Abstract
Purpose
To assess the feasibility, safety and clinical outcomes of image-guided percutaneous thermal ablation as salvage therapy for local recurrence of renal cell carcinoma (RCC) in patients initially treated surgically with curative intent.
Materials and Methods
A retrospective review of 11 consecutive patients (M/F = 8:3, mean age = 76 years) who underwent computed tomography (CT)-guided thermal ablation for locally recurrent RCC after partial (72%, 8/11) or radical nephrectomy (28%, 3/11) with a mean time to recurrence of 48 months (range 2–156). Assessment of technical success, complication (peri- and post-procedural), oncological outcome and survival analysis were performed. Patient baseline and follow-up renal function surrogates including creatinine level (Cr) and estimated glomerular filtration rate (eGFR) were statistically compared.
Results
Eleven biopsy-proven recurrent RCC measuring 1.4–3.9 cm (mean = 2.8 cm) were treated with CT-guided thermal ablation. Technical success was achieved in 100% (11/11) of the cases. There were no major complications except for one (9%) asymptomatic hemorrhage (Clavien–Dindo grade I complication). Complete response, local progression-free and overall survival rate were 91, 91 and 82% during the mean follow-up time of 2.5 years (range 0.1–7.1). Renal function was overall stable without significant change at 1 month and last follow-up (p = 0.21; GFR, p = 0.10; creatinine).
Conclusions
Image-guided percutaneous thermal ablation is a feasible, safe and effective for local recurrence after nephrectomy, representing a non-surgical alternative for unresectable disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection in the form of radical or partial nephrectomy is considered the definitive treatment for renal cell carcinoma (RCC) [1, 2]. While most of these patients benefited from favorable oncological outcome and will remain disease-free after surgery, approximately 2–4% of cases relapsed [3]. Treatment options are limited for locally recurrent RCC, and there are currently no consensus treatment protocols. Since standard radiotherapy and systemic therapy yielded little success, repeated surgery is generally regarded as the optimal available option [4,5,6,7]. However, repeat nephrectomy is not always feasible. Unfavorable tumor location invariably precludes a safe margin for resection in cases where local recurrence is proximal to vital anatomical structures, or dense perinephric fibrosis and altered anatomy plane secondary to previous operation [3, 7]. In addition, patient factors including advanced age and multiple comorbidities are not suitable for surgical resection. In these scenarios, less invasive approach that leverages image guidance to target recurrent lesions may represent an attractive option.
Percutaneous computed tomography (CT)-guided thermal ablation is a well-established treatment modality that is now routinely utilized for solid tumors [8]. Thermal energy using radiofrequency ablation (RFA), cryoablation (CA) or microwave (MWA) is effective non-surgical alternatives that are equivalent to nephrectomy for primary renal mass [9,10,11]. In addition, adjunctive maneuvers common in abdominal interventions can be readily adopt to treat anatomically complex tumors [12, 13]. The logical extension from these experiences is that thermal ablation may be equally beneficial for recurrent or metastatic RCC that are not amendable to surgery. Despite encouraging result from contemporary case series [14,15,16], the role of thermal ablation for recurrent RCC remains unclear. In the present study, we describe our single-institution experience to systemically examine the technique, safety and clinical outcomes of computed tomography (CT)-guided percutaneous thermal ablation for unresectable recurrent RCC following nephrectomy.
Materials and Methods
Written informed consent for thermal ablation was obtained from all patients at the time of initial clinical consultation. The internal review board waived the requirement of informed consent to conduct this retrospective study due to logistical challenges. This study was otherwise compliant with the Health Insurance Portability and Accountability Act. Between October 2006 and December 2017, 11 consecutive adult patients (8 males, 3 females; mean age = 77 years, range 60–86 years) underwent CT-guided percutaneous radiofrequency ablation (RFA), cryoablation (CA) or microwave ablation (MWA) for locally recurrent RCC following nephrectomy. All patients had preoperative CT or MR imaging, followed by percutaneous biopsy to establish the diagnosis of RCC. The medical records were reviewed to determine patient baseline characteristics including prior surgery and coexistent morbidities according to ECOG performance status and ASA classification systems [17, 18].
Patient Selection
Patients were referred for thermal ablation by urology when medical comorbidities or unfavorable tumor location precluded surgery. Patients were evaluated in the Interventional Radiology clinic to assess performance status and treatment planning. The choice of thermal ablation modality was made on a case-by-case basis and based on available devices at the time of treatment and operator preference. Tumor demographics including tumor size, location, and histological evaluation were recorded.
