERK1/2) presented a notable decrease. e, f Western blot analysis and statistical analysis showed a downregulation of GluR1, GluR2, and NR1 in the hippocampus, a significant difference was found in NR1 expression. Two tailed t test, *P < 0.05, **P < 0.01, all data presented as mean ± SEM; 3 mice per group
Dcf1 Deficiency Impairs Learning and Memory
Does Dcf1 play a functional role in generating the dendritic spine, which is closely associated with learning and memory [20]? Firstly, the object relocation test was executed to assess spatial memory in the cognitive process. During the training phase, Dcf1−/− and WT (wild-type) mice spent equal time playing with the two objects (Supplementary Fig. 4a), suggesting that they were interested in the two objects to the same degree. During the acquisition test, Dcf1−/− mice spent less time exploring the displaced object than the non-displaced object, this difference being greater in KO than in WT mice (Fig. 6a). In the Y-maze test, loss of Dcf1 decreased the length of time taken to recognize the novel arm after 60 min (Fig. 6b), indicating a reduced willingness of Dcf1−/− mice to explore a new environment. In the Morris water maze test, Dcf1−/− mice were only worse than WT mice at locating the hidden platform on the second trial of the first acquisition trial day (Supplementary Fig. 4b). However, Dcf1−/− mice were significantly impaired in performance on the reversal learning task (Fig. 6c). Detailed swimming tracks for trials 3 and 4 of the reversal learning task are shown in Supplementary Fig. 4c, and the distance covered to find the hidden platform was significantly different between Dcf1−/− mice and WT mice (Supplementary Fig. 4d, e), indicating that loss of Dcf1 affects memory function. Together, these tests demonstrate that mice lacking Dcf1 exhibit cognitive and memory deficits. Finally, the silencing of endogenous Lcn2 was confirmed to explain the behavioral mechanism. Transfection of neuro-2A cells with the shRNA-Lcn2 lentivirus was able to reduce the expression of Lcn2 by approximately 50% (Supplementary Fig. 5a), and the lentivirus was expressed in the target area (Supplementary Fig. 5b). When Lcn2 was silenced in Dcf1−/− animals, learning and memory were recovered to different degrees in the Morris water maze test on the acquisition trial day (Supplementary Fig. 5c) and in the reversal trial (Fig. 6d). These results further revealed that Dcf1 affected memory through the key gene Lcn2.
Dcf1 knockout reduces performance in memory tasks and impairment of mEPSC in the hippocampus. a In the object relocation test, both WT and Dcf1−/− mice spent equal time with the displaced and non-displaced object. b At an inter-trial interval of 60 min, Dcf1−/− mice exhibited reduced spatial memory in the Y-maze test. c In the Morris water maze test, Dcf1−/− mice showed significantly reduced reversal learning on the Morris water task compared with WT mice. Two-tailed t test. d Dcf1−/− mice injected with control vector took significantly longer to locate the hidden platform than WT mice, and KO mice injected with shRNA Lcn2 lentivirus can somehow rescue memory losing in KO mice. 5–10 mice per group. One-way ANOVA. e Representative traces of mEPSCs from hippocampus cells of control and Dcf1−/− mice. f The average frequency of mEPSC from the hippocampus cells of WT and KO mice. g The average amplitude of mEPSC from the hippocampus cells of WT and KO mice. 3 or 4 mice per group. Two-tailed t test, *P < 0.05, **P < 0.01; all data presented as mean ± SEM.
Dcf1−/− Mice Display Weaker mEPSC and Neuronal Activation
Considering the decreases in dendritic branching and spine density, and the damage to learning and memory observed in Dcf1−/− mice, we hypothesized that these reductions in dendritic complexity may alter synaptic plasticity and function. Then, whole-cell voltage clamp recordings of functional measurements, miniature excitatory postsynaptic currents (mEPSC) were performed in this area. We found that the frequency of mEPSC was significantly reduced in Dcf1−/− mice (Fig. 6e, f), although there was no apparent change in its amplitude (Fig. 6e, g), indicating a reduction in the number of functional synapses in Dcf1−/− CA3-CA1 Schaffer collaterals. Further, we used optogenetic techniques to activate neurons in the CA3 (Fig. 7a) to investigate the reason for the electrophysiology deficit in the CA3-CA1 Schaffer collateral pathway. We injected AAV-Chr2-mcherry (Obio technology) into the CA3 area. Two weeks later, mice were placed into the open field and exposed to blue light stimulation at 473 nm. Results indicated a significant decrease in movement distance and velocity in Dcf1−/− mice compared with their controls when the light was on, whereas no significant difference was observed between Dcf1−/− and control mice when the light was off (Fig. 7b, c), whereas no significant difference was observed between Dcf1−/− mice and control mice when the light was off, suggesting a reduction in neuronal activation in the absence of Dcf1. To test this, we calculated the number of c-Fos-positive neurons in the CA3 and found a significant reduction of positive neurons in this area in KO mice (Fig. 7d, e). The results presented here further reveal the disrupted function in area CA3 in KO mice.
