Abstract
Purpose
The purpose of the article is to evaluate the safety and oncologic efficacy of microwave ablation for metastatic renal cell carcinoma (mRCC).
Materials and methods
From September 2011 to December 2016, 33 mRCC were ablated in 18 patients using percutaneous microwave ablation. Sites of mRCC include retroperitoneum (n = 12), contralateral kidney (n = 6), liver (n = 6), lung (n = 5), adrenal gland (n = 5). Technical success, local, and distant tumor progression, and complications were assessed at immediate and follow-up imaging. The Kaplan–Meier method was used for survival analysis.
Results
Technical success was achieved for 33/33 (100%) mRCC tumors. Ablation provided durable local control for 28/30 (93%) mRCC tumors in 17 patients at a median duration of clinical and imaging follow-up of 1.6 years (IQR 0.7–3.6) and 0.8 years (IQR 0.5–2.7), respectively. In-hospital and perioperative mortality was 0%. There were 5 (15%) procedure-related complications including one high-grade event (Clavien–Dindo III). Four patients have died from mRCC at a median of 1.3 years (range 0.7–5.1) following ablation. Estimated OS (95% CI number still at risk) at 1, 2, and 5 years were 86% (53–96%, 11), 75% (39–92%, 8), and 75% (39–92%, 3), respectively.
Conclusions
Microwave ablation of oligometastatic renal cell carcinoma is safe and provides durable local control in appropriately selected patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Despite improved therapies for patients with metastatic renal cell carcinoma (mRCC) the median overall survival (OS) remains less than 2 years [1]. However, survival for individual patients is variable and approximately 10% of patients are alive 5 years after a mRCC diagnosis [2, 3]. Targeted drug therapies are expensive with estimated annual costs of $125,000–200,000 USD [4]. In addition, systemic mRCC therapies are associated with adverse events including severe fatigue, cardiac and liver toxicity, skin reactions, and pulmonary complications [5]. In some patients with a low volume of metastatic disease, local treatment of metastatic sites with surgery or thermal ablation may preserve quality of life by delaying initiation of systemic therapy. Furthermore, some patients with solitary sites of metastatic disease may have durable disease-free survival after complete metastasectomy [6, 7]. Although no randomized clinical trials have been conducted to demonstrate a survival benefit for metastasectomy, there is compelling retrospective data for local curative treatment of mRCC [8]. As a result, newer treatment guidelines have included recommendations to consider metastasectomy for patients with oligometastatic RCC [9].
While some patients may benefit from metastasectomy, surgery is associated with significant perioperative morbidity. A recent population-based study found that major complications (Clavien–Dindo III–IV) occurred in 25.1% of patients following metastasectomy [10]. In patients with metastasis, who likely have limited longevity, the fear of morbid complications from surgical metastasectomy is well founded. Thermal ablation may be an alternative curative intent treatment for select patients with mRCC, with prior reports demonstrating few procedural complications using percutaneous radiofrequency ablation (RF) and cryoablation [11]. While percutaneous microwave (MW) ablation has been used successfully for treatment of small renal masses, there are no prior studies that describe the use of MW for local treatment of mRCC [12, 13]. Therefore, the purpose of this study is to evaluate the safety and oncologic efficacy of microwave ablation for metastatic renal cell carcinoma.
Materials and methods
Patient selection
This HIPAA-compliant, single-center retrospective study was performed under a waiver of informed consent from the institutional review board. From September 2011 to December 2016, 33 mRCC tumors were treated in 18 patients during 24 ablation sessions. This cohort of consecutive patients was identified from an institutional database and the decision to offer ablation was made by a multidisciplinary team of medical oncologists, urologists, and radiologists experienced in systemic therapeutic options, metastasectomy, and tumor ablation. Clinical factors influencing decision-making were size and location of mRCC, histology of primary and mRCC (when available), extent of metastatic disease, disease-free interval (DFI), proximity of non-target anatomy, age, performance status, and comorbidities. Microwave ablation was performed by one of five radiologists in conjunction with urologists experienced in tumor ablation.
