Abstract
Renal cell carcinoma (RCC) local recurrence after radical nephrectomy is uncommon. When feasible, surgical removal remains the primary treatment strategy; nevertheless, local RCC relapse management is controversial, and less invasive procedures may represent an attractive option to achieve oncologic control. The aim of our study was to assess the feasibility, safety, and clinical outcomes of image-guided percutaneous microwave ablation (MWA) for RCC local recurrence in patients initially treated with nephrectomy with curative intent. 10 consecutive patients underwent CT-guided percutaneous MWA of a total of 10 retroperitoneal nodules. Inclusion criteria were: histologically verified retroperitoneal metastases, previous radical nephrectomy, lesion no larger than 3 cm, no other metastatic site elsewhere. All the procedures were performed under moderate sedation choosing the most favorable patient decubitus. If deemed necessary, pneumodissection was induced before ablation. After the antenna placement inside the target lesion, thermal ablation was achieved by maintenance of a power of 100 W for a total time between 2 and 4 min. All patients were observed overnight and discharged the following day if clinically stable. Technical success was obtained in 100% of patients. One patient was re-treated to complete oncologic response with repeat MWA. No major complications were observed. No patients demonstrated local recurrence at a mean follow-up of 26 months. MWA is a safe and effective treatment strategy for loco-regional relapse of RCC following radical nephrectomy. This technique may represent a valuable approach for patients who are not eligible for surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As renal cell carcinoma (RCC) represents approximately 3.8% of all new cancers diagnosed in the US population, it is the most common renal malignancy [1]. As a consequence of the spread of advanced diagnostic imaging in which asymptomatic renal cancers are incidentally detected, in the last decades, we have observed a constantly increasing incidence rate. After being diagnosed with RCC, the overall survival rate at 5 years for localized disease is 92.6% [1].
RCC treatment approach varies according to the tumor stage [2]; nonetheless, if practicable surgery remains the cornerstone of RCC treatment. Among all the available options, minimally invasive therapies have recently emerged as one of the finest developments in the care of patients with renal tumors, since interventional therapies can provide a valuable nephron-sparing alternative for those patients who are unfit for surgery.
In recent years, there have been reported different ablation technologies, and current thermal ablative modalities include microwave ablation (MWA), radiofrequency ablation (RFA), and cryoablation (CRA); in particular RFA and CRA are the most widely adopted for RCC treatment [2].
However, MWA has some theoretical advantages over the other available technologies, such as greater intratumoral temperatures, a more homogeneous and faster tissue damage over larger volumes, lesser susceptibility to local variation in tissue physical properties (e.g., electrical and thermal conductivity); these features may result in a more reliable and predictable coagulative effect [3, 4]. MWA technology is capable to induce coagulation necrosis when tissues are heated to lethal temperatures because of the agitation and friction of water molecules [5]; notably, bipolar water molecules within surrounding tissue are forced to oscillate by microwaves radiating from the exposed, non-insulated portion of the antenna, thus able to generate heath and consequent cellular death.
RCC local recurrence is defined as relapse within the renal fossa or elsewhere in the retroperitoneum in the case of radical nephrectomy, or at the resection margin in the case of partial nephrectomy. RCC local relapse is fairly uncommon since it occurs in almost 1.8% of cases without evidence of systemic metastatic disease [6]; nevertheless, it seems to be related to a poor prognosis.
When technically possible, surgery is still the primary curative approach [7]. However, treatment options for local RCC relapse are controversial, since the literature data on the natural history, patient outcome, and prognostic factors associated with local recurrence are limited, and no standard management strategy has been provided [7].
Furthermore surgical removal may be technically difficult due to many factors, including unfavorable nodule location, dense fibrosis or altered local anatomy resultant from previous intervention; also patient’s factors, including advanced age and multiple comorbidities may expose to a higher risk of peri-operative complications [8]. In these scenarios, less invasive treatment strategies, namely percutaneous ablation, may represent an attractive option to achieve oncologic control.
The aim of our study is to assess the feasibility, safety, and clinical outcomes of image-guided percutaneous MWA for local recurrence of RCC in patients initially treated with nephrectomy with curative intent.
