Abstract
An important aspect of differentiated thyroid cancer (DTC) management is disease localization by imaging. Functional imaging of thyroid cancer with iodinated radiotracers has been employed for metastatic disease detection for long. More recently, 2-deoxy-2-[18F] fluoro-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT), a non-iodinated ubiquitous PET tracer, has been used to detect non-radioiodine (RAI) avid disease. Advances in molecular imaging have led to the development of newer tracers like 18F-TFB (18F-tetrafluoroborate) that are transported through the sodium–iodide symporter (NIS) as well as 68 Ga-DOTATATE that image the somatostatin receptors sub-type 2 expressed in medullary thyroid cancer and some DTC. In coming years, there will be focus on newer receptor targets like prostate-specific membrane antigen expression and endoradiotherapies and theranostics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although there has been debate among experts whether this represents a real increase in disease incidence, rather than simply increased recognition of extant disease, recent evidence suggests that there has, in fact, been a 6.1% rise in annual incidence rates of advanced-stage PTCs since 1983 and that annual thyroid cancer mortality has risen by 1.1% since 1994 [1].
A key element of thyroid cancer management is disease localization by imaging. Functional imaging of thyroid cancer with radioiodine tracers has given birth to the entire field of nuclear medicine, and these radiotracers have been employed for metastatic disease detection for a long time as has, more recently, been 2-deoxy-2-[18F] fluoro-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT), a non-iodinated PET tracer. Important roles are also played by anatomic imaging with cervical ultrasonography commonly and with CT and MR of the neck, chest, liver, and skeleton in selected cases. In the era of precision medicine, it is imperative that imaging resources delivering significant radiation doses and significant cost be optimally targeted meet each patient’s diagnostic, prognostic, and therapeutic needs [2].
Newer radiotracers are now available that can detect residual disease in some challenging patients in whom radioiodine imaging and 18F-FDG PET/CT imaging fail to localize lesions. In this review, we have summarized the current state of thyroid cancer molecular imaging.
Sodium–iodide symporter (NIS) and role in thyroid cancer imaging
Most iodine radiotracers take advantage of the NIS to concentrate in thyroid follicular cells. NIS is not expressed in the parafollicular C cells or the Hurthle cells. NIS (Na+/I−) was molecularly characterized in 1996, and since then its role has been recognized as critical to the diagnosis and treatment of differentiated thyroid cancer (DTC). It transports I- and many other substrates (such as perchlorate (ClO4−) and chlorate (ClO3−)) with either an electronic or a neutral stoichiometry [3].
In a study done to characterize NIS expression in thyroid disease states, it was shown that NIS expression was decreased in over 90% of thyroid carcinomas by as much as 1200-fold [4]. Postulated mechanisms include damage to the DNA by ionizing radiation, decreased expression of SCL5A5 and/or diminished membrane targeting [5, 6].
Despite the decreased amount of symporter in thyroid carcinomas, NIS remains expressed at a level that allows for the localization of iodine radiotracers and the utility of those radiotracers for imaging and therapy. Indeed, autophagy activity strongly correlates with good response to radioiodine therapy (RAI), probably related to the ability to maintain differentiation and iodine uptake [7]. Cyclic-AMP-mediated increase in glycosylation has been shown to enhance the functionality of the NIS and is being investigated as a potential target [8].
Newer radiotracers that utilize the PET-based imaging systems processed through the NIS are being synthesized and investigated and will be discussed in further detail in subsequent sections of this review.
Planar and SPECT imaging of thyroid follicular cells and differentiated thyroid cancer
Diagnostic 123I scan: the first successful experiment in individualized medicine
The diagnostic 123I scan is performed prior to radioiodine therapy to evaluate the patient for the presence of locoregional nodal disease and/or distant metastases and to tailor the therapeutic radioiodine dose to the findings, although some centers prefer an empirical administration of 30–100 m Ci based on initial risk assessment. The American Thyroid Association guidelines state that postsurgical diagnostic whole-body scan (WBS) may be beneficial in cases where the extent of residual disease cannot be determined accurately by conventional imaging and/or the management (either decision to treat or the activity administered) of the patient may be altered by the additional information [9]. The diagnostic scan may be performed by low-dose 123I (1.5–3 mCi) or 131I (1–3 mCi) [9]. 123I diagnostic scans were shown to be superior to diagnostic 131I scans in terms of image quality and sensitivity [10].
