Abstract
It has been demonstrated that aberrant expression of microRNAs (miRNAs) is strongly associated with carcinogenesis. Recently, specific miRNAs may serve as potential biomarkers for the diagnosis and prognosis of various types of tumor. MiR-23a is known to play important role in the development of cancers and deregulated in various hematological malignancies. The aim of the present study is to explore miR-23a as potential diagnostic and/or prognostic marker of diffuse large B-cell lymphoma (DLBCL). We compared the expression level of miR-23a in DLBCL patients (n = 104) and reactive lymph nodes as controls (n = 28) from formalin-fixed, paraffin-embedded tissues using quantitative reverse transcription-polymerase chain reaction. The expression level of miR-23a was significantly higher in DLBCL patients than in controls (P = 0.001). No significant association was observed between the miR-23a expression level and clinical features such as age, gender, Ann Arbor stage, performance status, lactate dehydrogenase, extranodal sites and International Prognostic Index score (IPI). Kaplan–Meier analysis showed that higher expression level of miR-23a was significantly associated with a poor overall survival (OS) in DLBCL patients (log-rank test, P = 0.029), and multivariable Cox regression revealed the expression of miR-23a (adjusted P = 0.034) and IPI (adjusted P = 0.021) was independently associated with OS. To our knowledge, we provide here the first evidence that miR-23a may represent a diagnostic and prognostic marker for DLBCL. DLBCL patients with a high expression level of miR-23a had a shorter OS than patients with a lower expression level. Further investigation of the changes may be of prognostic significance in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs) are a class of small (~22 bp) endogenous, single-stranded noncoding RNAs, which modulate gene expression through binding to the 3′-UTR of the target mRNA, thus resulting in translational repression or degradation [1]. miRNAs orchestrate various cellular functions and play key roles in many biologic processes, including haematopoiesis developmental timing, cell proliferation, cell differentiation, apoptosis and cancer development [2–4], and they are frequently located at fragile sites or cancer-associated genomic regions, such as breakpoint regions in chromosome aberrations involving oncogenes or tumor-suppressor genes, minimal regions of loss of heterozygosity, and minimal regions of amplification [5]. miRNAs are proposed to play a direct role in oncogenesis, as they may serve as oncogenes or tumor suppressors [6]. Concordantly, miRNA expression deregulation has also been described in many types of cancer and been demonstrated to have diagnostic, prognostic and therapeutic potential in cancer [7–9].
The most common adult lymphoma is diffuse large B-cell lymphoma (DLBCL) which accounts for nearly 40 % of all lymphoid tumors [10]. Gene expression array technology and immunohistochemical studies demonstrated that DLBCL can be divided into two distinct molecular subtypes: germinal center B-cell-like (GCB) and non-GCB. Many studies have described altered expression of miRNAs in the malignant tissue compared with the corresponding nonmalignant tissues, suggesting that these miRNAs were involved in lymphomagenesis. Previous reports suggested that specific miRNAs might be associated with clinical parameters and outcome in DLBCL patients and represent as prognostic markers [11, 12].
The miR-23a/24-2/27a is a miRNA cluster which is located on human chromosome 19p13.2 [13]. Several studies have demonstrated that members of the cluster are involved in cell cycle, proliferation, differentiation, hematopoiesis and cardiac hypertrophy in various cell types [14–18]. Accumulating evidence indicated that the miR-23a/24-2/27a cluster may play a causal role in mammary tumorigenesis, and the members of this cluster are found to have altered expression in many diseased states [18]. In fact, this cluster was the first downstream miRNA target implicated in the regulation of myeloid versus lymphoid cells development of [19]. Recently, expression of miR-23a has been found to be up-regulated in acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) [20], cardiac hypertrophy [17], hepatocellular carcinoma (HCC) [14] and pancreatic cancer [21]. However, the expression and role of miR-23a in DLBCL remain to be explored.