Thermal Ablation Techniques
Patients received intravenous procedural sedation or general anesthesia based on the ASA guidelines, where patients with ASA score ≤ 2 received sedation and those with ASA > 3 received general anesthesia. Thermal ablations were performed by two radiologists with 20 years and 15 years of experience in imaged-guided percutaneous thermal ablation of renal masses, using CT guidance (LightSpeed; GE Medical System, Madison, Wisconsin). All RFAs were performed using 17-gauge internally cooled clustered electrode applicators (Covidien Cool-tip TM RF Ablation System & Switching Controller, Medtronic, Minneapolis, MN). Cryoablation were performed using cryoprobes that were 1.3–3.8 mm diameter (PerCryo ®, Endocare ® and Cryocare systems ®, HealthTronics™, Inc., Austin, TX). Microwave ablations were performed using 14- or 16-gauge antenna of the 2.4 GHz AMICA TM system (HS Medical, Boca Raton, Florida). Protective techniques were utilized in selective cases to minimize the risk of injury to adjacent vital organs. Hydrodissection using 0.9% saline was performed to displace adjacent retroperitoneal organs such as bowel, pancreas or psoas muscle [12]. Retrograde pyeloperfusion using 5% dextrose in water was performed in centrally located RCC to protect the ureter during ablation [13].
Assessment of Treatment Results and Clinical Follow-Up
Technical success as defined by CT imaging evidence of ablation zone entirely encapsulates the desired tumor lesions. Safety parameters were assessed based on immediate and 30-day complications according to the Clavien–Dindo classification system and the Society of Interventional Radiology guidelines [19, 20]. All patients were followed up with surveillance CT or MRI studies at the recommended intervals at 1, 3, 6 months and annually thereafter, to determine treatment response and oncologic outcomes using the RECIST criteria as previously described [21, 22]. Repeat thermal ablation was performed when follow-up imaging showed partial response. Local tumor progression was defined as new focal enhancement in the ablation zone on follow-up imaging. In addition, pre- and post-ablation (at 1 month and the last follow-up), blood urea nitrogen (BUN), creatinine levels (Cr) and estimated glomerular filtration rate (eGFR) were used to assess the stability of renal function.
Statistical Methods
Statistical analyses were performed using the Prism statistics software package (GraphPad Software, Inc. La Jolla, CA). One-way ANOVA repeated-measures analysis with Greenhouse–Geisser correction was performed to assess changes in renal laboratory values following salvage thermal ablation. The Kaplan–Meier method was applied to generate survival curves. All statistical tests reported were two-tailed, where p values < 0.05 were considered statistically significant.
Results
Eleven patients with eleven biopsy-proven RCCs were included in this study. Baseline patient demographics and tumor characteristics are summarized in Table 1. The median age was 76 years (range 60–86 years). The majority of patients (10/11 [9%]) had ECOG performance status of > 1. Of these, 7/11 (64%) had cardiovascular and respiratory comorbidities, 4/11 (36%) had non-metastatic extrarenal malignancy, 2/11 (18%) had impaired renal function. The median tumor size was 2.8 cm (range 1.4–3.9 cm). Four of the eleven (36%) recurrent disease were adjacent to the bowel; 2/11 (18%) were adjacent to the ureter; 1/11 (9%) was adjacent to the pancreas; 4/11 (36%) were adjacent to the psoas muscle and abdominal aorta. Histological subtypes were clear cell (8/11, 73%) and papillary (3/11, 27%).
Procedural data are also summarized in Table 1. Mean number of ablations per tumor was 2.5; mean ablation time per patient was 30 min (range 10–36 min); 9/11 (82%) tumors required ≥ 2 overlapping ablations. Adjuvant maneuvers were utilized in 4/11 (36%), including hydrodissection in 4/11 (36%) and pyeloperfusion in 1/11 (9%), respectively. A combined hydrodissection and pyeloperfusion was performed in one patient. There was 1/11 (9%) asymptomatic small hemorrhage (Clavien–Dindo grade I complication, SIR classification A-B) that does not require treatment. No significant changes were observed between pre- and post-ablation BUN (p = 0.77), creatinine (p = 0.63) and eGFR (p = 0.85) (Table 2 and Figs. 1, 2).