At our institution, the dose for the diagnostic scan (with radiotracer 123I) is administered 48 h before radioiodine therapy and images are obtained 24 h prior to the therapy dose. Typically, the patient receives a low-iodine diet for 2 weeks followed by two injections of recombinant thyroid-stimulating hormone (TSH) on day 1 and day 2. Recombinant TSH appears to be like thyroid hormone withdrawal (THW) in achieving adequate lesion 131I uptake [11,12,13]. 123I single-photon emission computed tomography (SPECT) imaging (i.e., volumetric, tomographic images acquired through rotation of the gamma camera heads around the patient) with low-dose computed tomography (CT) for attenuation correction and anatomic localization is not routinely performed unless there are regions of uncertainty in the planar images.
123I diagnostic scans appear to have a high concordance with 131I post-treatment scans for thyroid bed and bone metastases (89 and 86%, respectively), and a relative low concordance for lymph node disease (61%) and lung metastases (39%) as has previously been described [14]. In a study at Yale, it was shown that pre-therapy scans provided important information that changed management in 25% of cases, and for persons demonstrating increased uptake in midline lymph nodes, the percentage was even higher (about 50%) [15].
In summary, the 123I diagnostic scan is an excellent tool for determining treatment dose as well as excluding patients for RAI treatment with no uptake (suggestive of non-radioiodine avid disease).
Post-treatment 131I scan: the “gold standard”
The post-treatment 131I scan is performed after the patient has received a moderate- to high-dose 131I for treatment of disease that is limited to the thyroid bed or elsewhere in the body. The initial steps are outlined above. One day after completing the 123I diagnostic scan, he/she receives the treatment dose as per the American Thyroid Association guidelines based on eligibility and risk of recurrence and mortality [16]. There is debate about the timing of the post-treatment scan with conflicting results [17].
At our institution, the whole-body scan is acquired 1 week after therapy and involves both planar as well as SPECT/CT imaging of the head and neck.
Post-therapy 131I scan for long has been considered the definite test to visualize radioiodine avid DTC. Early studies showed that post-therapy scans may visualize additional lesions compared to the pre-therapy scan in as many as 40% of cases, especially involving lung and lymph node disease [18]. In another study, it was shown that post-therapy scanning changed the disease stage in 8.3% of the patients who were undergoing first ablation and provided valuable information for another 26% of patients who had had a prior ablation [19]. In a study at the Mayo Clinic involving 117 patients, 13% of patients showed additional foci on the post-therapy scan that were not seen on the pre-therapy scan and these findings resulted in change in management in 9% of patients [20].
Additionally, it has been shown that approximately 27% of post-treatment scans may differ from the pre-treatment scan [21]. A positive whole-body scan is related to disease recurrence as well as persistent disease and offers early assessment of long-term risk [22].
PET radiotracers for imaging the sodium–iodide symporter
PET offers better spatial resolution, and the images are more easily quantified for assessment of treatment response or progression when compared to SPECT [23]. Imaging the thyroid follicular cells with PET radiotracers that bind to the NIS offers the potential for high-resolution, high-quality images.
124I PET/CT
124I is a PET radiotracer that binds to the NIS, has a long half-life, and emits high-energy particles including gamma rays and positrons [24]. 124I has a half-life of 4.18 days, and 22% of its emission consists of positrons [25]. In a recent study involving 227 iodine avid metastatic lesions, there was a high level of agreement between pre-therapy 124I PET/CT and post-treatment 131I scan, with concordance rates of 97% (221/227) [26]. However, in another study involving a population that had elevated serum thyroglobulin levels, a negative diagnostic 123I/131I scan, and a negative 124I PET scan, post-treatment scan with 131I was frequently positive, especially if the patient had a prior positive 131I post-treatment scan (implying prior demonstration of radioiodine avidity and/or successful treatment) [27].
