Abstract
MicroRNAs (miRNAs) are regulatory RNA molecules that are deregulated in many disease types, including cancer. Recently, miRNAs have shown promise as markers for cancer diagnosis. The aim of this study was to investigate whether serum miRNAs can be used as biomarkers for the detection of diffuse large B cell lymphoma (DLBCL). We measured the levels of miRNAs (miR-15a, miR-16-1, miR-21, miR-29c, miR-34a, miR-155, and miR-223) in serum samples from patients with DLBCL and healthy controls using real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR). We show here that miRNAs are present in human serum in a remarkably stable form. Four of miRNAs (miR-15a, miR-16-1, miR-29c, and miR-155) were significantly elevated in DLBCL serum when compared with normal controls (P < 0.05), while miR-34a was downregulated in DLBCL serum when compared with controls (P < 0.05). Receiver operating characteristic analyses reflects strong discriminating DLBCL from controls, with area under the curves of 0.7722, 0.7002, 0.6672, 0.8538, and 0.7157 for miR-15a, miR-16-1, miR-29c, miR-34a, and miR-155, respectively. At the cut-off value of 0.0006 for miR-15a, the sensitivity was 80% and the specificity was 76%; at the cut-off value of 0.0886 for miR-16-1, the sensitivity was 94% and the specificity was 51%; at the cut-off value of 1.395 for miR-34a, the sensitivity was 100% and the specificity was 70%; at the cut-off value of 0.0022 for miR-155, the sensitivity was 83% and the specificity was 65%. In conclusion, these data suggest that serum miRNAs are potentially useful tools as novel noninvasive biomarker for the diagnosis of DLBCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common type of B cell lymphoma and represents a heterogeneous group of tumors on morphologic, phenotypic, molecular, and clinical grounds [1]. Enormous progress has been made in the treatment of DLBCL, mostly due to the anti-CD20 antibody rituximab (R), however, a large number of patients are not cured with conventional therapy. Detection of early stage cancer and precancerous lesions appears to be a key measure to reduce its mortality, and most DLBCL-related deaths can be preventable through early lesion detection and treatment of early stage cancer and precancerous lesions. Most patients are asymptomatic, but when symptoms are present, they are highly dependent on the site of involvement [2]. The diagnosis should always be confirmed by examination of a tissue specimen. An ideal screening method should have a high sensitivity and specificity for early stage cancers and precancerous lesions; it should be also safe and affordable so that it can be broadly accepted by patients. Thus, there is a pressing need for new non-invasive biomarkers to improve the detection of DLBCL.
MicroRNAs (miRNAs) are endogenous noncoding RNA molecules of 19–24 nucleotides in length. These small molecules have been shown to play an important regulatory role in a wide range of biological and pathological processes. miRNAs could now be added to the panel of potential biomarkers owing to their high stability and the availability of assays able to quantify their level [3]. Circulating RNAs have been identified in the serum and plasma of cancer patients for more than a decade. Recently, several studies have reported the occurrence of circulating miRNAs in serum and plasma samples from both cancer patients and healthy controls [4–8]. For example, Lawrie et al. [8] reported that miR-21 has the potential as a diagnostic biomarker for DLBCL and that the serum levels of miR-21 are associated with relapse-free survival in DLBCL patients. Therefore, the expression profiles of these circulating miRNAs in serum, plasma, and other body fluids herald immense potential for their use as novel minimally invasive biomarkers in diagnosing and monitoring human cancers.
However, the early diagnostic value of circulating miRNAs has not been reported to date. In this study, we focused on whether circulating miRNAs can be detected in serum and whether expression levels of specific miRNAs different between DLBCL and healthy individuals.
