Abstract
Patients with Huntington’s disease (HD) have an increased incidence of diabetes. However, the molecular mechanisms of pancreatic β-cell dysfunction have not been entirely clarified. Revealing the pathogenesis of diabetes can provide a novel understanding of the onset and progression of HD, as well as potential clues for the development of new therapeutics. Here, we demonstrated that the mouse pancreatic insulinoma cell line NIT-1 expressing N-terminal mutant huntingtin (mHTT) containing 160 polyglutamine (160Q cells) displayed lower cell proliferative ability than the cells expressing N-terminal wild-type HTT containing 20 polyglutamine (20Q cells). In addition, 160Q cells were more prone to apoptosis and exhibited deficient glucose-stimulated insulin expression and secretion. Furthermore, insulin signaling molecule insulin receptor substrate 2 (IRS-2) expression decreased and was recruited into mHTT aggregates. Consequently, glucose stimulation failed to activate the downstream molecule phosphatidylinositol-3 kinase (PI3K) in 160Q cells, leading to reduced phosphorylation levels of serine–threonine protein kinase AKT and forkhead box protein O1 (FoxO1). These data indicate that activation of the glucose-stimulated PI3K/AKT/FoxO1 signaling pathway is significantly blocked in pancreatic β-cells in HD. Importantly, insulin treatment inhibited the aggregation of mHTT and significantly improved the activation of PI3K/AKT/FoxO1 signaling in 160Q cells. These results suggest that the inhibition of the PI3K/AKT/FoxO1 pathway might be due to the recruitment of IRS-2 into mHTT aggregates in HD β-cells, ultimately contributing to the impairment of pancreatic β-cells. In conclusion, our work provides new insight into the underlying mechanisms of the high incidence of diabetes and abnormal glucose homeostasis in HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Huntington’s disease (HD) is a neurodegenerative disease underlying autosomal dominant pathology as a CAG expansion (encoding glutamine ≥ 36 repeats) located in exon 1 of the gene encoding huntingtin (HTT) (Walker 2007). The disease is characterized by psychiatric, motor, and cognitive disturbances and progressive weight loss. Importantly, a higher prevalence of diabetes has been observed among HD patients (Farrer 1985; Hu et al. 2014; Montojo et al. 2017) and transgenic mice (Andreassen et al. 2002; Björkqvist et al. 2005; Hunt and Morton 2005; Hurlbert et al. 1999). It was reported that the incidence of diabetes was 10.5% in a group of 620 HD patients (Farrer 1985), and 72.7% developed diabetes in a five-generation-large HD family (Hu et al. 2014). In addition, HD transgenic mice display impaired glucose tolerance (Björkqvist et al. 2005; Josefsen et al. 2008). These peripheral impairments not only may significantly affect the quality of life of HD patients, but may also aggravate the neurodegenerative process by disrupting glucose metabolism and systemic energy homeostasis (Craft and Watson 2004; Morea et al. 2017; van der Burg et al. 2009). However, the cause of abnormalities in glucose homeostasis and the higher incidence of diabetes is largely unknown. Consequently, determining the mechanisms of the effect of mutant HTT (mHTT) on glucose homeostasis is an important step toward developing effective therapeutic targets in HD.
Numerous studies have implicated impaired vesicles transport, apoptosis, and transcriptional repression caused by mHTT in HD-associated neurodegeneration (Niu et al. 2020; White et al. 2020; Yu et al. 2000). mHTT aggregates are viewed in both neurons and several other endocrine cells, such as pancreatic β-cells (Moffitt et al. 2009; Sathasivam et al. 1999), which play an important role in cytotoxicity of mHTT. Intranuclear aggregates accumulate with aging in pancreatic islets of HD R6/2 transgenic mice, concomitant with decreased glucose tolerance (Andreassen et al. 2002). The aggregates are involved in hyperglycemia and insulin-deficient diabetes in R6/2 transgenic mice (Hurlbert et al. 1999; Andreassen et al. 2002). Reduction of aggregates ameliorates the loss of insulin secretion in β-cells expressing mHTT (Ye and Li 2009). In addition, R6/2 transgenic mice exhibit deficient β-cell mass, reduced insulin mRNA level, and insulin exocytosis (Björkqvist et al. 2005; Smith et al. 2009). Several other pathogenic mechanisms have also been elucidated. For instance, mHTT disrupts vesicular transport and insulin secretion by interacting with microtubular β-tubulin (Smith et al. 2009). Beyond that, R6/2 mice develop diminished expression of key insulin gene transcription regulators including the pancreatic and duodenal homeobox-1 (Pdx-1), E2A proteins, and the coactivators CBP and p300 (Andreassen et al. 2002). Notably, DNA vaccination against mHTT ameliorated the diabetic phenotype in HD R6/2 transgenic mice, emphasizing the role of mHTT in the mechanism of diabetes (Miller et al. 2003). The underlying molecular mechanism of β-cell dysfunction has, however, not yet been rigorously investigated in HD. Elucidating the mechanism is vital to shed new light on the pathological processes of HD.