KO mice displayed weaker neuronal activation when neurons were evaluated using optogenetic techniques. a AAV-Chr2-mcherry was injected into area CA3. b Dcf1−/− and WT mice all traveled a longer distance when the light was on, and WT traveled farther than KO mice regardless of whether the light was on or off; n = 6 mice per group. c Dcf1−/− mice traveled at a lower velocity, regardless of whether the light was on or off; 6 mice per group. One-way ANOVA. d, e The number of c-Fos-positive neurons was much lower in area CA3 of KO mice. 5 mice per group. Scale bar 200 μm. One-way ANOVA. # P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001; all data are presented as mean ± SEM
Discussion
In this study, we have demonstrated the disruption of spine motility, dendritic morphology, memory deficiency, and mEPSC reduction in Dcf1−/− mice at the level of anatomy, behavior, and physiology to provide a comprehensive understanding of Dcf1 function. Our findings indicate for the first time that Dcf1 triggers dendritic spine formation and facilitates memory acquisition, contributing greatly to our understanding of brain development and function.
An increasing number of studies show that an altered epigenetic landscape may cause impairments in regulation of learning and memory-related genes and dendritic spine, such as SUV39H1 inhibition and the concomitant H3K9me3 downregulation mediate gene transcription in the hippocampus and reverse age-dependent deficits in hippocampal memory [21]. Abnormal acetylation of K274 and K281 on tau, identified in AD brains, promotes memory loss and disrupts synaptic plasticity by reducing postsynaptic KIdney/BRAin (KIBRA) protein, a memory-associated protein. Cyclic AMP (cAMP) signaling reduces phosphorylation of the Cdk5 sites in WAVE1, and increases spine density in a WAVE1-dependent manner [22]. Different from previous studies, this is a pioneering work that Dcf1 regulates dendritic spine development, completely answering the morphological details and molecular regulation mechanism. It is a novel study without any report previously. This study puts forward a new understanding of dendritic spines dysplasia for the brain structure and function.
In the cerebral cortex, KO mice showed a significant decrease in dendritic spine density in layers II/III and V/VI; KO mice also displayed simpler dendritic arborizations. Interestingly, in KO mice, the dendritic spine density in layer V/VI of the cortex was reduced at all three stages, and there was a significant increase in dendritic spine density in layer V/VI neurons across development in WT mice, whereas spine density was constant from P21 to P42 to P90 in Dcf1−/− mice. These results reveal that Dcf1 influences not only spine density but also the development of the dendritic spine in this area. Moreover, in the hippocampus, KO mice also showed a decrease in spine density and abnormal dendritic spine morphology in the CA3 area.
How does Dcf1 work? We found that Dcf1 interacts with Lcn2 thus sequentially causes a series of functional response. Lcn2 is a member of a family of over 20 small, secreted proteins that serve diverse cellular roles [23, 24] in regulating differentiation and maturation, tumor growth, and proliferation [25]. Dcf1 is differentially expressed during differentiation of primary NSCs from Sprague-Dawley rat striatal [9]. The downregulation of Dcf1 by RNAi interference in the mouse C17.2 neural stem cell line increases the differentiation of NSCs into neurons and astrocytes [10]. Nevertheless, its function in the central nervous system (CNS) has not yet been reported. Here, we have not only presented the significant role in spine formation and facilitation of memory acquisition but have also provided evidence that Dcf1 regulates Lcn2 in the performance of this function. Dcf1 seems to be a brake that controls this process. Removing the brake could lead to memory damage. Together, the results of this study provide new insight into dendritic spine structure and function and offer promise for an exciting new avenue for the treatment of memory loss.