Methods
This single-center prospective study was approved by the Institutional Review board and informed consent was given by all patients.
Patients
From June 2014 to June 2018, 10 consecutive patients (3 women and 7 men; average age 72 years, range 66–80 years) underwent CT-guided percutaneous MWA of a total of ten nodules in the renal fossa after radical nephrectomy for RCC.
Patient history was analyzed and images were completely reviewed within a multidisciplinary team, composed of an Urologist, Oncologist, and Interventional Radiologist.
Treatment options were discussed among the experts, and a consensus was achieved; the procedure was consequently proposed to the patient. Before treatment choice, all patients received a full explanation of the procedure focusing on benefits and risks. A complete check of their general clinical condition was performed by an anaesthesiologist.
Inclusion criteria for MWA treatment were as follows: histologically verified retroperitoneal metastases from RCC, previous surgery, lesion dimension no larger than 3 cm, no other metastatic site elsewhere.
All patients had preoperative CT or MR examination, and in all cases a preprocedural percutaneous biopsy was performed to confirm the diagnosis of retroperitoneal metastases from RCC.
Coagulation blood tests resulted within the reference values in all patients. Eventually ongoing anticoagulant or anti-platelet therapies were discontinued at least 7 days before the procedure, and low molecular weight heparin was started if necessary [9]. A first-generation cephalosporin (cefazolin 2 gr b.i.d.; Pfizer Srl, Milan, Italy) was administered at the beginning of every procedure as antibiotic prophylaxis.
The patient and tumoral nodule features are summarized in Table 1.
Procedure
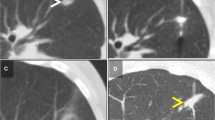
Percutaneous MWAs was performed under CT-guidance (GE LightSpeed VCT 64, General Electric Healthcare, Milwaukee, WI) with the patient under moderate sedation; the most favorable decubitus was chosen to enable a direct needle access to the lesion. If deemed necessary, pneumodissection was induced to displace adjacent organs before ablation to preserve them from heat damage (Fig. 1a–d). In 2 patients, the antenna was placed under US guidance (Philips iU 22; Philips Epic; Best, The Netherlands) and its correct location was confirmed by CT images.
All procedures were carried out by two Interventional Radiologists with more than 10 years of experience in thermal ablation procedures.
Routine vital signs (blood pressure, heart and respiratory rate), together with oxygen saturation and electrocardiographic tracing, were continuously recorded and monitored by an anaesthesiologist.
The ablation system comprises a microwave generator, capable of producing a power output of 100 W at 2450 MHz, which is connected to a 13.5 Gauge straight antenna with a 2.8 cm radiating section by coaxial cable (Emprint/Covidien-Medtronic, Boulder, CO, USA). After the antenna deployment into the target nodule, thermal energy was delivered to treat all study tumors by using a power output of 100 W maintained for a time between 2 and 4 min, as specified by the manufacturer, to produce the desired necrosis area (Fig. 2a–d).
After the completion of the procedure, all patients were admitted overnight for observation and discharged the following day, if clinically stable.
Outcome
Data were assessed for technical success, clinical success, and safety. Ablation time, complications, follow-up length, and disease relapse.
Technical success was defined as the correct placement of the MW probe into the target lesion, as planned before treatment.
Clinical success was defined on the basis of the absence of residual or recurrent disease. Notably, residual disease or incomplete ablation was defined as the evidence of enhancing tissue within or immediately adjacent to the ablative zone at CT scan performed at least after 1 month. In these cases, all partially ablated lesions would be treated with a second MWA session if indications and risks remained valid. Local recurrence instead was defined as the presence of viable tumor in the contest of the treated volume at CT scan obtained at least after 3 months.
Follow-up duration was measured from the ablative procedure to the last imaging evaluation.
The effectiveness of the technique was evaluated on the basis of imaging characteristics, using RECIST criteria [10]. Adverse events were graded into “major” and “minor” complications according to the SIR (Society of Interventional Radiology) classification [11]. Major complications were defined as events that, if untreated, could be life-threatening, or could lead to substantial morbidity and disability, or require hospital readmission, or substantially lengthen the patient’s hospital stay [12]. Minor complications included typical symptoms of the post-ablation syndrome (pain, fever, nausea, and vomiting) if lasting more than 4 days after the ablation procedure.