Insulin receptor (IR) and its downstream signal molecules play a critical role in regulating insulin secretion and maintaining β-cell proliferation and survival (Dearth et al. 2007; Shirakawa et al. 2017). Upon binding with insulin, IR is activated through autophosphorylation, further phosphorylating IR substrates (IRS) and regulating a variety of signaling pathways. IRS-2, one member of the IRS protein family, is the most prominently expressed in pancreatic β-cells (Lingohr et al. 2006). Above all, the importance of insulin signaling downstream of the phosphatidylinositol-3 kinase (PI3K)/serine–threonine protein kinase AKT (also known as protein kinase B) pathway has been highlighted, which is key to regulating glucose uptake, β-cell survival, and insulin gene transcription (Du et al. 2020; Martinez et al. 2008). The dysfunction of β-cells plays a potent role in the pathogenesis of both type 1 and type 2 diabetes mellitus (T2DM). Insulin produced in β-cells is a critical regulator of metabolism. Thus, β-cells are a metabolic hub in the body, connecting nutrient metabolism and the endocrine system. More importantly, insulin has proved effective in improving HD phenotypes via modulation of the PI3K/AKT pathway. Insulin treatment ameliorates mitochondrial function and clears mHTT aggregates, which improves motor abnormalities and prolongs survival (Bryan and Bowman 2017). Therefore, PI3K/AKT proteins as cellular components play an indispensable role in inhibiting the effects of HD (Chong et al. 2012; Sayed et al. 2020). Given that, we speculated that mHTT is likely to exert an adverse effect on pancreatic β-cell function by impairing the PI3K/AKT signaling pathway in HD.
In order to test the hypothesis, in this study, mouse pancreatic insulinoma NIT-1 cells were utilized as cell models to investigate the impairment effect of mHTT and the potential molecular mechanisms of diabetes in HD. We demonstrate that mHTT inhibits β-cell viability and diminishes insulin production and secretion by recruiting IRS-2 into the insoluble aggregates and disturbing the PI3K/AKT/FoxO1 signaling pathway. The results provide new insight into the pathogenesis of HD.
Materials and Methods
Cell Culture and Transfection
The mouse pancreatic insulinoma cell line NIT-1 (a kind gift from Professor Guan-xin Shen, Tongji Medical College, Wuhan, China) was cultured in DMEM (Invitrogen) containing 5.6 mM glucose supplemented with 10% fetal bovine serum. The cells were seeded in six-well plates and transfected with plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Plasmids including Cherry-exon-1 or GFP-exon-1 HTT with 160 CAG repeats (160Q) and 20 CAG repeats (20Q) were produced in our lab. An empty vector served as control. At 48 h after transfection, cells were collected for the detection of HTT expression.
Immunofluorescent Staining
At 48 h or 72 h after transfection, NIT-1 cells were fixed for 20 min in 2.5% paraformaldehyde and incubated overnight at 4 °C with primary antibody, EM48 (1:1000, a gift from Dr. Xiao-jiang Li, Jinan University), a rabbit antibody against amino-terminal fragment of huntingtin (Ye and Li 2009), or IRS-2 (1:200, Catalog #3089, Cell Signaling Technology). Subsequently, cells were incubated with FITC or rhodamine-conjugated secondary antibody (1:50, Catalog #111–095-003, #111–295-003, Jackson ImmunoResearch). Nuclei were stained with 1 µg/ml DAPI (4′,6-diamidino-2-phenylindole) for 10 min at room temperature. Images were obtained with a fluorescence microscope (Nikon Eclipse TE2000-U) or a laser scanning confocal microscope (Olympus FV1000).