In a meta-analysis, our group showed that 124I PET/CT detects residual disease with a very high sensitivity and also images many lesions not visualized by post-treatment 131I scan [28]. It is surmised that some lesions of DTC that have iodine avidity may be seen by the superior imaging characteristics and technology of the PET/CT systems but not visualized on post-treatment 131I scan, especially if the dose is small (30–50 m Ci range). On the other hand, the very high doses administered for a repeat therapy might detect residual disease on the 131I imaging as outlined above [27]. 124I appears to be superior to diagnostic 123I planar imaging in terms of sensitivity and specificity, though large-scale trials are lacking.
124I PET/CT may be useful for 3D dosimetry and planning adequate surgery in cases where the diagnostic 123I scan may be equivocal. Further large-scale studies are required before this expensive and high-radiation technology is incorporated as standard of care.
18F-Tetrafluoroborate—an upcoming promising agent
18F-Tetrafluoroborate (TFB) is a new agent that was recently discovered and has a biodistribution characteristic of NIS expression [29]. After an injection of 24.93 ± 0.05 MBq/kg of 18F-TFB, dosimetry demonstrated that the compound had an effective radiation dose higher than 99m Tc pertechnetate (a very common single-photon-emitting radiotracer that can be used to image the thyroid) but lower than 123I and 131I [29]. It shows accumulation in cells derived from animal models that are stimulated by TSH, achieving an SUV of 72 within 1 h of injection within the thyroid [30]. Compared to 123I SPECT/CT, it has better and faster uptake and better clearance from circulation [31]. 18F-TFB appears to be pharmacologically and radiobiologically safe in humans, and some investigators are currently recommending phase 2 trials [32].
18F-TFB appears to hold promise in the diagnosis and treatment guidance of DTC, and we look forward to the results of further studies with this agent.
18F-FDG PET
18F-FDG PET/CT is most useful for the detection of thyroid cancer that is not radioiodine avid and more aggressive in its behavior. For whole-body-scan-negative patients with persistently elevated thyroglobulin, 18F-FDG PET may detect disease in approximately 60–70% of patients (especially when combined with diagnostic CT scan) [33, 34]. Some other studies report even higher sensitivities [35, 36]. 18F-FDG PET/magnetic resonance imaging (MRI) appears to be less sensitive than 18F-FDG PET/CT for detection of residual disease [37].
The optimal thyroglobulin (Tg) cutoffs for achieving the maximum sensitivity and specificity in the receiver operator characteristic (ROC) curves for this modality range from 12 to 32 ng/ml [33, 34]. Recombinant TSH stimulation prior to PET/CT might improve the diagnostic sensitivity, increase the number of lesions detected, and change the management in a small percentage of cases [38, 39]. However, in another study, 20% of positive PET/CT cases were in persons with Tg less than 10 ng/ml, giving credence to the opinion that it is difficult to establish exact Tg cutoffs for 18F-FDG PET/CT [40].
18F-FDG PET/CT uptake that is detected incidentally but focally within the thyroid (in patients being scanned for reasons unrelated to thyroid cancer) is associated with significantly higher risk of thyroid cancer (ranges from 15 to 40%) [41,42,43]. A higher SUV (> 5.5) coupled with suspicious US features increases the sensitivity to as high as 82% [44]. High 18F-FDG PET/CT uptake has been associated with poor survival in persons with mediastinal metastatic lymph nodes [45]. A negative 18F-FDG PET/CT performed early in intermediate- to high-risk thyroid cancer patients is associated with excellent response to therapy by modified Hicks criteria [35].
18F-FDG PET/CT has long been the imaging agent for medullary thyroid cancer (MTC) with high calcitonin levels. The combination of calcitonin doubling time and 18F-FDG PET/CT positivity has been shown to be a good prognostic factor for MTC [46].
In summary, 18F-FDG PET/CT is an invaluable tool for imaging non-radioiodine avid DTC and MTC. It can also be used as a prognostic marker for morbidity and recurrence.