Comparison of renal function outcomes following salvage thermal ablation. Related to Table 2. Paired line plots are shown to compare individual patients’ renal function measurements between pre- and post-ablation, and last laboratory follow-up. eGFR is estimated glomerular filtration rate, and BUN is blood urea nitrogen
Analysis of chronic kidney disease (CKD) following salvage thermal ablation. Related to Table 2. Clustered column graph is shown to illustrate no significant progression of CKD in patient cohorts following salvage thermal ablation. CKD stages are defined by KDOQI-US classification system
Treatment outcome is also summarized in Table 1 and Fig. 3. Technical success was achieved for 100% of the tumors. At 1-month imaging follow-up, complete response (CR) was achieved in 10/11 (91%) of tumors (Table 1 and Fig. 4). Partial response (PR) was achieved in 1/11 (9%) of tumor (Fig. 5). This one case was re-treated to complete response with repeat ablation, for an overall complete response rate of 100%. The mean imaging follow-up time was 2.5 years (range 0.1–7.1 years) to assess intermediate-term loco-regional tumor control. During the imaging follow-up time, there was one case of locally recurrent disease detected at 6 months. All but two patients (82%) are alive; two patients died after 30- and 31-month follow-up of cardiovascular death unrelated to the thermal ablation or RCC (Table 3).
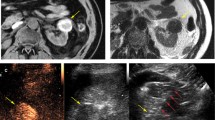
A Axial oral contrast enhanced CT scan of the abdomen in a patient following prior left nephrectomy for RCC. White asterisk indicates recurrent RCC in the left nephrectomy bed. B Unenhanced CT scan of the abdomen obtained at the time of RFA for recurrent RCC in the left renal fossa. White arrow indicates cluster electrodes within the recurrent RCC. Patient is in prone position. C Axial unenhanced CT scan of the abdomen one month after RFA for recurrent RCC in the left renal fossa, (white asterisk). White arrows demarcate zone of ablation. D Axial unenhanced CT scan of the abdomen 7 years after RFA for recurrent RCC in the left renal fossa, (white asterisk). The tumor has undergone significant reduction in size
A Axial contrast material enhanced CT scan of the abdomen in a patient who is s/p right nephrectomy for RCC. Asterisk indicates tumor recurrence in the right renal fossa. B Axial unenhanced CT image at the time of cryoablation of recurrent RCC in the right renal fossa. White arrows demarcate the ice ball. C Axial contrast material enhanced CT scan of the abdomen 1 month after cryoabaltion of recurrent RCC in the right renal fossa. White arrows indicate residual disease
Discussion
Loco-regional recurrence after nephrectomy occurs infrequently but carries a poor prognosis [3, 23, 24]. While surgical management with expiration of recurrent disease offers acceptable oncological outcome, this treatment option is not necessarily feasible for every case. Patients with existing comorbidities, end-stage renal disease or advanced age are generally poor surgical candidates. Similarly, anatomic limitations imposed by postoperative fibrosis, altered anatomy from prior surgery, and recurrent disease adjacent to retroperitoneal structures make it inaccessible for complete resection. An imaged-guided approach with minimally nature of percutaneous ablative therapy may have the potential to circumvent these limitations. This technique has been successful for treating primary renal tumor in non-surgical patients who might otherwise be managed expectantly [9, 10]. However, it remains obscure whether this same therapeutic strategy could be effective for locally recurrent disease that is not amenable to surgery. The results of our present study demonstrate that thermal ablation is a safe and effective treatment modality that offers favorable clinical outcomes for locally recurrent RCC after nephrectomy.
Our study expanded upon the limited knowledge of image-guided thermal ablation for locally recurrent RCC [14,15,16] (Table 3). The first case report by McLaughlin et al. [14] described the use of percutaneous RFA after radical nephrectomy for non-resectable recurrent disease due to close proximity to the abdominal aorta. Although adjunctive maneuver was not performed, this patient successfully underwent the procedure without any complications and remained disease-free during the follow-up time of 16 months. Similarly, Hegg et al. [16] reported the feasibility and efficacy of percutaneous CA after partial nephrectomy. While majority of cases were identified as de novo tumors, there were only three cases of local tumor recurrence that were effectively treated. The feasibility and oncological efficacy of this procedure were further elaborated by the largest case series by Monfardini et al. [15], which consisted of six patients who were treated with percutaneous RFA for local recurrence after radical nephrectomy or partial nephrectomy. There was no complication reported, likely attributed to pneumodissection as a protective measure during the procedure. Although most cases were small (mean size = 1.6 cm) nodal recurrence, the early oncological outcome is encouraging as there was no residual disease after a mean follow-up of 12 months. Drawing from the experience of larger patient cohort, longer follow-up time and all three modalities (RFA, CA and MWA), we aim to provide an update assessment to corroborate the values of imaged-guided percutaneous ablative therapy for unresectable local recurrence after nephrectomy.