MTT Assay
Cellular viability was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Briefly, cells in plates were incubated in DMEM containing MTT (0.5 mg/ml) for 4 h at 37 °C. Subsequently, all media were replaced by 100 μl/well of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) to dissolve the formazan crystals for another 30 min. The absorbance was measured at 490 nm using an EnSpire® Multimode Plate Reader (PerkinElmer).
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
NIT-1 cells were seeded on coverslips. At 48 h after transfection, NIT-1 cells were treated with 1 µM apoptotic inducer staurosporine for 3 h. Apoptotic cells were assessed by using the One Step TUNEL Apoptosis Assay Kit (Catalog #C1086, Beyotime) according to the manufacturer’s instructions. Cells were fixed with 4% paraformaldehyde for 30 min and then permeabilized with 0.3% Triton X-100 in 0.1% sodium citrate for 5 min. TUNEL reaction mixture (100 µl) was added to the coverslips. After incubation for 1 h at 37 °C, nuclei were stained with DAPI for 10 min. Images were obtained with a laser scanning confocal microscope (Olympus FV1000). The number of TUNEL-positive cells was determined in 10 randomly selected fields. The experiment was performed three times.
Insulin Secretion Tests
After glucose treatment for 30 min, insulin release in Krebs–Ringer bicarbonate (KRB) buffer was assayed using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Abcam) according to the manufacturer’s instructions. All standards, controls, and samples were run in duplicate. The insulin concentrations were determined with dedicated software (CurveExpert), plotting the absorbance of the standards against the standard concentrations to derive the sample concentrations. The experiment was performed three times.
Quantitative Real-Time PCR
After glucose treatment for 30 min, total RNA of NIT-1 cells was extracted and reverse-transcribed as previously described (Niu et al. 2020). Real-time polymerase chain reaction (PCR) was performed using the SYBR green method. Fluorescent quantitative PCR was executed for 45 rounds (95 °C 10 min, 95 °C 15 s, 60 °C 60 s). Primer sequences were as follows: insulin forward: 5′-ACGAACACTTTGCCATTGCC-3′, 5′-CCTTTGCCCGATTATGCAGC-3′; β-actin forward: 5′-GCCCTGAGGCTCTCTTCCA-3′, reverse: 5′-GCGGATGTCGACGTCACA-3′. The experiments were carried out three times.
Western Blotting
For the assay of cleaved caspase-3 expression, at 48 h after transfection, NIT-1 cells were treated with 1 µM apoptotic inducer staurosporine for 3 h. For detecting IRS-2 expression, at 48 h after transfection, NIT-1 cells were incubated in KRB buffer (120 mM NaCl, 2.5 mM CaCl2, 4.8 mM KCl, 24 mM NaHCO3, 1.2 mM MgCl2, and 1 g/L bovine serum albumin [BSA]) containing different concentrations of glucose (0 mM, 5.6 mM, or 11.1 mM) for 12 h. The expression of IRS-2 in NIT-1 cells at 5.6 mM glucose concentration was also detected at 72 h after transfection. For the detection of insulin and PI3K signaling molecules expression, transfected NIT-1 cells were pre-incubated for 2 h in glucose-free KRB buffer and then incubated for 30 min in KRB buffer containing different concentrations of glucose (5.6 mM, 11.1 mM, 24.2 mM) or incubated for 30 min in KRB buffer containing 100 nM insulin (Sigma-Aldrich). Then, cells were harvested and lysed in RIPA buffer including 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS) (Catalog #P0013k, Beyotime) supplemented with protease and phosphatase inhibitors (cocktail, 1:1000, Sigma-Aldrich; phenylmethylsulfonyl fluoride, 100 µg/mL, Sigma-Aldrich). Protein samples were separated by SDS- polyacrylamide gel electrophoresis (PAGE) and transferred onto NC membranes. Blots were incubated overnight at 4 °C in primary antibodies: 1:10,000 for γ-tubulin (Catalog #SAB4701030, Sigma-Aldrich), 1:5000 for GAPDH (Catalog #SAB2103104, Sigma-Aldrich), and 1:1000 for insulin (Catalog #SAB4200691, Sigma-Aldrich), cleaved caspase-3 (Catalog #9661, Cell Signaling Technology), IRS-2 (Catalog #3089, Cell Signaling Technology), phospho-AKT (Ser473) (Catalog #9271, Cell Signaling Technology), AKT (Catalog #9272, Cell Signaling Technology), phospho-FoxO1 (Ser256) (Catalog #9461, Cell Signaling Technology), FoxO1 (Catalog #9454, Cell Signaling Technology), and EM48, and then incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The immunoreactive bands were visualized by exposure to enhanced chemiluminescence (ECL) (Pierce Thermo Scientific).