Newer agents targeting other receptors
Ga 68 DOTATATE PET/CT in thyroid cancer
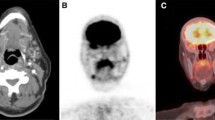
Somatostatin receptor (SST) expression in medullary thyroid cancer (MTC) has now been established [47]. In a study by Papotti et al. [48] looking at the distribution of SST 1–5, 49% of the MTC tumors were positive for sst1, 43% for sst2, 47% for sst3, 4% for sst4, and 57% for sst5. Many of the tumors express more than one receptor types [47]. Ga 68 DOTATATE PET/CT is a radionuclide molecular imaging agent that binds to SST 2 with a very high affinity [49]. A patient with advanced MTC imaged with Ga 68 DOTATATE PET/CT is shown in Fig. 1.
In a small series of patients who had all the mentioned diagnostic tests, Ga 68 DOTATATE PET/CT has been found to be superior to CT, US, MRI, FDG PET/CT as well as MIBG scan in detecting new lesions of MTC especially in patients with very high serum calcitonin[50]. In a study comparing Ga 68 DOTATATE PET/CT with FDG PET/CT and another agent 99mTc-(V) DMSA, Ga 68 DOTATATE PET/CT performed better and detected many more lesions of MTC [51].
Ga 68 DOTATATE PET/CT has a potential to image non-radioiodine tumors like Hurthle cell adenomas that show increased SST-2 receptor expression [52]. Its potential use in non-radioiodine avid DTC needs to be evaluated in clinical trials.
In summary, Ga 68 DOTATATE PET/CT that has been recently FDA-approved is a useful imaging agent for MTC.
PSMA expression and imaging in thyroid tissue
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein that has primarily been investigated as a target for the development of antibodies and small molecules for the detection and treatment of sites of prostate cancer [53, 54]. However, despite the specificity implicit in its name, PSMA is expressed in a variety of normal tissues as well as the tumor neovasculature of many non-prostate cancers [55,56,57]. On a histologic level, PSMA expression occurs on the endothelial cells of tumor neovasculature in both benign and malignant thyroid lesions, although a higher rate of malignant tumors have been found to be PSMA-positive [58]. In a series of case reports, in vivo findings on PET scans with PSMA-targeted agents (labeled with 68Ga) in localized thyroid tumors have demonstrated radiotracer uptake in both papillary and follicular carcinomas as well as follicular adenomas [59,60,61,62].
In the context of metastatic disease, Verburg et al. reported strong PSMA-targeted radiotracer accumulation in a patient with 131I-negative, 18F-FDG-positive poorly differentiated disease affecting cervical lymph nodes and the lungs. A series of six patients (all with iodine-negative, 18F-FDG-positive metastatic differentiated thyroid cancer) were imaged with 68Ga-HBED-CC-PSMA by Lütje and colleagues [63]. Those authors found that 5 of 6 (83%) patients had definable lesions that were avid for the PSMA-targeted radiotracer, although in 2 out of 5 (40%) of those patients, 18F-FDG PET identified more lesions. Nonetheless, the preponderance of the evidence to date would suggest that many patients with metastatic thyroid cancer have lesions that express PSMA. Indeed, distant metastatic disease and radioactive iodine-refractory tumors appear to have some of the highest rates of expression [64].
In summary, PSMA-based agents offer potential for further research and evaluation.
Future directions
Imaging of differentiated and other forms of thyroid cancer is offering new opportunities for clinicians and radiologists. The indolent and slow progression of the disease helps the physician in localizing and treating advanced disease for many years and in some cases decades. It is unclear now whether these technologies offer survival benefit. However, they offer invaluable insight into the molecular biology of these tumors and could serve as laboratory for other malignancies. The theranostics of 131I would continue to be the driving force for the excellent results seen with differentiated thyroid cancer. The use of 18F-based agents may reduce scan time and radiation exposure as well as improve the quality of the images.
In coming years, there will almost certainly be investigations of PSMA-targeted endoradiotherapeutics for the treatment of patients with metastatic thyroid cancer that no longer responds to iodine therapy, in much the same way that endoradiotherapies derived from DOTATATE are being used for metastatic medullary thyroid cancer.