Materials and methods
Study population
This study was approved by the institutional review board of Nanjing Medical University, and the written informed consent was obtained from each participant or from the patient representatives. This also conducted according to the Declaration of Helsinki. After obtaining informed consent, 75 patients with de novo DLBCL were recruited in this study. Histologic diagnoses were established according to the World Health Organization classification [9]. Seventy-seven age-matched healthy subjects were collected as the control based on their negative results of health examination including blood test, chest X-ray, abdominal ultrasound examination, and CT scan. None of these controls had previously been diagnosed with any types of malignancy previously. The population controls were unrelated ethnic Han Chinese.
Samples processing and miRNAs extraction
The whole blood was separated into serum and cellular fractions within 24 h after sample collection by centrifugation at 1,600 × g for 15 min. Then, cellular fractions and serum were immediately stored at −80°C. To normalize the difference of extraction efficiency and reverse transcription efficiency among different samples, synthetic versions of the Caenorhabditis elegans miRNA (cel-miR-39) was spiked into serum after the addition of Trizol LS reagent (Invitrogen, Carlsbad, CA) to the samples. RNA was extracted using Trizol LS reagent (Invitrogen, Carlsbad, CA) as described by the manufacturer. For RNA isolation from serum, 200 μl of serum was homogenized in 250 μl of Trizol LS reagent. Then, 200 μl of chloroform was added to the sample, and the mixed solution was centrifugated. After an additional chloroform extraction and precipitation with isopropanol, the RNA sample was suspended in 20 μl of nuclease-free water. In general, we obtained 400 ng of RNA from 1 ml of serum.
MiRNAs quantification by real-time quantitative RT-PCR
To evaluate mature miRNAs in a specific manner, we modified a previously described looped real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) methodology to quantify the expression of mature miR-15a, miR-16-1, miR-21, miR-29c, miR-34a, miR-155, and miR-223 [10]. Twenty nanograms of total RNA from each sample was reverse-transcribed using the reverse transcription reagents (Applied Biosystems). Reverse transcriptase reactions contained 20 ng of purified total RNA, 5 × M-MLV buffer (Invitrogen, USA), M-MLV (200 U/μl, Invitrogen, USA) 1.0 μl, DTT 1.0 μl, stem-loop RT primer (Table 1; 10 μmol/l) 1.0 μl, RNase Inhibitor (40 U/μl) 0.5 μl, dNTP (10 mmol/l) 1.0 μl. Twenty-microliter mixtures were incubated at 42°C for 60 min and 85°C for 5 min and then held at 4°C. Subsequently, quantitative real-time PCR was performed using the ABI 7300 Real-Time PCR system and software (SDSv2.0, Applied Biosystems). Each amplification reaction was performed in a final volume of 20 μl containing 1 μl of the cDNA, 0.5 μmol/l of each primer (Table 1) and 1 × SYBR Green PCR Master mix. The reaction was first incubated at 95°C for 5 min, followed by 35 cycles of 95°C for 5 s and 60°C for 30 s. All reactions were run in triplicate. Sequences of amplified production were verified by DNA sequencing.
Statistical analysis
The statistical significance of differences in clinical characteristics between patients with DLBCL and controls was assessed using the χ 2 test. Expression levels of serum miRNAs were compared using the Mann–Whitney U test. The ΔCt method was used for analysis \( \left[ {\Delta {\text{Ct}} = {\text{mean}}\;{\text{Ct}}\left( {{\text{cel}} - {\text{miR}} - 39} \right) - {\text{mean}}\;{\text{C}}\left( {{\text{miRNAs}}\;{\text{of}}\;{\text{interest}}} \right)} \right] \). The relative amount of miRNAs was calculated by the equation 2−ΔCt. Receiver-operating characteristics (ROC) curves were established to evaluate the diagnostic value of serum miRNAs for differentiating between DLBCL and controls. A P value of less than 0.05 was considered statistically significant. All statistical analysis was performed with GraphPad Prism 5.0 (GraphPadSoftware, San Diego, CA).