Ablation procedure
All procedures were performed in a CT suite (GE Optima 580W, Waukesha, WI) under general anesthesia. Immediately prior to the procedure, a single dose of intravenous prophylactic antibiotics was administered to cover skin flora according to the routine standard of care at our institution. Ultrasound (GE LOGIQ, Waukesha, WI) and/or computed tomography fluoroscopy (GE LightSpeed 580, Waukesha, WI) was utilized for applicator placement. The MW device (Certus 140, NeuWave Medical; Madison, WI) was a 2.45 GHz, gas-cooled system with 17-gauge antennas (PR) that can be powered simultaneously. Our heuristic for treating mRCC, depending on the shape of the tumor, was 1 antenna for tumors < 2 cm, 2 antennas for tumors 2-3 cm, and 3 antennas for tumors > 3 cm. Hydrodisplacement was performed when non-target anatomy was within 1 cm of the expected zone of ablation. Faintly radiopaque normal saline or D5 W (2% iohexol solution) was infused through an 18- or 20-gauge introducer positioned between the tumor and non-target structure until adequate displacement was achieved.
Immediately following the ablation procedure, contrast-enhanced CT (CECT) was performed to evaluate technical success and complications. Patients were either discharged on the day of the procedure or admitted overnight for observation. Restaging CECT and/or magnetic resonance (MRI) was performed at target intervals of 3 months.
Data collection and analysis
Clinical, pathological, and procedural data were collected for each patient. Clinical data collected included patient age, gender, treatment of primary RCC, onset and extent of mRCC, and timing of systemic therapy. Charlson comorbidity index (CCI) excluding age and ECOG (Eastern Cooperative Oncology Group) status at the time of the ablation procedure were recorded [14, 15]. Pathologic data collected of the primary renal carcinoma included histology, nuclear grade, and stage according to the American Joint Committee on Cancer TNM system [16]. Pathologic data collected of the mRCC included tumor size, location, histology, and nuclear grade when available. Percutaneous biopsy confirmed the diagnosis of mRCC in 19 tumors (19/33, 58%) in 13 patients (13/18, 72%). The presumptive diagnosis of mRCC was made in the remaining patients based upon new or enlarging soft tissue mass on surveillance imaging. Procedural data included volume of hydrodisplacement, complications, and duration of hospitalization. Complications were classified according to the revised Clavien–Dindo system [17].
Continuous features were summarized with medians and interquartile ranges (IQR) and categorical features were summarized with frequency and percentages. Two fellowship trained abdominal radiologists experienced in tumor ablation (SAW, SLA) reviewed imaging in consensus for technical success, complications, and disease progression. Established criteria were used to define treatment success [18]. Local progression-free survival and overall survival (OS) were estimated using the Kaplan–Meier method. Survival was defined from the date of the first ablation procedure to the date of local progression, progressive metastatic disease on follow-up imaging, and death. Statistical analysis was performed using STATA® (Version 14, College Station, TX) software package.
Results
Patient and procedure data
Median age at time of ablation was 66 years (IQR 62–75). Patients were obese (median BMI 33 kg/m2, IQR 28–36), predominantly male (15/18, 83%) with a median CCI of 7 (range 6–14), and an ECOG performance status of 0 (13/18, 72%) or 1 (5/18, 28%) at the time of ablation.
Clear cell subtype (13/18, 72%) was the predominant primary RCC histology. Nuclear grade was variable and included 5 patients with grade II (28%), 3 patients with grade III (17%), and 5 patients with grade IV (28%). Stratified by T stage, there were 2 patients with T1a (11%), 2 patients with T1b (11%), 2 patients with T2a (11%), 5 patients with T3a (28%), and 2 patients with T4 (11%). The primary RCC were treated with nephrectomy (14/18, 78%), partial nephrectomy (3/18, 17%), and embolization (1/18, 6%).