Change history
12 December 2017
The original version of this article unfortunately contained a mistake. The middle name of the author Steven B. Rowe is incorrect. The corrected name is Steven P. Rowe.
References
Kitahara CM, Devesa SS, Sosa JA. Increases in thyroid cancer incidence and mortality-reply. JAMA. 2017;318(4):390–1.
Ladenson PW. Precision medicine comes to thyroidology. J Clin Endocrinol Metab. 2016;101(3):799–803.
Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N. The sodium/iodide symporter (NIS): molecular physiology and preclinical and clinical applications. Annu Rev Physiol. 2017;79:261–89.
Lazar V, Bidart JM, Caillou B, Mahe C, Lacroix L, Filetti S, et al. Expression of the Na+/I− symporter gene in human thyroid tumors: a comparison study with other thyroid-specific genes. J Clin Endocrinol Metab. 1999;84(9):3228–34.
Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2014;2(10):830–42.
Lyckesvard MN, Kapoor N, Ingeson-Carlsson C, Carlsson T, Karlsson JO, Postgard P, et al. Linking loss of sodium-iodide symporter expression to DNA damage. Exp Cell Res. 2016;344(1):120–31.
Plantinga TS, Tesselaar MH, Morreau H, Corssmit EP, Willemsen BK, Kusters B, et al. Autophagy activity is associated with membranous sodium iodide symporter expression and clinical response to radioiodine therapy in non-medullary thyroid cancer. Autophagy. 2016;12(7):1195–205.
Chung T, Youn H, Yeom CJ, Kang KW, Chung JK. Glycosylation of sodium/iodide symporter (NIS) regulates its membrane translocation and radioiodine uptake. PLoS ONE. 2015;10(11):e0142984.
Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, et al. American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):481–3.
Mandel SJ, Shankar LK, Benard F, Yamamoto A, Alavi A. Superiority of iodine-123 compared with iodine-131 scanning for thyroid remnants in patients with differentiated thyroid cancer. Clin Nucl Med. 2001;26(1):6–9.
Freudenberg LS, Jentzen W, Petrich T, Fromke C, Marlowe RJ, Heusner T, et al. Lesion dose in differentiated thyroid carcinoma metastases after rhTSH or thyroid hormone withdrawal: 124I PET/CT dosimetric comparisons. Eur J Nucl Med Mol Imaging. 2010;37(12):2267–76.
Jimenez-Hoyuela Garcia JM, Garcia Almeida JM, Delgado Garcia A, Aguilar Fernandez I, del Valle Martinez, Torres MD, Ortega Lozano S, et al. Application of recombinant human TSH in the diagnostic protocol of differentiated thyroid carcinoma. Rev Esp Med Nucl. 2005;24(3):152–60.
Kovatcheva RD, Hadjieva TD, Kirilov GG, Lozanov BS. Recombinant human TSH in radioiodine treatment of differentiated thyroid cancer. Nucl Med Rev Cent East Eur. 2004;7(1):13–9.
Iwano S, Kato K, Nihashi T, Ito S, Tachi Y, Naganawa S. Comparisons of I-123 diagnostic and I-131 post-treatment scans for detecting residual thyroid tissue and metastases of differentiated thyroid cancer. Ann Nucl Med. 2009;23(9):777–82.
Chen MK, Yasrebi M, Samii J, Staib LH, Doddamane I, Cheng DW. The utility of I-123 pretherapy scan in I-131 radioiodine therapy for thyroid cancer. Thyroid. 2012;22(3):304–9.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
Salvatori M, Perotti G, Villani MF, Mazza R, Maussier ML, Indovina L, et al. Determining the appropriate time of execution of an I-131 post-therapy whole-body scan: comparison between early and late imaging. Nucl Med Commun. 2013;34(9):900–8.
Spies WG, Wojtowicz CH, Spies SM, Shah AY, Zimmer AM. Value of post-therapy whole-body I-131 imaging in the evaluation of patients with thyroid carcinoma having undergone high-dose I-131 therapy. Clin Nucl Med. 1989;14(11):793–800.