Materials and Methods
Production and Genotyping of Dcf1 Knockout Mice
Dcf1 mouse DNA was cloned from a BAC clone (Clone-ID: RP23-423N6) using PCR methods. The gene targeting vector pLNT-Dcf1 was constructed as follows: the 5′ homologous arm (F1 fragment) included 1.2-kb genomic DNA (Not I/Sal I digest) upstream from exon 1 of Dcf1. The 3′ homologous arm (F3 fragment) was a 4.8-kb Cla I-Kpn I fragment amplified from intron 1 of Dcf1. The targeted allele (F2 fragment) was mainly exon 1 (1.4-kb BamHI genomic part between 5′ and 3′ homologous sequence) and neo selecting gene, containing the ATG of Dcf1. A PGK-TK gene outside of the 3′ homologous arm was used as the negative selection gene. Targeting vector pLNT-Dcf1 was linearized by NotI and electroporated into 129/Ola embryonic stem (ES) cells. ES clones were cultured in G418 and Ganciclovir conditions and screened by PCR. ES clones were injected into C57BL/6 blastocysts and four chimeric mice were obtained. Neo gene containing Dcf1+/− mice (neo Dcf1+/−) were generated by crossing chimeric mice with C57BL/6 to generate neo Dcf1+/− F1 litters, which contain a C57BL/6 and 129/Ola mixed genetic background. Excision of neo from the mouse germline was accomplished by crossing neo Dcf1+/− mice with EIIa-Cre transgenic mice to generate Dcf1+/− mice (neo gene null). Dcf1+/− mice were then bred to generate Dcf1−/−, Dcf1+/− and Dcf1+/+ mice, which were genotyped by PCR. Two pairs of primers, Up/Low and Up/F3 Low, were used to identify different genotypes. The removal of the F2 fragment was represented by a 700-bp band versus a 2.1-kb band for WT (Up/F3 Low), and the absence of a 400-bp band (Up/Low) indicated the double null.
Hippocampal Neuron Tests
The Hippocampal neuron tests were performed as previously described [7]. Hippocampal neurons were detected from mice of P0 and were plated at 20000 cells on 20 mm coverslips coated with 0.1 mg/ml poly-dl-lysine. The cells were cultured in neurobasal-A medium containing 2 mM glutaMAX, 1% penicillin-streptomycin, and B27 supplement (Invitrogen) in 5% CO2/10% O2 atmosphere at 37 °C. The hippocampal neurons were transfected at DIV4 and DIV7 using the calcium phosphate method. And Spine motility was analyzed using ‘Motility Index’ as previously described [26]. For calculations of motility index, seven frames representing maximal spine displacement were chosen and superimposed. The motility index is defined as the ratio of the difference between accumulated and smallest areas occupied by the spine divided by the average area of the spine.
Morphology of Dendritic Spines
Thy1 EGFP-expressing male mice (JAX Mice Database - 007788 STOCK TgMJrs-J) aged P21, P42 and P90 were anesthetized deeply with Nembutal and perfused with 0.9% NaCl, followed by 4% paraformaldehyde in 0.1 M PB. After fixation, mouse brains were removed and post-fixed in 4% paraformaldehyde for 24 h. After fixation, brains were removed and post-fixed in 4% paraformaldehyde for 24 h. The brains were then transferred into 20% sucrose and then 30% sucrose, incubated in OCT overnight at −80 °C, and then cut into 30 μm sections at −20 °C. The sections were imaged using a 488-nm wavelength laser on a Zeiss confocal microscope. The number of dendritic spines in layer II/III and layer V/VI was counted in 30–50-μm segments from the distal apical dendrites, and 10–20 dendrites or neurons were used per mouse. The simplicity of dendritic arborization was analyzed according to previous study [14]. For Golgi staining, brains of 3-month-old wild-type and Dcf1−/− adult mice were immersed with Golgi-Cox solution according to the Golgi procedure (Hito Golgi-Cox Optim Stain™ Kit). Sections were cut at a thickness of 100 μm and mounted in bright-field z-series. Dendritic spines in the mouse cerebral cortex and hippocampus were visualized with a Zeiss confocal microscope. The number of dendritic spines in these regions was counted in at least 30-μm segments. All the pictures taken and analyzed were done by a person who was blind to the genotype.