Four patients (22%) had synchronous metastatic disease at initial diagnosis of RCC and 14 patients (78%) developed mRCC at a median of 3.4 years (IQR 1.7–7.4) following surgery. Clear cell (n = 7) and unspecified (n = 6) were the most common histologic mRCC subtypes from the metastatic tumors. Four patients (22%) received systemic therapy prior to ablation which was continued after ablation, four patients (22%) were initiated on systemic therapy at a median of 1.6 years (range 1.1–2.0) after ablation and 10 patients (56%) have not received any systemic therapies. Nine patients (50%) had metastatic tumors in a solitary anatomic site and 9 (50%) had oligometastatic disease. Microwave ablation was performed at a median of 2.6 years (IQR 0.6–5.4) following the onset of metastatic disease. In the four patients who were on systemic therapy prior to ablation, MW ablation was performed at a median of 1.3 years (IQR 0.3–3.0) from the onset of metastatic disease. For the 14 patients who did not receive systemic therapy before ablation, median time from diagnosis of mRCC to ablation was 3.0 years (IQR 0.3–5.6).
A total of 33 mRCC tumors were ablated in 18 patients during 24 treatment sessions. Hydrodisplacement was used prior to the ablation of 15 mRCC (44%) with a median volume of 525 mL (range 80–1300 mL). Twelve patients (67%) had a single session treatment with 1 mRCC tumor ablated. The remaining 6 patients (33%) had more than 1 mRCC tumor ablated including 4 patients (12%) who developed new sites of mRCC that were treated with ablation, after the initial ablation procedure. Median tumor diameter was 1.7 cm (IQR 1.2–2.5). Sites of mRCC include retroperitoneum (n = 12, 30%; median tumor diameter 1.6 cm), contralateral kidney (n = 6, 18%; median tumor diameter 1.7 cm), lung (n = 5, 15%; median tumor diameter 1.2 cm), adrenal gland (n = 5, 15%; median tumor diameter 2.0 cm), and liver (n = 5, 15%; median tumor diameter 1.0 cm). Clinical and pathological characteristics are summarized in Table 1.
Patients were discharged within 1 day after 23/24 (94%) ablation treatment sessions. One patient was hospitalized for observation for 2 days following ablation.
Follow-up
Immediate technical success of MW ablation for mRCC was achieved for 33/33 (100%) tumors (Figs. 1, 2). Clinical follow-up was available for all patients (100%) and imaging follow-up was available for 17 patients (94%) with 30 (91%) ablated mRCC tumors. The one patient with three ablated mRCC without imaging follow-up was excluded from survival analysis. Median duration of clinical and imaging follow-up was 1.6 years (IQR 0.7–3.6) and 0.8 years (IQR 0.5–2.7).
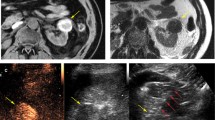
Six months post-nephrectomy surveillance chest CT (A) demonstrates a new solitary 1.3 cm pulmonary metastasis (white arrowhead). A single microwave antenna was placed with CT fluoroscopy guidance (B). Immediate post-procedure chest CT (C) demonstrates dramatic tumor contraction and ground glass in and around the ablation zone. Follow-up chest CT (D) shows a scar without evidence of local tumor progression (yellow arrowhead)
Surveillance enhanced abdomen CT (A, B) demonstrates slow growth of a solitary right retroperitoneal metastasis (white arrowhead). Three microwave antennas were placed with CT fluoroscopy guidance (C). Hydrodisplacement was used to protect the colon. Follow-up enhanced abdomen CT (D) demonstrates decreased size and lack of enhancement of the index tumor (yellow arrowhead) without evidence of local tumor progression
Oncologic efficacy
Microwave ablation provided durable local control for 28/30 (93%) mRCC. Local tumor progression (LTP) occurred in 50% (2/4) of patients who received systemic therapy prior to ablation and 0% (0/13) of patients who have not received systemic therapy or received systemic therapy only after ablation. Of the two patients with LTP, both had biopsy-proven mRCC (4.5 cm retroperitoneum, 3.6 cm adrenal) with aggressive primary tumor RCC histology (renal vein invasion, clear cell RCC with sarcomatoid and rhabdoid features). Local progression was evident on initial imaging follow-up (3 months) for both patients. Both patients proceeded to systemic therapy, without repeat ablation, due to progression of metastatic disease elsewhere. Stratified patient disease status, ablation results, and survival status are summarized in Table 2. The estimated 2-year local progression-free survival rate was 80% (95% CI 59–91%). (Fig. 3).