Souza Rosario PW, Barroso AL, Rezende LL, Padrao EL, Fagundes TA, Penna GC, et al. Post I-131 therapy scanning in patients with thyroid carcinoma metastases: an unnecessary cost or a relevant contribution? Clin Nucl Med. 2004;29(12):795–8.
Fatourechi V, Hay ID, Mullan BP, Wiseman GA, Eghbali-Fatourechi GZ, Thorson LM, et al. Are posttherapy radioiodine scans informative and do they influence subsequent therapy of patients with differentiated thyroid cancer? Thyroid. 2000;10(7):573–7.
Sherman SI, Tielens ET, Sostre S, Wharam MD Jr, Ladenson PW. Clinical utility of posttreatment radioiodine scans in the management of patients with thyroid carcinoma. J Clin Endocrinol Metab. 1994;78(3):629–34.
Ciappuccini R, Heutte N, Trzepla G, Rame JP, Vaur D, Aide N, et al. Postablation (131)I scintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur J Endocrinol. 2011;164(6):961–9.
Garcia EV. Physical attributes, limitations, and future potential for PET and SPECT. J Nucl Cardiol. 2012;19(Suppl 1):S19–29.
Sgouros G, Hobbs RF, Atkins FB, Van Nostrand D, Ladenson PW, Wahl RL. Three-dimensional radiobiological dosimetry (3D-RD) with 124I PET for 131I therapy of thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38(Suppl 1):S41–7.
Eschmann SM, Reischl G, Bilger K, Kupferschlager J, Thelen MH, Dohmen BM, et al. Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. Eur J Nucl Med Mol Imaging. 2002;29(6):760–7.
Ruhlmann M, Jentzen W, Ruhlmann V, Pettinato C, Rossi G, Binse I, et al. High level of agreement between pretherapeutic 124I PET and intratherapeutic 131I imaging in detecting iodine-positive thyroid cancer metastases. J Nucl Med. 2016;57(9):1339–42.
Khorjekar GR, Van Nostrand D, Garcia C, O’Neil J, Moreau S, Atkins FB, et al. Do negative 124I pretherapy positron emission tomography scans in patients with elevated serum thyroglobulin levels predict negative 131I posttherapy scans? Thyroid. 2014;24(9):1394–9.
Santhanam P, Taieb D, Solnes L, Marashdeh W, Ladenson PW. Utility of I-124 PET/CT in identifying radioiodine avid lesions in differentiated thyroid cancer: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017;86(5):645–51.
Marti-Climent JM, Collantes M, Jauregui-Osoro M, Quincoces G, Prieto E, Bilbao I, et al. Radiation dosimetry and biodistribution in non-human primates of the sodium/iodide PET ligand [(18)F]-tetrafluoroborate. EJNMMI Res. 2015;5(1):70.
Jauregui-Osoro M, Sunassee K, Weeks AJ, Berry DJ, Paul RL, Cleij M, et al. Synthesis and biological evaluation of [(18)F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur J Nucl Med Mol Imaging. 2010;37(11):2108–16.
Diocou S, Volpe A, Jauregui-Osoro M, Boudjemeline M, Chuamsaamarkkee K, Man F, et al. [18F]tetrafluoroborate-PET/CT enables sensitive tumor and metastasis in vivo imaging in a sodium iodide symporter-expressing tumor model. Sci Rep. 2017;7(1):946.
O’Doherty J, Jauregui-Osoro M, Brothwood T, Szyszko T, Marsden PK, O’Doherty MJ, et al. 18F-tetrafluoroborate (18F-TFB), a PET probe for imaging sodium-iodide symporter expression: whole-body biodistribution, safety and radiation dosimetry in thyroid cancer patients. J Nucl Med. 2017;58(10):1666–71.
Bertagna F, Albano D, Bosio G, Piccardo A, Dib B, Giubbini R. 18F-FDG-PET/CT in patients affected by differentiated thyroid carcinoma with positive thyroglobulin level and negative 131I whole body scan. It’s value confirmed by a bicentric experience. Curr Radiopharm. 2016;9(3):228–34.