Electron Microscopy
The brains were removed from the anesthetized mice, then dissected and the CA3 area were separated and about 1 mm3 tissue were placed in a fixative containing 2.5% glutaraldehyde for 2 h, then rinsed in phosphate buffer, and then post-fixed for 2 h in 1% osmium tetroxide in phosphate buffer at 4 °C. Following this, the brains were dehydrated and embedded in 618# resin. Ultrathin sections were cut using a Reichert ultramicrotome, and then contrasted with uranyl acetate and lead citrate, and examined under a FEI 5018/11 electron microscope at 11000X at last. Excitatory synapses were identified in electron micrographs by the presence of a dendritic spine containing a clear electron dense postsynaptic density apposed to an asymmetric presynaptic compartment containing at least three synaptic vesicles. All measurements were made by a single experimenter who was blinded to sample genotype. Dendritic spines that had a visible neck were used to measure spine head area, beginning at the thinnest part of the neck.
In Utero Electroporation
Pregnant mice were anesthetized with 2% sodium pentobarbital (3.3 ml per kg, intraperitoneally). Plasmids were transfected into multiparous KO and WT mice at 14.5 days of gestation and later observed at P21. Uteruses were exposed, and 8–12 μg of plasmid mixed with Fast Green (2 mg/ml; Sigma) was injected by trans-uterus pressure microinjection into the lateral ventricle of the embryos. Next, electric pulses generated by an ECM830 (BTX) were delivered to the cerebral wall in sequences of five pulses at 30 V for 50 ms, with an interval of 100 ms.
Western Blot
Protein analysis of the cortex and hippocampus from mice and cultured cells was performed using Western blot. Protein was denatured at 100 °C for 15 min in 5× loading buffer. The nitrocellulose membranes were blocked with 5% BSA before incubation with primary antibodies, which included anti-PSD95 (1:800, ABclonal), anti-Lcn2 (1:800, ABclonal), anti-Rac1(1:800, ABclonal), anti-CDC42 (1:800, ABclonal), anti-GAPDH (1:1000, Santa Cruz), anti-β-actin (1:800, Santa Cruz), anti-Snap25 (1:800, ABclonal), anti-DCF1 (1:1000), anti-GluR1 (1:800, ABclonal), anti-GluR2 (1:800,ABclonal), and anti-NR1 (1:800,ABclonal), anti-LC3B (1:1000, CST), anti-JNK (1:1000, CST), anti-p-JNK (1:1000, CST), anti-ERK1/2 (1:1000, CST), anti-p-ERK/2 (1:1000, CST), and anti-Lcn2 (1:800, ABclonal). Bound proteins were visualized with the Odyssey infrared imaging system at wavelengths of 700 and 800 nm. For the overexpression experiments in HEK293 cells, pCAGGS-EGFP, pCAGGS-Dcf1, pCAGGS-Rac1, pCAGGS-Cdc42, pEGFP-N2, pEGFP-N2-D1, Trans-Dcf1 (the deletion mutant corresponding to transmembrane of DCF1), pCDNA3.1-Dcf1-His, pCAGGS-Lcn2-1D4 were transfected into HEK293 cell. For the detecting of shRNA experiments, three shRNA Lcn2 plasmids cotransfected with Lcn2, 48 h later, proteins were extracted using cell lysis buffer, mercaptoethanol, and 5× SDS buffer. Then, proteins were used to do the western blot.
For the co-IP test, HEK293 cells (10 cm X 4) were cotransfected with pcDNA-Dcf1-His (50 μg), pCAGGS-Lcn2-1D4 (50 μg). Cells were lysised at 48 h post-transfection and added 0.5 ml 1× ice-cold cell lysis buffer plus (RIPA) 1 mM PMSF to each dish. Then, 500 μg total protein were taken and added 6 μg antibody, place the tube in an ice bucket for 1 h, added 20ul of resuspended volume of the appropriate agarose conjugate (proteinA/Gbeads, washed and diluted 1:1 by PBS). The antibody used was anti-His (mouse, abclonal) and anti-1D4 (mouse, Corning).
Spatial Object Relocation Task
Spatial object recognition was performed in an open field setup according to methods described previously [27, 28]. To test memory for the spatial location of objects, Dcf1−/− mice and control mice were allowed to investigate two identical novel objects. First, in the acquisition trial, mice investigated the two objects twice for 7 min, separated by a 15 min interval during which the objects were placed in two adjacent corners of the square. Thirty minutes following the last acquisition trial, the mice had a 7-min retrieval trial in which one object remained in its original location(non-displaced object) and the other object was placed in the opposite corner (displaced object). If the animals are able to recall the initial spatial rearrangement of the objects, they will spend more time exploring the displaced object during the retrieval trial.