Median OS was shorter for patients who received systemic therapy prior to ablation compared to patients who have not received systemic therapy or received systemic therapy after ablation (2.0 vs. 5.1 years, p = 0.03) (Fig. 4). Four patients (22%) died at a median of 1.1 years (range 0.7–5.0) after ablation. All deaths were attributed to distant progression of mRCC. Estimated OS (95% CI number still at risk) at 1, 2, and 5 years were 86% (53–96%, 11), 75% (39–92%, 8), and 75% (39–92%, 3), respectively (Fig. 5).
Kaplan–Meier estimate of overall survival from date of first ablation for 4 patients who received systemic therapy before ablation and 14 patients who received ablation before systemic therapy/patients who have not received systemic therapy. The median OS was significantly shorter for patients who received systemic therapy prior to ablation (2.0 vs. 5.1 years, p = 0.03)
Complications
There were 5 (15%) procedure-related complications including four Clavien–Dindo Grade I complications and one Clavien–Dindo Grade III complication. The Grade III complication was a retroperitoneal abscess that occurred after the ablation of a nephrectomy bed metastasis, due to a colonic injury. The abscess was managed with percutaneous drain and oral antibiotics and the colon injury healed without further intervention. One other patient required readmission within 30 days of ablation for chest pain and hypoxia after a technically successful lung metastasis ablation. There were no grade II, IV, or V complications. Procedure-related and 30-day mortality were 0%.
Discussion
Patients with mRCC are generally treated with systemic therapy to slow down disease progression, improve disease related symptoms, and ultimately prolong survival. While anti-VEGF tyrosine kinase inhibitors have improved OS in patients with mRCC, they are not curative and are associated with a wide spectrum of side effects that may erode patients’ quality of life. Therefore, in carefully selected patients with slow growing and limited metastatic disease, local curative therapies can improve patient’s quality of life and survival. In a retrospective analysis of outcomes in 278 patients with mRCC by Kavolius et al., there was a significant improvement in 5-year OS for metastasectomy (44%) over non-surgical treatment (11%) [19]. Multiple other retrospective studies have also shown some improvement in OS after metastasectomy [20,21,22,23,24]. However, careful patient selection is the key to avoid invasive procedures that provide neither survival advantage nor palliative benefit. DE novo metastatic disease, presence of constitutional symptoms at the time of initial diagnosis, DFI of less than 2 years following nephrectomy and high-grade primary RCC are associated with poor prognosis and shorter OS [19,20,21,22,23,24,25,26]. In our study, we observed a lower rate of local control for patients with mRCC from high-grade primaries and DFI < 2 years compared to patients with mRCC from low-grade primaries and a DFI > 2 years (60% vs. 100%, respectively).
Historically, percutaneous ablation of metastatic tumors was reserved for patients with small renal, liver, and lung tumors who were unfit for surgery. As ablation technology has evolved and procedural expertise matured, the size of tumors that can be successfully treated with percutaneous ablation has increased. Cryoablation and modern MW ablation devices harness thermal synergy to create larger, more confluent ablations, and adjuvant maneuvers, such as hydrodisplacement, can be used to displace non-target anatomy, in order to ablate larger tumors safely [27,28,29,30]. In our study, a 93% rate of local control at a median follow-up of 1.6 years was achieved for tumors with a median diameter of 1.7 cm. This resulted in an improved median OS of 5.1 years with estimated 1-, 2-, and 5-year OS rates of 86, 75, and 75%, respectively. Atwell et al. reported a similar rate of local control (92%) at a median of 1.4 years following RF and cryoablation of bone and soft tissue mRCC [31]. At a mean follow-up of just over 2 years, Sago et al. reported a local recurrence rate of 33% in patients with metastatic pulmonary RCC treated for curative intent with RF [32]. Our improved local control of pulmonary mRCC treated with MW is likely due to the physical advantages of MW. Delivery of RF power in the lung is limited by intrinsically high impedance of the air-filled lung, limited even further by charring. In an attempt to reduce charring, RF power is either ramped or pulsed. These factors combine to limit size of RF ablations in the lung. Microwaves, on the other hand, use electromagnetic heating, and not electrical current, to heat a volume of tissue around the applicator. As a result, MW heating is not limited by aerated lung or charring. Further, MW applicators can be powered continuously and simultaneously at maximum power. These factors combine to create hotter, larger, and more confluent ablations that are more likely to encompass the index tumor and margin [33].