Results
Patient characteristics
Patient characteristics are summarized in Table 2. A total of 152 participants including 75 patients with de novo DLBCL and 77 age-matched healthy subjects were recruited into this study. There were no significant differences of age and sex distribution between patients with DLBCL with healthy controls (P = 0.627, ANOVA; P = 0.744, χ 2 test).
Expression levels of seven selection miRNAs on a small set of DLBCL serum samples
We research the expression levels of miRNAs (miR-15a, miR-16-1, miR-21, miR-29c, miR-34a, miR-155, and miR-223) based on previous reports by using qRT-PCR on 40 serum samples (20 patients with DLBCL and 20 controls) [8, 11–13]. Five of miRNAs (miR-15a, miR-16-1, miR-21, miR-29c, and miR-155) were significantly elevated in DLBCL serum when compared with normal controls (P = 0.0001 for miR-15a, P = 0.0003 for miR-16-1, P = 0.0049 for miR-21, P = 0.0020 for miR-29c, and P = 0.0023 for miR-155, respectively; Mann–Whitney U test; Fig. 1). No significant difference was observed in the levels of miR-223 (P = 0.2557), while miR-34a was downregulated in DLBCL serum when compared with controls (P = 0.0002, Fig. 1).
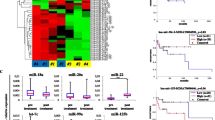
MicroRNA selection and validation by quantitative reverse-transcription polymerase chain reaction (RT-PCR) analysis. Box plots of serum levels of a miR-15a, b miR-16-1, c miR-21, d miR-29c, e miR-34a, and f miR-155 in those who were healthy and normal (n = 20) and patients with DLBCL (n = 20). Expression levels of the miRNAs (log10 scale at y-axis) are normalized to cel-miR-39. The lines inside the boxes denote the medians. Statistically significant differences were determined using Mann–Whitney U tests
Independent large-scale validation on serum samples
To further verify the discriminating power of the six miRNAs (miR-15a, miR-16-1, miR-21, miR-29c, miR-34a, and miR-155) markers identified in the stage of preliminary marker selection, the levels of these six miRNAs were measured on a total of 152 serum samples including 75 DLBCL and 77 normal controls (Table 2). Because of the limited of sample size in this study, these 40 cases in preliminary marker selection were also been included in the large-scale validation. Four miRNAs were significantly elevated in serum of patients with DLBCL than those in controls (P < 0.0001 for miR-15a, P = 0.0001 for miR-16-1, P = 0.0031 for miR-29c, and P = 0.0003 for miR-155, respectively; Mann–Whitney U test; Fig. 2), while miR-34a was downregulated in DLBCL serum when compared with controls (P < 0.0001, Fig. 2). Representation of the data using a ROC plot reflects strong separation between the two groups, with an area under the curve (AUC) of 0.7722 for miR-15a (95% CI, 0.6839 to 0.8605), 0.7002 for miR-16-1 (95% CI, 0.6027 to 0.7978), 0.6672 for miR-29c (95% CI, 0.5612 to 0.7733), 0.8538 for miR-34a (95% CI, 0.7714 to 0.9362), and 0.7157 for miR-155 (95% CI, 0.6090 to 0.8224), respectively. At the cut-off value of 0.0006 for miR-15a, the sensitivity was 80% and the specificity was 76%; at the cut-off value of 0.0886 for miR-16-1, the sensitivity was 94% and the specificity was 51%; at the cut-off value of 1.395 for miR-34a, the sensitivity was 100% and the specificity was 70%.; at the cut-off value of 0.0022 for miR-155, the sensitivity was 83% and the specificity was 65% (Fig. 3).