The aim of the present study was to investigate: (1) the expression status of miR-23a in DLBCL by quantitative real-time polymerase chain reaction (qRT-PCR), (2) the association of miR-23a with clinical features and outcome of the patients and (3) whether miR-23a can be treated as a predictive marker of progression-free survival (PFS) or overall survival (OS) in patients with DLBCL. In the past few years, we have collected 104 cases of DLBCL with clinical follow-up information. We conducted the present study to analyze the expression of miR-23a in archived formalin-fixed, paraffin-embedded (FFPE) lymph node specimens. These findings may help us to improve the understanding of the biologic function of miR-23a in DLBCL and provide prospective candidate targets for the treatment of human DLBCL.
Materials and methods
Study subjects
A total of 104 patients diagnosed with DLBCL and 28 individuals with reactive lymph nodes as controls were enrolled in this study. All the FFPE lymph node specimens are obtained between 2008 and 2012 from the Pathology Department of First Affiliated Hospital of Xinxiang Medical University, Zhengzhou, Henan. None of these controls had been diagnosed with any types of malignancy previously. The controls were unrelated ethnic Han Chinese. The DLBCL diagnosis was made independently by three senior pathologists on the basis of morphology, immunophenotypic and molecular findings in accordance with the World Health Organization classification criteria. The Ann Arbor Staging System was used to determine disease stage. Details of clinical, histopathologic parameters, immunophenotypic findings and follow-up data about these patients were retrieved from medical records, pathology reports and referring clinicians. The main clinical characteristics of DLBCL patient details are summarized in Table 1. Sample collection and analysis were conducted after informed consent. The study protocol was performed under the supervision of the Institutional Review Board (IRB).
Immunohistochemical staining
DLBCL cases were classified as GCB- or non-GCB-type by immunohistochemistry. Monoclonal antibodies including CD10, CD20, CD3, CD7, CD79a, BCL6 and Ki67 were used for tumor immunotypes. Appropriate positive and negative controls were run concurrently. All the antibodies were purchased from Dako company and were used in accordance with manufacturer’s protocols. The expression of each immunohistochemistry marker was determined according to cutoff level for each marker based on the intensity of staining and the percentage of positive results by three senior pathologists.
RNA preparation and cDNA synthesis
Total RNA was extracted from 4 × 20 μm FFPE sections using the Recoverall kit from Ambion (Huntington, UK) following the manufacturers’ instructions. The concentrations of all RNA samples were determined by measurement of the optical density at 260 nm using NanoDrop 1000 (Nanodrop, Wilmington, Delaware, USA), and the RNA was stored at −80 °C until use. Total RNA was exposed to RNAase-free DNAase I, and cDNA was synthesized using random hexamer primers and miScript Reverse Transcription Kit (Qiagen, Germany) according to the manufacturer’s instructions.
Real-time quantitative reverse transcription-PCR
The expression of the miR-23a was undertaken with FFPE tissues using qRT-PCR. qRT-PCR reaction was carried out using the TaqMan MicroRNA Reverse Transcription Kit on a LightCycler 480 real-time PCR system an software (SDSv2.0, Applied Biosystems). RNU66 (PN 4373382) was used for normalization as an internal control. All qRT-PCRs were run in duplicates. Sequences of amplified production were verified using DNA sequencing. All of the reagents including primers and probes for the TaqMan MicroRNA assays were provided by Applied Biosystems.
The data were analyzed using an automated baseline. Mean threshold cycle (Ct) values and standard deviations were calculated. The Ct was defined as the cycle number at which the fluorescence exceeded the given threshold. Relative expression level of miR-23a was analyzed using the comparative Ct (2−ΔΔCt) method with RNU66 as the endogenous control. The amount of miR-23a was normalized relative to the amount of RNU66. Briefly, for the equation 2−ΔΔCt, ΔΔCt (miR-23a−RNU66) = CtmiR-23a−CtRNU66.
Statistical and survival analysis
The statistical differences in clinical characteristics between DLBCL patients and controls were determined by chi-square test or Student’s t test. The difference in expression level of miR-23a was compared with the Mann–Whitney U test. Receiver-operating characteristics (ROC) curves were established in order to evaluate the diagnostic value of miR-23a for discriminating patients with or without DLBCL. The cutoff point of miR-23a level for the prediction of the OS was assessed using the Youden Index (sensitivity + specificity − 1).