The major advantages of MW ablation compared to metastasectomy are favorable morbidity and recovery time in a patient population that has a limited lifespan. Patients are understandably reluctant to pursue metastasectomy when they may be faced with pain and activity limitations during a prolonged recovery after surgery, worse if complications ensue. Percutaneous ablation procedures are well tolerated, can be performed on an outpatient basis or with overnight in-hospital observation, have an abbreviated convalescent period, and are associated with very low complication and readmission rates [11,12,13, 27,28,29,30,31]. The complication profile in our study certainly compares favorably to metastasectomy where overall and major complication rates of 30–48% and 25%, respectively, have been reported [10]. Our overall complication rate was 15% with only one high-grade (Clavien–Dindo III) complication. The other 4 complications were minor (Clavien–Dindo I) and did not prolong recovery time. Also, percutaneous ablation was associated with lower morbidity in sicker patients, when compared to metastasectomy. Meyer et al. reported that a high comorbidity burden (CCI ≥ 2) (OR 2.41, 95% CI 1.60–3.62) was a predictor of major complications following metastasectomy [10]. Our favorable complication profile was achieved in the face of a highly comorbid cohort with a median CCI of 7. In-hospital, perioperative and ablation-related mortality in our study were 0%, which also compares favorably to metastasectomy where in-hospital and perioperative mortality rates of 2.4% and 0.9–2.3%, respectively, have been reported [10]. Further, 94% (17/18) of patients were discharged within 1 day and only 2 patients were readmitted within 30 days.
Limitations of our study include a risk for selection bias, which should be acknowledged in all studies evaluating the efficacy and/or survival of metastasectomy or local treatment for mRCC. Patients who have surgery, radiation, or ablation of mRCC are selected for better prognosis; therefore, it is difficult to clearly demonstrate an impact on survival. Our patients were selected by a multidisciplinary team and represent a contemporary practice at a tertiary referral center. Importantly, ablation may extend the time from diagnosis of metastatic disease to initiation of systemic therapy, acknowledging that most patients ultimately progress to systemic therapy [34]. Finally, similar to other early reports of RF and cryoablation for mRCC, the follow-up interval may be relatively short to assess durable oncologic efficacy [10]. However, duration of follow-up should be interpreted in light of a median OS of less than 2 years for patients with mRCC [1].
In conclusion, MW ablation offers durable local oncologic control in appropriately selected patients and could be considered as an alternative treatment option to metastasectomy for patients with mRCC. Further, microwave ablation procedures appear safe and well tolerated even in comorbid mRCC patients.
References
Heng DY, Xie W, Regan MM, et al. (2013) External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol 14(2):141–148
Choueiri TK, Rini BI, Garcia JA, et al. (2007) Prognostic factors associated with long-term survival in previously untreated metastatic renal cell carcinoma. Ann Oncol 18(2):249–255
National Cancer Institute (2014) SEER cancer stat facts: kidney and renal pelvis cancer. Bethesda, MD: National Cancer Institute.