In our study, thermal ablation yielded consistent technical success with an overall complete ablation rate of 100%. This demonstrates the feasibility of this technique in locally recurrent RCCs despite difficult anatomy or unfavorable tumor location that preclude complete resection (Table 1). No major peri-procedural complication was encountered, but one (10%) minor complication of hemorrhage was observed that did not require transfusion or unanticipated escalation of care or other long-term consequence. This compares favorably to the 20–35% complication rate reported for repeated surgery [3, 4, 25]. One potential explanation for this difference is that thermal ablation has the technical advantage of image guidance and numbers of adjunctive maneuvers available to facilitate less invasive approach. In this study, local recurrence was mapped and targeted using CT guidance, while adjacent non-targeted tissues such as bowel and ureter were protected by utilizing hydrodissection and retrograde pyeloperfusion. Although limited by a small patient cohort, our result highlights the value of CT-guided ablative therapy to be a minimally invasive approach to treat locally recurrent RCC.
Because of its poor disease prospect, locally recurrent RCC necessitates effective treatment intervention. Using the RECIST criteria to objectively assess treatment response, complete response rate can be achieved in almost all patients (91%, 10/11) except in one case that showed residual disease at 1-month follow-up. Nevertheless, repeat ablation was performed to achieve complete response. Notably, all of the patients achieved RCC-specific survival and remained free of metastases during the 2.5-year follow-up (range 0.1–7.1 years). Similarly, the 5-year disease-free survival rate is promising at 91%, which is at least equivalent, if not more favorable to the 50–100% survival rate of repeat nephrectomy [3, 4, 6, 7, 24]. Our result also showed that all three ablative modalities (RFA, CA and MWA) achieved favorable treatment response and oncological outcome despite their different mechanisms of energy deposition. Despite these encouraging outcomes, our findings must be interpreted with caution since our experience is limited with small patient cohorts. Additionally, future long-term studies are necessary to critically assess the therapeutic durability given the indolent nature of RCC.
Renal function decline is undesirable consequence of surgical expiration of renal parenchyma, predisposing patient to end-stage renal disease and adverse cardiovascular outcome [26]. In this study, no significant decline in renal function was observed between patient’s baseline and one-year post-ablation follow-up. Importantly, this renal outcome remained unchanged at an intermediate follow-up time at 2.5 years. Because our patient underwent nephrectomy and thus predispose to CKD, one critical analysis is to assess disease progression following thermal ablation. There was no significant upstaging of CKD in patients with preexisting disease and minimal new onset of CKD in healthy patients. However, one patient (9%, 1/11) with solitary kidney due to prior radical nephrectomy experienced progressive disease from stage 3A to 4. Given no significant decrease in renal function post-ablation, this particular case may represent the natural expected course of renal decline from solitary kidney and may not necessarily indicative of an adverse impact of thermal ablation on renal function. Regardless, none of the patients requires either temporary or permanent dialysis because of end-stage renal disease. The result of this study confers the renal protective property of thermal ablation for recurrence disease, similar to those reported for primary RCC in patients with preexisting CKD, solitary kidney or multifocal disease requiring multiple ablations [11, 27, 28].
Our study has several limitations. Firstly, the retrospective design and small patient cohort inherently limit the strength of our results. Given the rarity of cases limited to loco-regional recurrence after nephrectomy, a multicenter collaboration would be necessary to conduct prospective clinical trials or retrospective database queries to more clearly define the roles of salvage thermal ablation in sizable patient cohorts. Secondly, direct comparison between thermal ablation and other treatment modalities such as surgery, active surveillance and systemic therapies was not made in the present study; therefore, we cannot draw definite conclusions to recommend the use of salvage thermal ablation. However, our experience yielded encouraging results that may stimulate prospective clinical trials, can rigorously evaluate treatment outcomes and identify patient populations that respond better to certain therapies, ultimately establishing a rational treatment protocols for locally recurrent RCC.
Conclusion
Image-guided percutaneous thermal ablation is a safe and effective salvage treatment option for recurrent renal masses following nephrectomy. Prospective trials should be prioritized to provide quality evidence to establish the clinical outcomes of salvage thermal ablation in comparison with existing treatment regimens for locally recurrent RCCs following nephrectomy.
References
Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–9.
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–24.