Co-Immunoprecipitation
At 48 h after transfection, cells treated with 100 nM insulin or different concentrations of glucose were collected and lysed in lysis buffer at pH 7.4 (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM MgCl2, 1 mM NaF, 1 mM Na3VO4, 1% NP 40, 10% glycerol) supplemented with protease and phosphatase inhibitors (PMSF and cocktail) for 20 min on ice. After centrifugation at 13,500 × g for 15 min, protein lysate was pre-cleared with protein A/G Sepharose beads for 1 h and incubated with anti-IRS-2 antibody (1:50, Catalog #3089, Cell Signaling Technology) overnight at 4 °C. After additional incubation with protein A/G Sepharose beads, immune complexes were harvested by centrifugation and thoroughly washed with washing buffer and boiled with SDS loading buffer. Then, the samples were subjected to SDS-PAGE and immunoblot with anti-PI3K p85 (1:1000, Catalog #4292, Cell Signaling Technology) or anti-IRS-2 antibody. The experiments were replicated three times.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software for one-way analysis of variance (ANOVA) followed by Student’s t test. Differences were considered significant for values of p < 0.05. All values were represented as mean ± SD.
Results
-
1.
Mutant huntingtin inhibited proliferation and induced apoptosis in pancreatic NIT-1 cells.
To estimate the effect of mHTT on cell viability of pancreatic β-cells, we transfected the Cherry-fused HD exon 1 protein with 20Q and 160Q into pancreatic NIT-1 β-cells. At 48 h after transfection, we detected the expression of HTT by using immunofluorescent staining and Western blotting. 160Q mHTT was located predominantly in the cytoplasm of NIT-1 cells, and aggregates were visible in the cytoplasm of some cells (Fig. 1a). The mHTT aggregates that stayed in the stacking gel were also identified by Western blotting (Fig. 1b).
Next, the cell proliferative ability of NIT-1 cells expressing 160Q mHTT or 20Q HTT (160Q or 20Q cells) was evaluated by MTT assay. NIT-1 cells were cultured in medium containing 5.6 mM glucose for 48 h after transfection. mHTT led to significant inhibition of cell proliferation in 160Q cells (Fig. 1c). Moreover, after stimulation with apoptotic inducer staurosporine for 3 h, 160Q cells highly expressed cleaved caspase-3 in comparison with control or 20Q cells (Fig. 1d). The increasing apoptosis in 160Q cells was further demonstrated by TUNEL assay (Fig. 1e and f). These data imply that mHTT inhibits cell proliferation and makes β-cells more susceptible to apoptosis.
-
2.
Mutant huntingtin reduced glucose-stimulated insulin production and secretion in NIT-1 cells.
As glucose has emerged as a key stimuli of insulin secretion in pancreatic β-cells, we assessed the effect of mHTT on glucose-stimulated insulin expression and secretion in NIT-1 cells. At 48 h after transfection, NIT-1 cells were pre-incubated with glucose-free KRB buffer for 2 h and then treated with different concentrations of glucose (5.6 mM, 11.1 mM, or 24.2 mM) for 30 min. Insulin secretion was measured by ELISA methods. Insulin release significantly enhanced with the increase of glucose concentration from 5.6 mM to 24.2 mM in controls and 20Q cells. However, at the same glucose concentrations (11.1 mM and 24.2 mM), insulin secretion was considerably lower in 160Q NIT-1 cells than that in matched controls and 20Q cells (Fig. 2a). These results verify that mHTT damages glucose-stimulated insulin secretion in pancreatic β-cells.