Stangierski A, Kaznowski J, Wolinski K, Jodlowska E, Michaliszyn P, Kubiak K, et al. The usefulness of fluorine-18 fluorodeoxyglucose PET in the detection of recurrence in patients with differentiated thyroid cancer with elevated thyroglobulin and negative radioiodine whole-body scan. Nucl Med Commun. 2016;37(9):935–8.
Trivino Ibanez EM, Muros MA, Torres Vela E, Llamas Elvira JM. The role of early 18F-FDG PET/CT in therapeutic management and ongoing risk stratification of high/intermediate-risk thyroid carcinoma. Endocrine. 2016;51(3):490–8.
Pathak KA, Goertzen AL, Nason RW, Klonisch T, Leslie WD. A prospective cohort study to assess the role of FDG-PET in differentiating benign and malignant follicular neoplasms. Ann Med Surg (Lond). 2016;12:27–31.
Vrachimis A, Burg MC, Wenning C, Allkemper T, Weckesser M, Schafers M, et al. [(18)F]FDG PET/CT outperforms [(18)F]FDG PET/MRI in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016;43(2):212–20.
Leboulleux S, Schroeder PR, Busaidy NL, Auperin A, Corone C, Jacene HA, et al. Assessment of the incremental value of recombinant thyrotropin stimulation before 2-[18F]-Fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography imaging to localize residual differentiated thyroid cancer. J Clin Endocrinol Metab. 2009;94(4):1310–6.
Saab G, Driedger AA, Pavlosky W, McDonald T, Wong CY, Yoo J, et al. Thyroid-stimulating hormone-stimulated fused positron emission tomography/computed tomography in the evaluation of recurrence in 131I-negative papillary thyroid carcinoma. Thyroid. 2006;16(3):267–72.
Vera P, Kuhn-Lansoy C, Edet-Sanson A, Hapdey S, Modzelewski R, Hitzel A, et al. Does recombinant human thyrotropin-stimulated positron emission tomography with [18F]fluoro-2-deoxy-d-glucose improve detection of recurrence of well-differentiated thyroid carcinoma in patients with low serum thyroglobulin? Thyroid. 2010;20(1):15–23.
Chun AR, Jo HM, Lee SH, Chun HW, Park JM, Kim KJ, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Endocrinol Metab (Seoul). 2015;30(1):71–7.
Jamsek J, Zagar I, Gaberscek S, Grmek M. Thyroid lesions incidentally detected by (18)F-FDG PET-CT—a two centre retrospective study. Radiol Oncol. 2015;49(2):121–7.
Hassan A, Riaz S, Zafar W. Fluorine-18 fluorodeoxyglucose avid thyroid incidentalomas on PET/CT scan in cancer patients: how sinister are they? Nucl Med Commun. 2016;37(10):1069–73.
Demir O, Kose N, Ozkan E, Unluturk U, Aras G, Erdogan MF. Clinical significance of thyroid incidentalomas identified by 18F-FDG PET/CT: correlation of ultrasonograpy findings with cytology results. Nucl Med Commun. 2016;37(7):715–20.
Kwon SY, Choi EK, Kong EJ, Chong A, Ha JM, Chun KA, et al. Prognostic value of preoperative 18F-FDG PET/CT in papillary thyroid cancer patients with a high metastatic lymph node ratio: a multicenter retrospective cohort study. Nucl Med Commun. 2017;38(5):402–6.
Yang JH, Camacho CP, Lindsey SC, Valente FOF, Andreoni DM, Yamaga LY, et al. The combined use of calcitonin doubling time and 18F-FDG PET/CT improves prognostic values in medullary thyroid carcinoma: the clinical utility of 18F-FDG PET/CT. Endocr Pract. 2017;23(8):942–8.
Mato E, Matias-Guiu X, Chico A, Webb SM, Cabezas R, Berna L, et al. Somatostatin and somatostatin receptor subtype gene expression in medullary thyroid carcinoma. J Clin Endocrinol Metab. 1998;83(7):2417–20.