Y-maze
The Y-maze was made of PVC tubing, oriented with an angle of 120° each between three equal-length arms. The Y-maze test consisted of two trials. In the first trial (the acquisition trial), the mice were allowed to explore only two of the three arms for 10 min while the third arm was blocked. After a 30-, 60-, or 120-min inter-trial interval (ITI), the mice were placed back into the maze for 5 min and allowed to access all three arms (the retrieval trial). These trials were recorded using a video-tracking system (Noldus), and the percentage of time the mice spent in the novel arm compared to the familiar arms was measured. The apparatus was cleaned between trials to ensure that olfactory cues between mice were not present.
Morris Water Maze
The Morris water maze test was performed as described previously, with some adaptations for mice [29]. A circular tank (122 cm in diameter) was filled with opaque water by adding milk (22 ± 1 °C), and the walls visible from the tank had cues attached to them at a distance of approximately 1 m from the pool. After a 60 s free swim trial, mice were trained to find a visible platform (10 cm in diameter) 1 cm above the surface of the water in four trials (visual training phase). The mice then received four spatial training sessions per day for three consecutive days in which they located a platform which was in a fixed position 1 cm under the water (spatial training phase). The starting locations were varied across the different tests. On the day after spatial training was completed, the mice were assessed in a 60s probe trial in which the platform was removed. On the final day, the hidden platform was placed in the quadrant opposite its original location, and the mice completed four trials in which they located the platform in the new location (reversal learning). In all the sessions, the tests were terminated once the mouse touched or went up the platform or once 60 s had elapsed. The ITI was 10 min.
Whole Cell Recording
For recording mEPSC, 14–15 postnatal day mice were used. Whole cell voltage-clamp recordings were done under the CA3-CA1 Schaffer collaterals in area CA1 using a microscopy (Nikon) by random. 300-μm acute slices were prepared in ice cold buffer bubbled with 95% O2/5% CO2, which contained: (in mM): 2.5 KCl; 26 NaHCO3; 1.25 NaH2PO4; 2.5 CaCl2; 2 MgSO4; 20 Glucose; 248 Sucrose. Slices were kept in artificial cerebrospinal fluid (ACSF) which contain (in mM): 124 NaCl; 2.5KCl; 1.25 NaH2PO4; 2 MgSO4; 2.5 CaCl2; 26 NaHCO3; 10 Glucose for 1 h at 35 °C. Patch electrodes (7–8 MΩ) were pulled from 1.5-mm OD borosilicate glass using a micropipette puller (P-97). The internal solution containing (in mM): 130 K-gluconate, 5 NaCl, 10 Na-phosphocreatine, 1 MgCl2, 10 HEPES, 0.02 EGTA, 2 MgATP, 0.5 Na2GTP. Cells were held in voltage clamp at −70 mV with TTX (1 uM) included in the external solution (ACSF).
Immunohistochemistry
The c-Fos detection test was performed as described previously [30, 31]. First, mice were placed into a novel open field, 5 min of stimulation (10 Hz, 20 ms pulses, 8 mw light power) then returned to their home cage. 1.5 h later, mice were perfused, and then slices were used to label c-Fos.