Glucose serves as a master regulator of insulin biosynthesis, and also maintains insulin mRNA stability. The observed role in inhibiting insulin secretion could be due to decreased insulin expression in 160Q cells. We next measured total insulin content in 160Q cells, 20Q cells, and control cells. Western blotting assay showed that both control cells and 20Q NIT-1 cells markedly upregulated insulin content with increasing glucose concentration. In contrast, glucose stimulation failed to promote insulin content in 160Q NIT-1 cells (Fig. 2c and d).
To further clarify the insulin content deficiency observed, we investigated insulin mRNA levels in these cells treated with glucose at different concentrations. Similarly, insulin mRNA levels increased significantly in control cells and 20Q cells treated with glucose, whereas there was no dramatic increase in insulin mRNA levels in 160Q cells after 24.2 mM glucose treatment (Fig. 2b). These data demonstrate that the decrease in glucose-stimulated insulin secretion is associated with deficient insulin expression in pancreatic β-cells in HD.
-
3.
Mutant huntingtin recruited IRS-2 into aggregates in NIT-1 cells.
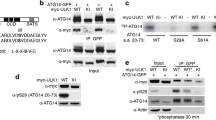
Glucose affects insulin expression and survival of pancreatic β-cells via activating the insulin signaling pathway. We next detected the expression of insulin signaling molecules IRS-2 in 160Q and 20Q NIT-1 cells. The results showed that the expression of IRS-2 decreased significantly at 48 h and 72 h after transfection in 160Q cells at 5.6 mM glucose concentration, compared with 20Q cells (Fig. 3a and b). On the other hand, the level of IRS-2 expression was lower in 160Q cells than that in 20Q cells at the same glucose concentration (5.6 mM and 11.1 mM) (Fig. 3c and d).
Glucose can upregulate the expression of IRS-2 in islet β-cells, which is not mediated via secreted insulin (Lingohr et al. 2006). Therefore, the decreased IRS-2 protein level might not be due to the deficient insulin release. It has been showed that mHTT can recruit proteins into aggregates, contributing to the pathogenesis of HD (Chiang et al. 2007). Therefore, we performed the immunofluorescent staining to identify the expression of IRS-2 in NIT-1 cells transfected with GFP-fused 160Q mHTT or 20Q HTT, respectively. At 48 h after transfection, GFP-fused mHTT formed aggregates in the cytoplasm of NIT-1 cells. Importantly, IRS-2 immunofluorescent staining was strongly positive in mHTT aggregates in 160Q cells, whereas 20Q cells mainly exhibited positive IRS-2 staining on the cytomembrane (Fig. 3e). The results suggest that mHTT can recruit IRS-2 into the aggregates and block the role of IRS-2 in mediating insulin signaling in pancreatic β-cells.
-
4.
Mutant huntingtin inhibited activation of the glucose-stimulated PI3K/AKT signaling pathway in NIT-1 cells.
PI3K is a critical node between IRS and AKT in insulin signaling (Fernández et al. 2007; Shirakawa and Terauchi 2020). Once phosphorylated, IRS-2 binds the p85 regulatory subunit of PI3K and activates PI3K signaling (Fernández et al. 2007). To evaluate the association of IRS-2 with the p85 subunit of PI3K, NIT-1 cells were treated with different concentrations of glucose for 30 min after pre-incubation with glucose-free KRB buffer. Then, we investigated the activity of PI3K/AKT signaling molecules. As expected, co-immunoprecipitation showed that high glucose (24.2 mM) upregulated the binding of PI3K p85 to IRS-2 in both control cells and 20Q cells, but not in 160Q cells (Fig. 4a). Correspondingly, AKT phosphorylation also increased in both control cells and 20Q cells after high glucose stimulation (Fig. 4b and c). In contrast, neither PI3K nor AKT was activated by glucose in 160Q NIT-1 cells (Fig. 4a–c). Furthermore, we checked the expression of FxoO1. FxoO1 is a downstream target of AKT kinase, which can affect pancreatic β-cell survival and the transcription of insulin gene and glucose transporter (GLUT) (Shirakawa and Terauchi 2020). Phosphorylation of FxoO1 was elevated by glucose in both control cells and 20Q cells, whereas glucose failed to promote the phosphorylation of FxoO1 in 160Q cells (Fig. 4d and e). Together, these data signify that mHTT inhibits the activation of glucose-stimulated PI3K/AKT signaling via recruiting IRS-2 protein into insoluble aggregates, finally leading to the reduction of insulin secretion and poor cell survival of pancreatic β-cells in HD.