The follow-up information including OS and PFS was obtained from hospital information systems and the patients or patients’ relatives. The OS was defined as the date of pathological diagnosis to death from any cause or last contact. PFS was calculated from the time of diagnosis until the date of clinical recurrence, death or last contact. Data were censored if the DLBCL patients were alive at last follow-up. Multivariate Cox regression analysis was carried out to assess the OS time and the PFS time of the DLBCL cases. Kaplan–Meier method was used to estimate using the survival distributions. Log-rank analysis was used to compare the survival rates between different groups.
All above statistical analyses were conducted with the SPSS software version 17.0 (SPSS, Chicago, IL, USA). A two-sided P value less than 0.05 was considered to indicate a statistically significant result.
Results
Study groups and clinicopathological features
Clinical characteristics of 104 DLBCL cases and 28 controls analyzed in the present study are shown in Table 1. The median age of 104 patients with DLBCL at diagnosis was 55 years (range 17–85 years), with a male-to-female ratio of 1.3:1 (58 males and 46 females). There were no significant differences in age and gender distribution between DLBCL patients with controls (P > 0.05, χ 2 test).
According to the Ann Arbor staging system for lymphoma, 45 patients were at stages I and II, and 59 patients were at stages III and IV. Increased serum lactate dehydrogenase (LDH) (>250 IU/L) level was detected in 29 of the 104 patients.
Correlation of miR-23a expression with clinicopathologic characteristics
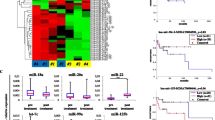
miR-23a level in the FFPE lymph nodes in both DLBCL patients and control subjects was detected by qRT-PCR. After normalization with RNU66, miR-23a expression level converted from Ct values in DLBCL patients was significantly higher than in controls (P = 0.001, Fig. 1). Besides, we measured the correlation between miR-23a expression and clinical parameters. However, statistical analysis showed that there were no significant association between miR-23a level and any of the listed parameters including age, gender, Ann Arbor stage, performance status, LDH, extranodal sites and International Prognostic Index score (IPI) (P > 0.05, Mann–Whitney U test, data not shown).
As shown in Fig. 2, receiver-operating characteristic (ROC) curve analysis showed a separation between DLBCL patients and controls for miR-23a (95 % CI, 0.590–0.807), miR-23a yielded an area under the curve (AUC) of 0.698 with the sensitivity of 62.5 % and specificity of 67.9 %.
miR-23a is an independent prognostic indicator in DLBCL
The median follow-up period of the 104 patients was 412 days (range from 92 to 1,024 days). At the last follow-up, 24 patients had disease progression, and 29 patients died from DLBCL, treatment toxicity, or unrelated causes. Median survival was 365 days (range 71–981 days) for DLBCL, Median PFS was 196 days.
To investigate the prognostic value of miR-23a in DLBCL, Kaplan–Meier survival analysis was used to compare OS and PFS of DLBCL patients between the high and low miR-23a groups, and the log-rank test was used to examine the statistical significance between stratified survival groups according to the relative expression level of miR-23a. As shown in Fig. 3, higher expression level of miR-23a was significantly associated with a poor outcome (i.e., poor prognosis/short-term OS) in DLBCL patients (log-rank test, P = 0.029). However, no significant association was found between miR-23a and PFS (data not shown). Of note, the difference in the OS was not due to the different treatment for patients in each group received different treatment regimens (DHAP, IMVP-16, GMOX and CHOP-like regimens) assigned solely based on their clinical features that were independent of their miR-23a level. Thus, miR-23a probably can serve as a prognostic marker for patients with DLBCL.
In addition, univariate Cox regression was conducted to analyze whether miR-23a and other clinical features could be independent OS factors for DLBCL. High level of miR-23a expression was a “risky” factor in DLBCL. Next, we carried out multivariate Cox regression incorporating miR-23a expression, age, gender, Ann Arbor stage, performance status, LDH, extranodal sites and IPI. This analysis revealed miR-23a expression (adjusted P = 0.034, HR 3.776, 95 % CI 1.106–12.892) and IPI (adjusted P = 0.021, HR 1.595, 95 % CI 1.072–2.371) were significantly associated with OS (Table 2). These results indicated that miR-23a could be used as an independent prognostic biomarker for DLBCL.