Procedure parameters were registered in all patients (Table 1).
Results
In our cohort of patients, mean time to recurrence after radical nephrectomy was 14 months (range 8–20 months), and the mean nodule diameter was 20 mm (range 10–30 mm). Histological RCC subtypes were clear cell (6/10) and papillary (4/10).
Technical success was obtained in 100% of patients; all procedures were carried out successfully using the established protocol. Mean ablation time per patient was 3.2 min (range 2–4 min).
In one patient, residual enhancing tissue was noticed on post-procedural contrast-enhanced CT, therefore, this one case was re-treated to complete oncologic response with repeat MWA.
No major complications were recorded; one patient developed an asymptomatic retroperitoneal hematoma that did not require any specific treatment, and one patient reported transient neuralgic pain.
Mean follow-up length was 26 months (range 12–48 months); no patients demonstrated local recurrence after thermal treatment at the last follow-up evaluation.
Discussion
To the best of our knowledge, this is the first case series evaluating the feasibility, efficacy, and clinical outcome of image-guided percutaneous MWA for RCC local recurrence in patients initially treated with radical nephrectomy with curative intent. This technique proved to be feasible, safe, and effective in the cohort of patients involved in this study; no major complications were observed, all procedures were successfully performed, and no patient developed local recurrence at a mean follow-up evaluation of 26 months.
RCC relapse in the renal fossa after nephrectomy is fairly uncommon; therefore, given that the existing data on the natural history and outcome of these patients are limited, it represents a significant therapeutic dilemma. In fact, in the recent literature, no consensus treatment strategy has been proposed [7].
The findings from retrospective studies suggest that a significant proportion of patients without evidence of systemic disease may benefit from a surgical approach in terms of long-term oncologic control and longer overall survival [13]. Likewise, in light of the moderate rate of peri-operative complications, surgical excision of loco-regional relapse should be regarded in selected patients as a potentially curative option.
Nevertheless, due to patient’s comorbidities or unsuitable tumor site, repeating surgery is not always applicable: in these scenarios, it is timely to consider whether minimally invasive approaches, namely percutaneous thermal ablative procedures, could be encouraged.
Over the last years, in an effort to provide a less invasive, nephron-sparing treatment for subjects who cannot or do not wish to undergo surgery [2], percutaneous thermal ablation has been increasingly employed in routine clinical practice for RCC treatment, especially for small cortical tumors (≤ 3 cm) in patients who are at high surgical risk, with a single functioning kidney or renal impairment, hereditary, or multiple bilateral tumors [14]. Nonetheless, due to lack of evidences, it still remains unclear if the same therapeutic efficacy could be applied to loco-regional relapse not suitable for surgical re-intervention. Our work yields new insights into this complex and uncertain field.
Previous studies have investigated the role of percutaneous ablation in this setting. McLaughlin et al. described the first published experience with percutaneous RFA therapy under CT-guidance of an isolated recurrence in the nephrectomy bed [15]; the patient well tolerated the procedure and remained free from disease at 16 months follow-up evaluation.
Hyun et al. [16] favorably examined the feasibility, outcomes, and cost-effectiveness of percutaneous CRA of metastatic RCC performed with palliative intent; they treated 7 patients with 11 lesions in the renal fossa, reporting no major complications or further local recurrence. Moreover, the authors concluded that CRA may achieve positive results in terms of oncologic control as an adjunctive tool, while remaining cost-effective.
Monfardini and colleagues further investigated the role of RFA in this context [17]. The authors looked at 8 patients previously treated with radical (n = 4) or partial (n = 4) nephrectomy for RCC; in 6 patients the procedure was carried out percutaneously under US guidance, with the final placement of the probe confirmed by CT, whereas in the two other patients presenting pancreatic metastases a laparotomic approach was preferred. All tumoral nodes were completely ablated with no evidence of residual enhancing tissue at post-procedural CT and at the last CT examination. No procedural complications were observed among patients treated percutaneously. After a mean follow up of 12 months, 5 patients were disease-free, 1 developed a single relapsed nodule not suitable for further percutaneous therapy, and the 2 last patients demonstrated metastatic lung disease.