References
Banerjee J, Fischer CC, Wedegaertner PB (2009) The amino acid motif L/IIxxFE defines a novel actin-binding sequence in PDZ-RhoGEF. Biochemistry 48(33):8032–8043. doi:10.1021/bi9010013
Tschida KA, Mooney R (2012) Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron 73(5):1028–1039. doi:10.1016/j.neuron.2011.12.038
Bian WJ, Miao WY, He SJ, Qiu Z, Yu X (2015) Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes. Cell 162(4):808–822. doi:10.1016/j.cell.2015.07.018
Merino-Serrais P, Benavides-Piccione R, Blazquez-Llorca L, Kastanauskaite A, Rabano A, Avila J, DeFelipe J (2013) The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer's disease. Brain : a journal of neurology 136(Pt 6):1913–1928. doi:10.1093/brain/awt088
Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD et al (2009) Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462(7276):1065–1069. doi:10.1038/nature08628
Kondratiuk I, Leski S, Urbanska M, Biecek P, Devijver H, Lechat B, Van Leuven F, Kaczmarek L et al (2016) GSK-3beta and MMP-9 cooperate in the control of dendritic spine morphology. Mol Neurobiol. doi:10.1007/s12035-015-9625-0
Chen Y, Wang F, Long H, Chen Y, Wu Z, Ma L (2011) GRK5 promotes F-actin bundling and targets bundles to membrane structures to control neuronal morphogenesis. J Cell Biol 194(6):905–920. doi:10.1083/jcb.201104114
Li J, Gu J, Wang B, Xie M, Huang L, Liu Y, Zhang L, Xue J et al (2015) Activation of dopamine D1 receptors regulates dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons. Mol Neurobiol 51(3):1024–1037. doi:10.1007/s12035-014-8762-1
Wen T, Gu P, Chen F (2002) Discovery of two novel functional genes from differentiation of neural stem cells in the striatum of the fetal rat. Neurosci Lett 329(1):101–105
Wang L, Wang J, Wu Y, Wu J, Pang S, Pan R, Wen T (2008) A novel function of dcf1 during the differentiation of neural stem cells in vitro. Cell Mol Neurobiol 28(6):887–894. doi:10.1007/s10571-008-9266-1
Li X, Feng R, Huang C, Wang H, Wang J, Zhang Z, Yan H, Wen T (2012) MicroRNA-351 regulates TMEM 59 (DCF1) expression and mediates neural stem cell morphogenesis. RNA Biol 9(3):292–301. doi:10.4161/rna.19100
Boada-Romero E, Letek M, Fleischer A, Pallauf K, Ramon-Barros C, Pimentel-Muinos FX (2013) TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3. EMBO J 32(4):566–582. doi:10.1038/emboj.2013.8
Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 26(1):3–11. doi:10.1523/JNEUROSCI.3648-05.2006
Cubelos B, Sebastian-Serrano A, Beccari L, Calcagnotto ME, Cisneros E, Kim S, Dopazo A, Alvarez-Dolado M et al (2010) Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex. Neuron 66(4):523–535. doi:10.1016/j.neuron.2010.04.038
Ohnuma T, Kato H, Arai H, Faull RL, McKenna PJ, Emson PC (2000) Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport 11(14):3133–3137
Beresewicz M, Kowalczyk JE, Zablocka B (2008) Kalirin-7, a protein enriched in postsynaptic density, is involved in ischemic signal transduction. Neurochem Res 33(9):1789–1794. doi:10.1007/s11064-008-9631-y
Liu RX, Wang WQ, Ye L, Bi YF, Fang H, Cui B, Zhou WW, Dai M et al (2010) p21-activated kinase 3 is overexpressed in thymic neuroendocrine tumors (carcinoids) with ectopic ACTH syndrome and participates in cell migration. Endocrine 38(1):38–47. doi:10.1007/s12020-010-9324-6
Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284(23):15857–15866. doi:10.1074/jbc.M808986200
Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304(5673):1021–1024. doi:10.1126/science.1096615
Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, Wolf M, Liebl C et al (2013) Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci 16(6):706–713. doi:10.1038/nn.3395
Snigdha S, Prieto GA, Petrosyan A, Loertscher BM, Dieskau AP, Overman LE, Cotman CW (2016) H3K9me3 inhibition improves memory, promotes spine formation, and increases BDNF levels in the aged hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 36(12):3611–3622. doi:10.1523/JNEUROSCI.2693-15.2016
Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP et al (2006) Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442(7104):814–817. doi:10.1038/nature04976
Flower DR (1996) The lipocalin protein family: structure and function. The Biochemical journal 318(Pt 1):1–14
Flower DR, North AC, Sansom CE (2000) The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta 1482(1–2):9–24
Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H et al (2002) An iron delivery pathway mediated by a lipocalin. Mol Cell 10(5):1045–1056
Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R (1999) Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A 96(23):13438–13443
Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, Tobler I (2006) Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem 85(3):263–271. doi:10.1016/j.nlm.2005.11.005
Schmidt MV, Schulke JP, Liebl C, Stiess M, Avrabos C, Bock J, Wochnik GM, Davies HA et al (2011) Tumor suppressor down-regulated in renal cell carcinoma 1 (DRR1) is a stress-induced actin bundling factor that modulates synaptic efficacy and cognition. Proc Natl Acad Sci U S A 108(41):17213–17218. doi:10.1073/pnas.1103318108
Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W et al (2011) Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(38):13625–13634. doi:10.1523/JNEUROSCI.2259-11.2011
Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA et al (2013) Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77(5):955–968. doi:10.1016/j.neuron.2012.12.038
Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S (2013) Creating a false memory in the hippocampus. Science 341(6144):387–391. doi:10.1126/science.1239073
Acknowledgments
This work was funded by the National Science Foundation of China (31070954, 81271253, 81471162), the Science and Technology Commission of Shanghai (14JC1402400), and the Key Innovation Project of Shanghai Municipal Education Commission (Grant No.14ZZ090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
The generation of Dcf1 knockout mice. a Verification of F2 deletion at the genomic level. A 700-bp band and lack of a 400-bp band indicate removal of F2 from the genomic DNA. b RT-PCR analysis of Dcf1 expression. The absence of the Dcf1 fragment confirmed gene knockout. c Thy1-EGFP mice were identified by PCR with a transgene (TR) that has a 415-bp band and an internal positive control (IP) that has a 324-bp band. (GIF 19 kb.)