Discussion
Accumulating evidences indicate that about 10–30 % of human genes are the targets of miRNA regulation [22]. Overwhelming researches demonstrated that the expression level of miRNA genes is deregulated in various cancers. The overexpression of miRNA may lead to the down-regulation of tumor-suppressor genes, and on the contrary, the oncogene up-regulation can be caused by the underexpression of miRNA [23–25]. It is showed the majority of human miRNAs are located at cancer-associated genomic regions, which indicates miRNAs have a potential critical role in cancer. Cancer-associated miRNAs have oncogenic charcteristics [26]. A large number of cancer-associated miRNAs have emerged as important modulators in cellular pathways, and some of them have been demonstrated to be predictive markers of the clinical behavior for several types of hematological malignancies [27–29]. For example, decreased miR-34a expression predicts a poor OS for DLBCL [30]. DLBCL patients with low miR-155 and miR-146a expression levels achieved a higher complete remission rate, higher overall response rate and longer PFS time [31]. Thus, discovery of new biomarkers is needed for prognosis and clinical management. Deregulation of miR-23a was associated with many types of cancer, including a wide range of solid and hematological malignancies, such as ALL, AML [20], HCC [14], pancreatic cancer [21]. This phenomenon suggests that miR-23a plays a fundamental role in the establishment of a general malignant phenotype. However, the expression and role of miR-23a in DLBCL remain to be explored.
To our knowledge, in the current study, we firstly describe the expression level of miR-23a in FFPE lymph nodes of DLBCL patients. We found that miR-23a expression level in DLBCL patient tissue was higher than in reactive hyperplasia lymphoid nodes. The differential expression of miR-23a between DLBCL patient and healthy controls suggests that miR-23a may be a potential tool for future molecular diagnostics in DLBCL. Next, we examined whether the expression level of miR-23a was associated with clinical features, including age, gender, Ann Arbor stage, performance status, LDH, extranodal sites and IPI. However, there was no significant association between miR-23a and the clinical parameters. Many studies have shown that miRNA expression levels have potential prognostic value in malignant diseases, such as CLL, DLBCL and pancreatic cancer. Therefore, in order to ascertain whether miR-23a expression is able to predict clinical outcome, we analyzed PFS and OS in the DLBCL patients. We found that high expression of miR-23a was associated with a shorter OS time. Besides, we carried out multivariate analysis and found that miR-23a and IPI were statistically significant independent prognostic indicators. These findings suggest that the expression of miR-23a has prognostic significance in DLBCL and higher level of miR-23a had a worse clinical outcome.
During carcinogenesis, oncogenic transcription factor c-myc regulates the cell cycle, glucose metabolism and glutamine catabolism. It has been demonstrated that the repression of miR-23a and mir-23b implicated in the glutamine catabolism stimulation by Myc’s role [18]. Thus, we presume that the tumorigenesis of miR-23a in DLBCL may be influenced either by the reposition of some genes close to its promoter regions such as myc, or by the relocalization of some regulatory elements. It has been stated, the behaviors of the same gene, as an oncogene or suppressor gene, also can be influenced by the transcriptional and/or post-transcriptional events. Further studies are needed to clarify the mechanisms of the carcinogenesis of miR-23a in DLBCL.
Seeking new markers for the diagnosis, prognosis and therapy of DLBCL have been an ongoing endeavor. FFPE blocks represent a rich archive of well-characterized tissue specimens, which offer a valuable source of patient data. Numerous studies have been undertaken using FFPE specimens. Compare to mRNA, the small size of miRNAs is relatively resistant to RNase degradation and can be recovered intact from archival FFPE material [32]. Siebolts et al. [33] carried a high-quality assessment and proved FFPE tissues are suitable for miRNA analyses using qRT-PCR. In our present study, we have successfully analyzed miR-23a expression in FFPE lymph node specimens from patients with DLBCL. Further studies exploring a potential biologic function of circulating miR-23a, including serum and plasma are needed, it will be expected that circulating miR-23a can be used as a marker for the diagnosis and predict clinical outcome of DLBCL [8].
Although our findings are promising, there are several limitations in our study. First, the sample size is relatively small, further investigations of miR-23a in larger populations and in independent studies are needed. Second, qRT-PCR by relative quantification approach testing low levels of miRNAs is less accurate, in which they may not fall into the linear range of the assay; therefore, absolute quantification approach with standard curve calibration would be more appropriate for further validation of our results.