Finally, Zhou et al. [8] reported a slightly larger but less homogeneous cohort of patients (n = 11) treated by RFA, CRA, or MWA under CT-guidance following radical (n = 3) or partial (n = 8) nephrectomy. The procedures yielded high technical success and no major complications were observed; regarding complications, they noted a single asymptomatic hematoma that did not require any specific treatment. In one case, a repeat ablation was able to achieve complete oncologic response.
This work has the following limitations; firstly, given the rarity of local relapse after radical nephrectomy, the patient cohort was not statistically significant: prospective randomized clinical studies are required to define the clinical benefits of this technology in comparison with conventional surgical treatment and the other thermal ablation modalities in terms of safety and patient outcomes. Therefore, our data do not provide unequivocal answers about the value of MWA therapy for loco-regional RCC recurrence; however, our results are encouraging, and may stimulate further studies to clarify this relevant issue.
Image-guided percutaneous MWA is a safe and effective option for loco-regional relapse of RCC following nephrectomy. Hence, we believe that it could be considered among the treatment regimens for locally recurrent RCC, in patients who are not eligible for surgery.
References
SEER Cancer Statistics. Kidney and Renal Pelvis Cancer - Cancer Stat Facts [Internet]. NIH. 2018 [cited 2019 Feb 18]. https://seer.cancer.gov/statfacts/html/kidrp.html
Krokidis ME, Orsi F, Katsanos K, Helmberger T, Adam A. CIRSE Guidelines on percutaneous ablation of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2017;40:177–91.
Bartoletti R, Cai T, Tosoratti N, Amabile C, Crisci A, Tinacci G, et al. In vivo microwave-induced porcine kidney thermoablation: results and perspectives from a pilot study of a new probe. BJU Int. 2010;106:1817–21.
Chan P, Vélasco S, Vesselle G, Boucebci S, Herpe G, Debaene B, et al. Percutaneous microwave ablation of renal cancers under CT guidance: safety and efficacy with a 2-year follow-up. Clin Radiol Elsevier. 2017;72:786–92.
Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6:S65–S6969.
Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol. 2000;164:322–5.
Acar Ö, Şanlı Ö. Surgical management of local recurrences of renal cell carcinoma. Surg Res Pract. 2016;2016:1–6.
Zhou W, Herwald SE, Uppot RN, Arellano RS. Image-guided thermal ablation for non-resectable recurrence of renal cell cancer following nephrectomy: clinical experience with eleven patients. Cardiovasc Intervent Radiol. 2018;41:1743–50.
Malloy PC, Grassi CJ, Kundu S, Gervais DA, Miller DL, Osnis RB, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20:S240–S249249.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191191.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–6.
Herout R, Graff J, Borkowetz A, Zastrow S, Leike S, Koch R, et al. Surgical resection of locally recurrent renal cell carcinoma after nephrectomy: Oncological outcome and predictors of survival. Urol Oncol Semin Orig Investig. 2018;36:11.e1–.e6.
Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;25:49–56.
McLaughlin CA, Chen MY, Torti FM, Hall MC, Zagoria RJ. Radiofrequency ablation of isolated local recurrence of renal cell carcinoma after radical nephrectomy. Am J Roentgenol. 2003;181:93–4.
Bang HJ, Littrup PJ, Goodrich DJ, Currier BP, Aoun HD, Heilbrun LK, et al. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. J Vasc Interv Radiol. 2012;23:770–7.
Monfardini L, Varano GM, Foà R, Della Vigna P, Bonomo G, Orsi F. Local recurrence of renal cancer after surgery: prime time for percutaneous thermal ablation? Cardiovasc Intervent Radiol. 2015;38:1542–7.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study has obtained the Institutional Review Board (IRB) approval.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ierardi, A.M., Carnevale, A., Rossi, U.G. et al. Percutaneous microwave ablation therapy of renal cancer local relapse after radical nephrectomy: a feasibility and efficacy study. Med Oncol 37, 27 (2020). https://doi.org/10.1007/s12032-020-01354-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01354-0