Validation of miR-15a, miR-16-1, miR-21, miR-29c, and miR-155 on an independent group of serum samples (n = 152). Box plots of serum levels of a miR-15a, b miR-16-1, c miR-29c, d miR-34a, and e miR-155 in those who were healthy and normal (n = 77) and patients with DLBCL (n = 75). Expression levels of the miRNAs (log10 scale at y-axis) are normalized to cel-miR-39. The lines inside the boxes denote the medians. Statistically significant differences were determined using Mann–Whitney U tests
Receiver operating characteristic(ROC) plot reflects strong separation between the two groups, with an AUC of 0.7722 for miR-15a (95% CI, 0.6839 to 0.8605) (a), 0.7002 for miR-16-1 (95% CI, 0.6027 to 0.7978) (b), 0.6672 for miR-29c (95% CI, 0.5612 to 0.7733) (c), 0.8538 for miR-34a (95% CI, 0.7714 to 0.9362) (d), and 0.7157 for miR-155 (95% CI, 0.6090 to 0.8224) (e), respectively. At the cut-off value of 0.0006 for miR-15a, the sensitivity was 80% and the specificity was 76%; at the cut-off value of 0.0886 for miR-16-1, the sensitivity was 94% and the specificity was 51%; at the cut-off value of 1.395 for miR-34a, the sensitivity was 100% and the specificity was 70%; at the cut-off value of 0.0022 for miR-155, the sensitivity was 83% and the specificity was 65%
Relationship between serum levels of selection miRNAs and clinical characteristics
We examined the correlation between the expression of miR-15a, miR-16-1, miR-29c, miR-34a, and miR-155 with clinical parameters. No significant association was found between these five miRNAs and age, gender, stage, extranodal involvement, International Prognostic Index score (P > 0.05, Mann–Whitney U test, data not shown).
Discussion
The presence of altered miRNA profiles in plasma or serum has been reported for several types of solid tumors [4–7, 14]. In association with other markers, the changes in the levels of specific circulating miRNAs in plasma or serum offer the potential for high sensitivity and specificity in tumor detection and classification, because access to plasma or serum markers is certainly more direct and simple.
We demonstrate that three tumor-associated miRNA (miR-15a, miR-16-1, and miR-155) expression levels were not only to be apparently upregulated in patient serum compared with the normal controls, but also that these miRNAs have strong separation between the two groups. Lawrie et al. [8] reported that miR-155, miR-21, and miR-210 have potential as diagnostic biomarkers for DLBCL and that the serum levels of miR-21 were associated with relapse-free survival in DLBCL patients. Baraniskin et al. [15] found that miRNAs were the most abundant miRNA in cerebrospinal fluid of patients with primary central nervous system lymphoma (histopathologic diagnosis of diffuse large B cell type lymphoma). miR15 and miR16 lie within a small region of chromosome 13q14 that is deleted in more than 65% of chronic lymphocytic leukemia and that allelic loss in this region correlates with downregulation of both miR15 and miR16 expression [16]. miR-15a and miR-16a induce apoptosis by targeting the mRNA of the antiapoptotic gene B cell leukemia/lymphoma-2. miR-15a and miR-16a were “up-expressions” in DLBCL patient serum, maybe related with gains of 13q14 [17]. mRNA targets of miR-21 have been described as phosphatase and tensin homolog and programmed cell death 4, which regulate important cellular processes, including cell growth, proliferation, and apoptosis [18, 19]. miR-155 directly downregulates one of the MYC antagonists, for example, MAD1, MXI1, ROX/MNT, or any other. MiR-155 could act in cooperation with MYC or its related pathways in the transformation of B cells [20].
Extracellular miRNAs circulating in the peripheral blood are obviously included in cell membrane-derived particles, such as apoptotic bodies, microvesicles, and exosomes [21]. Caby et al. [22] has recently reported that 50 to 90 nm membrane-bound exosome-like particles, which are abundant in plasma, contain, in addition to other nucleic acids, mature miRNAs. Because miRNAs are exported from cells under some circumstances, the changes of the most abundant serum miRNAs species from miR-15a to miR-155 may be the result of the changing composition of lymphoid cells in circulation [23]. This raises the question of whether tumor-associated miRNAs are directly derived from tumor cells, but additional studies exploring a potential biologic function of miRNAs circulating in body fluids such as peripheral blood are required.