In conclusion, our data firstly suggest that the expression level of miR-23a in FFPE lymph nodes can distinguish patients with DLBCL from control subjects and may be a potential diagnostic marker for DLBCL. Furthermore, the high expression level of miR-23a may serve as a poor prognostic marker for DLBCL. Since miR-23a could silence or modulate gene expression by regulation of transcription factors in humans, we also propose that targeted therapies to inhibition miR-23a might represent as potential strategies for controlling DLBCL tumorigenesis.
References
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Vasilatou D, Papageorgiou S, Pappa V, Papageorgiou E, Dervenoulas J. The role of microRNAs in normal and malignant hematopoiesis. Eur J Haematol. 2010;84(1):1–16.
Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66(15):7390–4.
Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11(4):441–50.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69.
Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353(17):1793–801.
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141(5):672–5.
Kota SK, Balasubramanian S. Cancer therapy via modulation of micro RNA levels: a promising future. Drug Discov Today. 2010;15(17–18):733–40.
Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol. 2001;13(5):325–34.
Roehle A, Hoefig KP, Repsilber D, Thorns C, Ziepert M, Wesche KO, et al. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br J Haematol. 2008;142(5):732–44.
Jung I, Aguiar RC. MicroRNA-155 expression and outcome in diffuse large B-cell lymphoma. Br J Haematol. 2009;144(1):138–40.
Fujita PA, Rhead B, Zweig AS, Hinrichs AS, Karolchik D, Cline MS, et al. The UCSC Genome Browser database: update 2011. Nucleic Acids Res. 2011;39((Database issue)):D876–82.
Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, et al. Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-beta-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int J Cancer. 2008;123(4):972–8.
Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67(22):11001–11.
Lal A, Pan Y, Navarro F, Dykxhoorn DM, Moreau L, Meire E, et al. miR-24-mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat Struct Mol Biol. 2009;16(5):492–8.
Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106(29):12103–8.
Chhabra R, Dubey R, Saini N. Cooperative and individualistic functions of the microRNAs in the miR-23a ~ 27a ~ 24-2 cluster and its implication in human diseases. Mol Cancer. 2010;9:232.
Kong KY, Owens KS, Rogers JH, Mullenix J, Velu CS, Grimes HL, et al. MIR-23A microRNA cluster inhibits B-cell development. Exp Hematol. 2010;38(8):629–40.
Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly MB, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104(50):19971–6.
Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297(17):1901–8.
Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20.
Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7.
Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2005;65(9):3509–12.
McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13(4):253–8.
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101(9):2999–3004.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–8.
Fang C, Zhu DX, Dong HJ, Zhou ZJ, Wang YH, Liu L, et al. Serum microRNAs are promising novel biomarkers for diffuse large B cell lymphoma. Ann Hematol. 2012;91(4):553–9.
Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42(8):1273–81.
He M, Gao L, Zhang S, Tao L, Wang J, Yang J et al. Prognostic significance of miR-34a and its target proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and DLBCL. Gastric Cancer. 2013.
Zhong H, Xu L, Zhong JH, Xiao F, Liu Q, Huang HH, et al. Clinical and prognostic significance of miR-155 and miR-146a expression levels in formalin-fixed/paraffin-embedded tissue of patients with diffuse large B-cell lymphoma. Exp Ther Med. 2012;3(5):763–70.
Li J, Smyth P, Flavin R, Cahill S, Denning K, Aherne S, et al. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007;7:36.
Siebolts U, Varnholt H, Drebber U, Dienes HP, Wickenhauser C, Odenthal M. Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J Clin Pathol. 2009;62(1):84–8.
Acknowledgments
We would like to thank all the participants and staff of the First Affiliated Hospital of Xinxiang Medical University, for their valuable contributions on collecting specimens and sorting out clinical data.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Wl., Yang, C., Han, Xl. et al. MicroRNA-23a expression in paraffin-embedded specimen correlates with overall survival of diffuse large B-cell lymphoma. Med Oncol 31, 919 (2014). https://doi.org/10.1007/s12032-014-0919-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0919-2