Bratslavsky G, Linehan WM. Long-term management of bilateral, multifocal, recurrent renal carcinoma. Nat Rev Urol. 2010;7(5):267–75.
Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol. 2008;180(1):89–93 (discussion).
Sandhu SS, Symes A, A’Hern R, Sohaib SA, Eisen T, Gore M, et al. Surgical excision of isolated renal-bed recurrence after radical nephrectomy for renal cell carcinoma. BJU Int. 2005;95(4):522–5.
Master VA, Gottschalk AR, Kane C, Carroll PR. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005;174(2):473–7 (discussion 7).
Psutka SP, Heidenreich M, Boorjian SA, Bailey GC, Cheville JC, Stewart-Merrill SB, et al. Renal fossa recurrence after nephrectomy for renal cell carcinoma: prognostic features and oncological outcomes. BJU Int. 2017;119(1):116–27.
Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–9.
Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156–61.
Wehrenberg-Klee E, Clark TW, Malkowicz SB, Soulen MC, Wein AJ, Mondschein JI, et al. Impact on renal function of percutaneous thermal ablation of renal masses in patients with preexisting chronic kidney disease. J Vasc Interv Radiol. 2012;23(1):41–5.
Arellano RS, Garcia RG, Gervais DA, Mueller PR. Percutaneous CT-guided radiofrequency ablation of renal cell carcinoma: efficacy of organ displacement by injection of 5% dextrose in water into the retroperitoneum. Am J Roentgenol. 2009;193(6):1686–90.
Cantwell CP, Wah TM, Gervais DA, Eisner BH, Arellano R, Uppot RN, et al. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol. 2008;19(7):1034–40.
McLaughlin CA, Chen MY, Torti FM, Hall MC, Zagoria RJ. Technical innovation—radiofrequency ablation of isolated local recurrence of renal cell carcinoma after radical nephrectomy. Am J Roentgenol. 2003;181(1):93–4.
Monfardini L, Varano GM, Foa R, Della Vigna P, Bonomo G, Orsi F. Local recurrence of renal cancer after surgery: prime time for percutaneous thermal ablation? Cardiovasc Interv Radiol. 2015;38(6):1542–7.
Hegg RM, Schmit GD, Boorjian SA, McDonald RJ, Kurup AN, Weisbrod AJ, et al. Percutaneous renal cryoablation after partial nephrectomy: technical feasibility, complications and outcomes. J Urol. 2013;189(4):1243–8.
Kochhar G, Mehta P, Kalra S, Singh G, Maurer W, Tetzlaff J, et al. Does the ASA physical status classification predict airway or cardiopulmonary complications during routine EGD or colonoscopy utilizing anesthesiologist-directed propofol sedation? A prospective cohort study. Am J Gastroenterol. 2012;107:S751.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, Mcfadden ET, et al. Toxicity and response criteria of the eastern-cooperative-oncology-group. Am J Clin Oncol Cancer. 1982;5(6):649–55.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications—a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2002;13(9):879–81.
Thumar AB, Trabulsi EJ, Lallas CD, Brown DB. Thermal ablation of renal cell carcinoma: triage, treatment, and follow-up. J Vasc Interv Radiol. 2010;21(8):S233–41.
Eisenhauer E, Therasse P, Bogaerls J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline version 1.1. EJC. 2008;6(12):13.
Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol. 2000;164(2):322–5.
Chow JJ, Ahmed K, Fazili Z, Sheikh M, Sheriff M. Solitary renal fossa recurrence of renal cell carcinoma after nephrectomy. Rev Urol. 2014;16(2):76–82.
Magera JS, Frank I, Lohse CM, Leibovich BC, Cheville JC, Blute ML. Analysis of repeat nephron sparing surgery as a treatment option in patients with a solid mass in a renal remnant. J Urol. 2008;179(3):853–6.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
Zhou WH, Uppot RN, Feldman AS, Arellano RS. Percutaneous image-guided thermal ablation for multifocal renal cell carcinoma: 10-year experience at a single center. Am J Roentgenol. 2017;209(4):733–9.
Raman JD, Raj GV, Lucas SM, Williams SK, Lauer EM, Ahrar K, et al. Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int. 2010;105(4):496–500.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, W., Herwald, S.E., Uppot, R.N. et al. Image-Guided Thermal Ablation for Non-resectable Recurrence of Renal Cell Cancer Following Nephrectomy: Clinical Experience with Eleven Patients. Cardiovasc Intervent Radiol 41, 1743–1750 (2018). https://doi.org/10.1007/s00270-018-1976-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1976-2