Geynisman DM, Hu JC, Liu L, Tina Shih YC (2015) Treatment patterns and drug costs for patients with metastatic renal cell carcinoma in the United States. Clin Genitourin Cancer 13(2):E93–E100
Eisen T, Sternberg CN, Robert C, et al. (2012) Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst 104(2):93–113
Karam JA, Wood CG (2011) The role of surgery in advanced renal cell carcinoma: cytoreductive nephrectomy and metastasectomy. Hematol Oncol Clin N Am 25(4):753–764
Alt AL, Boorjian SA, Lohse CM, et al. (2011) Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 117(3):2873–2882
Dabestani S, Marconi L, Hofmann F, et al. (2014) Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol 15(12):E549–E561
Ljungberg B, Bensalah K, Canfield S, et al. (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5):913–924
Meyer CP, Sun M, Karam JA, et al. (2017) Complications after metastasectomy for renal cell carcinoma: a population-based assessment. Eur Urol 72(2):171–174
Thompson RH, Atwell T, Schmit G, et al. (2015) Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2:252–259
Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. (2017) Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology 284(1):272–280
Wells SA, Wheeler KM, Mithqal A, et al. (2016) Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single-session treatment. Abdom Radiol 41(6):1203–1211
Oken MM, Creech RH, Tormey DC, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Charlson ME, Pompei P, Alex KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC cancer staging manual, 7th edn. New York: Springer
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Ahmed M, Solbiati L, Brace CL, et al. (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol 25(11):1691–1705
Kavolius JP, Mastorakos DP, Pavlovich C, et al. (1998) Resection of metastatic renal cell carcinoma. J Clin Oncol 16:2261–2266
Kanzaki R, Higashiyma M, Fujiwara A, et al. (2011) Long-term results of surgical resection for pulmonary metastasis from renal cell carcinoma: a 25-year single-institution experience. Eur J Cardiothorac Surg 39:167–172
Staehler MD, Kruse J, Haseke N, et al. (2010) Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J Urol 28:543–547
Piltz S, Meimarakis G, Wichmann MW, et al. (2002) Long-term results after pulmonary resection of renal cell carcinoma metastases. Ann Thorac Surg 73:1082–1087
Friedel G, Hurtgen M, Penzenstadler M, Kyriss T, Toomes H (1999) Resection of pulmonary metastases from renal cell carcinoma. Anticancer Res 19(2C):1593–1596
Fourquier P, Regnard JF, Rea S, Levi JF, Levasseur P (1997) Lung metastases of renal cell carcinoma: results of surgical resection. Eur J Cardiothorac Surg 11(1):17–21
Leibovich BC, Cheville JC, Lohse CM, et al. (2005) A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol 17:1759–1763
Leibovich BC, Han KR, Bui MH, et al. (2003) Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 12:2566–2575
Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr (2010) Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 8:S192–S203
Atwell TD, Vlaminck JJ, Boorjian SA, et al. (2015) Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol 26(6):792–799
Ziemlewicz TJ, Hinshaw JL, Lubner MG, et al. (2015) Percutaneous microwave ablation of hepatocellular carcinoma with a gas-cooled system: initial clinical results with 107 tumors. J Vasc Interv Radiol 1:62–68
Egashira Y, Singh S, Bandula S, Illing R (2016) Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: a retrospective single-center experience. J Vasc Interv Radiol 4:474–479
Welch BT, Callstrom MR, Morris JM, et al. (2014) Feasibility and oncologic control after percutaneous image guided ablation of metastatic renal cell carcinoma. J Urol 192(2):357–363
Soga N, Yamakado K, Gohara H, et al. (2009) Percutaneous radiofrequency ablation of unresectable pulmonary metastases from renal cell carcinoma. BJU Int 104(6):790–794
Brace CL, Hinshaw JL, Laeseke PF, Sampson LA, Lee FT Jr (2009) Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 251(3):705–711
van der Zanden LFM, Vermeulen SH, Oskarsdottir A, et al. (2017) Description of the EuroTARGET cohort: an European collaborative project on targeted therapy in renal cell cancer genetic and tumor-related biomarkers for response and toxicity. Urol Oncol 35(8):529.e9–529.e16
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
TJZ, JLH, FTL, and SAW are paid consultants for Ethicon Inc. MGL receives grant support from Phillips and Ethicon Inc. FTL is on the board of directors of HistoSonics. The remaining authors have no relevant disclosures.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Maciolek, K.A., Abel, E.J., Best, S.L. et al. Percutaneous microwave ablation for local control of metastatic renal cell carcinoma. Abdom Radiol 43, 2446–2454 (2018). https://doi.org/10.1007/s00261-018-1498-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1498-z