Papotti M, Kumar U, Volante M, Pecchioni C, Patel YC. Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin Endocrinol (Oxf). 2001;54(5):641–9.
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34(7):982–93.
Tran K, Khan S, Taghizadehasl M, Palazzo F, Frilling A, Todd JF, et al. Gallium-68 Dotatate PET/CT is superior to other imaging modalities in the detection of medullary carcinoma of the thyroid in the presence of high serum calcitonin. Hell J Nucl Med. 2015;18(1):19–24.
Ozkan ZG, Kuyumcu S, Uzum AK, Gecer MF, Ozel S, Aral F, et al. Comparison of (6)(8)Ga-DOTATATE PET-CT, (1)(8)F-FDG PET-CT and 99mTc-(V)DMSA scintigraphy in the detection of recurrent or metastatic medullary thyroid carcinoma. Nucl Med Commun. 2015;36(3):242–50.
Sood A, Singh H, Sood A, Basher RK, Mittal BR. Incidentally detected thyroid follicular neoplasm on somatostatin receptor imaging and post-therapy scan. Indian J Nucl Med. 2017;32(3):224–6.
Rowe SP, Gorin MA, Allaf ME, Pienta KJ, Tran PT, Pomper MG, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19(3):223–30.
Kiess AP, Banerjee SR, Mease RC, Rowe SP, Rao A, Foss CA, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 2015;59(3):241–68.
Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5(10):2674–81.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–8.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(12):2117–36.
Bychkov A, Vutrapongwatana U, Tepmongkol S, Keelawat S. PSMA expression by microvasculature of thyroid tumors—potential implications for PSMA theranostics. Sci Rep. 2017;7(1):5202.
Derlin T, Kreipe HH, Schumacher U, Soudah B. PSMA expression in tumor neovasculature endothelial cells of follicular thyroid adenoma as identified by molecular imaging using 68 Ga-PSMA ligand PET/CT. Clin Nucl Med. 2017;42(3):e173–4.
Damle NA, Tripathi M, Chakraborty PS, Sahoo MK, Bal C, Aggarwal S, et al. Unusual uptake of prostate specific tracer 68 Ga-PSMA-HBED-CC in a benign thyroid nodule. Nucl Med Mol Imaging. 2016;50(4):344–7.
Kanthan GL, Drummond J, Schembri GP, Izard MA, Hsiao E. Follicular thyroid adenoma showing avid uptake on 68 Ga PSMA-HBED-CC PET/CT. Clin Nucl Med. 2016;41(4):331–2.
Taywade SK, Damle NA, Bal C. PSMA expression in papillary thyroid carcinoma: opening a new horizon in management of thyroid cancer? Clin Nucl Med. 2016;41(5):e263–5.
Lutje S, Gomez B, Cohnen J, Umutlu L, Gotthardt M, Poeppel TD, et al. Imaging of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68 Ga-HBED-CC-PSMA PET/CT. Clin Nucl Med. 2017;42(1):20–5.
Moore M, Panjwani S, Mathew R, Crowley M, Liu YF, Aronova A, et al. Well-differentiated thyroid cancer neovasculature expresses prostate-specific membrane antigen—a possible novel therapeutic target. Endocr Pathol. 2017. https://doi.org/10.1007/s12022-017-9500-9.
Acknowledgements
Our sincere thanks to Dr. Paul W Ladenson and John Eager Howard, Professor in Endocrinology, Diabetes and Metabolism at the Johns Hopkins University School of Medicine, for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors. The image used to demonstrate findings of interest to the reader has been approved by the Johns Hopkins IRB for use since the person is not a research subject and is completely de-identified.
Additional information
The original version of this article is revised: “The middle name of the author Steven B. Rowe is incorrect. The corrected name is Steven P. Rowe”.
A correction to this article is available online at https://doi.org/10.1007/s12032-017-1063-6.
Rights and permissions
About this article
Cite this article
Santhanam, P., Solnes, L.B. & Rowe, S.P. Molecular imaging of advanced thyroid cancer: iodinated radiotracers and beyond. Med Oncol 34, 189 (2017). https://doi.org/10.1007/s12032-017-1051-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1051-x