Supplementary Figure 2
Dcf1−/− mice exhibit abnormal spine synapse ultrastructure in area CA3. a Electron micrographs of synapses in WT and Dcf1−/− mice. b Dcf1−/− mice have significantly decreased spine head area. c Frequency histograms of spine head area for WT and Dcf1−/− neurons in area CA3. *P < 0.05, **P < 0.01, one way ANOVA; all data are presented as mean ± SEM; 4 mice per group. (GIF 105 kb.)
Supplementary Figure 3
Dcf1 knockout causes the loss of dendritic spines in layer V/VI at different ages. a Neuronal development at P21, P42, and P90 in layer II/III. Neurons labeled with EGFP appeared at P90 but not at P21 and P42 in this layer. Scale bar: 100 μm. b The density of dendritic spines in layer V/VI of Dcf1−/− mice is decreased in comparison with that of WT mice at P21, P42, and P90. Scale bar: 5 μm. c Statistical comparison of dendritic spine density in layer V/VI of WT and Dcf1−/− mice demonstrates significant differences at P42 and P90. Analysis of dendritic spine density also showed a significant increase in WT mice from P21 to P42 and P90, whereas there was no increase in Dcf1−/− mice across ages. *P < 0.05, **P < 0.01, ***P < 0.001, two way ANOVA; all data are presented as mean ± SEM; 4 or 5 mice per group. (GIF 171 kb.)
Supplementary Figure 4
Dcf1 knockout reduces performance in memory tasks. a In the training trial of the spatial object relocation test, WT and Dcf1−/− mice spent equal time exploring the objects. b In the Morris water maze test, Dcf1−/− mice took significantly longer to locate the hidden platform only in the second trial on the first spatial training day. c-e Swimming path when searching for the hidden platform was much less directed in Dcf1−/− mice c, and the distance they swam on trial 3 d and trial 4 e of the reversal learning task was much longer compared with WT mice. Two tailed t-test, # P < 0.1, *P < 0.05,**P < 0.01,***P < 0.001; all data presented as mean ± SEM. 7–10 mice per group. (GIF 70 kb.)
Supplementary Figure 5
Knocking down the level of Lcn2 in DG area of the hippocampus of KO mice can rescue the learning and memory of KO mice. a The lentivirus of pLKD-Ubc-EGFP-U6-shRNA Lcn2 can reduce the level of Lcn2 contrast the control in the neuro-2A cells. b Pictures showed that the lentivirus was expressed in the DG area of the hippocampus. Two tailed t-test. c Dcf1−/− mice injected with control only took significantly longer to locate the hidden platform contrast WT mice injected with control and KO mice injected with shRNA Lcn2 lentivirus in the second trial on the first spatial training day. Scale bar: 20 μm. # P < 0.1, *P < 0.05,**P < 0.01,***P < 0.001; all data presented as mean ± SEM. 6–9 mice per group. (GIF 99 kb.)
ESM 1
(PDF 47.9 kb.)
Rights and permissions
About this article
Cite this article
Liu, Q., Feng, R., Chen, Y. et al. Dcf1 Triggers Dendritic Spine Formation and Facilitates Memory Acquisition. Mol Neurobiol 55, 763–775 (2018). https://doi.org/10.1007/s12035-016-0349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0349-6