Although our results are promising, there are several limitations in this study. First, as the sample size is still small, further validations of this marker in large cohorts and in independent studies are necessary. Second, qRT-PCR by the relative quantification approach becomes less accurate if measuring with low levels of miRNAs, in which they may not fall into the linear range of the assay, yet absolute quantification approach with standard curve calibration would be preferable for further validation of our approach.
In conclusion, serum miRNAs appear to be novel biomarkers for early detection of DLBCL. Our data serve as basis for further investigation, preferably in large prospective studies before these miRNAs can be used as a noninvasive screening tool for DLBCL in routine clinical practice.
References
Abramson JS, Shipp MA (2005) Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood 106(4):1164–1174
Pfreundschuh M (2010) How I treat elderly patients with diffuse large B-cell lymphoma. Blood 116(24):5103–5110
Ferracin M, Veronese A, Negrini M (2010) Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn 10(3):297–308
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Nat Acad Sci 105(30):10513–10518
Moussay E, Wang K, Cho JH, van Moer K, Pierson S, Paggetti J, Nazarov PV, Palissot V, Hood LE, Berchem G, Galas DJ (2011) MicroRNA as biomarkers and regulators in B-cell chronic lymphocytic leukemia. Proc Nat Acad Sci U S A 108(16):6573–6578
Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ (2009) Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 58(10):1375–1381
Tanaka M, Oikawa K, Takanashi M, Kudo M, Ohyashiki J, Ohyashiki K, Kuroda M (2009) Down-regulation of miR-92 in human plasma is a novel marker for acute leukemia patients. PLoS One 4(5):e5532
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141(5):672–675
SH S, E C, NL H (2008) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12
Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, Cattan H, Enver T, Mager R, Boultwood J, Wainscoat JS, Hatton CS (2007) MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer 121(5):1156–1161
Zhu DX, Miao KR, Fang C, Fan L, Zhu W, Zhu HY, Zhuang Y, Hong M, Liu P, Xu W, Li JY (2011) Aberrant microRNA expression in Chinese patients with chronic lymphocytic leukemia. Leuk Res 35(6):730–734
Sampath D, Calin GA (2009) miRs: fine-tuning prognosis in CLL. Blood 113(21):5035–5036
Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE (2009) The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112(1):55–59
Baraniskin A, Kuhnhenn J, Schlegel U, Chan A, Deckert M, Gold R, Maghnouj A, Zollner H, Reinacher-Schick A, Schmiegel W, Hahn SA, Schroers R (2011) Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 117(11):3140–3146
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM (2002) Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 99(24):15524–15529
Tagawa H, Suguro M, Tsuzuki S, Matsuo K, Karnan S, Ohshima K, Okamoto M, Morishima Y, Nakamura S, Seto M (2005) Comparison of genome profiles for identification of distinct subgroups of diffuse large B-cell lymphoma. Blood 106(5):1770–1777
Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H (2008) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27(15):2128–2136
Medina PP, Nolde M, Slack FJ (2010) OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature 467(7311):86–90
Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A (2004) High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 39(2):167–169
Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101(10):2087–2092
Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17(7):879–887
Wang K, Zhang S, Weber J, Baxter D, Galas DJ (2010) Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 38(20):7248–7259
Acknowledgments
This study was supported by National Natural Science Foundation of China (30871104, 30971296, 81170488), Natural Science Foundation of Jiangsu Province (BK2010584), “Qing Lan” project of Jiangsu Province, “Liu Da RenCaiGaoFeng” of Jiangsu Province, University Doctoral Foundation of the Ministry of Education of China (20093234110010), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, C., Zhu, DX., Dong, HJ. et al. Serum microRNAs are promising novel biomarkers for diffuse large B cell lymphoma. Ann Hematol 91, 553–559 (2012). https://doi.org/10.1007/s00277-011-1350-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1350-9