-
5.
Insulin activated the PI3K/AKT signaling pathway by suppressing the aggregation of mHTT in NIT-1 cells.
NIT-1 cells expressing mutant huntingtin display decreased cell viability and increased apoptosis. a Identification of huntingtin expression in transfected NIT-1 cells. Left column shows the expression of huntingtin tagged with Cherry (red), and middle column shows the expression of huntingtin labeled by immunofluorescent stain (green). Right column shows the merged images. Scale bar, 100 µm. The boxed area indicates aggregates of mHTT, and is shown in the upper-right corner at a higher magnification. b Western blotting assay of mHTT aggregates. All samples are extracts from cellular lysates without insoluble precipitates. Aggregated huntingtin remains in the stacking gel. c MTT assay shows that the proliferative ability of 160Q NIT-1 cells is lower than that of control or 20Q cells. Mean ± SD, 0.69 ± 0.07 (Con), 0.73 ± 0.06 (20Q), 0.42 ± 0.04 (160Q). Con, control, NIT-1 cells transfected with empty vector. *p < 0.05, compared with control or 20Q NIT-1 cells. n = 3. d Western blotting and statistical analysis show that 160Q NIT-1 cells highly express cleaved caspase-3, compared with control or 20Q NIT-1 cells. Mean ± SD, 0.13 ± 0.05 (Con), 0.14 ± 0.09 (20Q), 0.53 ± 0.09 (160Q). **p < 0.01, compared with control or 20Q NIT-1 cells. n = 3. e, f TUNEL staining and statistical analysis show that there are more TUNEL-positive cells in 160Q NIT-1 cells than those in 20Q NIT-1 cells. The boxed area indicates TUNEL-positive cells, and is shown in the upper-right corner at a higher magnification. Scale bar, 50 µm. Mean ± SD, 5.07 ± 2.00 (20Q), 14.67 ± 4.16 (160Q). *p < 0.05, compared with 20Q NIT-1 cells. n = 3
Mutant huntingtin inhibits glucose-stimulated insulin expression and secretion in NIT-1 cells. a ELISA analysis shows that glucose-stimulated insulin secretion significantly decreases in 160Q cells, compared with control or 20Q cells. Mean ± SD, 2.26 ± 0.15 (Con, 5.6 mM), 2.33 ± 0.25 (20Q, 5.6 mM), 1.87 ± 0.21 (160Q, 5.6 mM), 5.13 ± 0.30 (Con, 11.1 mM), 4.81 ± 0.36 (20Q, 11.1 mM), 3.11 ± 0.46 (160Q, 11.1 mM), 5.87 ± 0.25 (Con, 24.2 mM), 5.83 ± 0.32 (20Q, 24.2 mM), 3.87 ± 0.31 (160Q, 24.2 mM). b Quantitative real-time PCR shows that glucose-stimulated insulin mRNA level is significantly decreased in 160Q cells, compared with control or 20Q cells. Mean ± SD, 1 (Con, 5.6 mM), 1.13 ± 0.21 (20Q, 5.6 mM), 0.93 ± 0.32 (160Q, 5.6 mM), 3.47 ± 0.15 (Con, 11.1 mM), 3.33 ± 0.38 (20Q, 11.1 mM), 2.21 ± 0.26 (160Q, 11.1 mM), 5.63 ± 0.25 (Con, 24.2 mM), 5.07 ± 0.21 (20Q, 24.2 mM), 2.33 ± 0.31 (160Q, 24.2 mM). c, d Western blotting and quantitative measure show that glucose-stimulated insulin protein expression significantly decreases in 160Q cells, compared with control or 20Q cells. Mean ± SD, 0.19 ± 0.04 (Con, 5.6 mM), 0.18 ± 0.04 (20Q, 5.6 mM), 0.23 ± 0.04 (160Q, 5.6 mM), 0.39 ± 0.07 (Con, 11.1 mM), 0.44 ± 0.04 (20Q, 11.1 mM), 0.24 ± 0.05 (160Q, 11.1 mM), 0.58 ± 0.05 (Con, 24.2 mM), 0.65 ± 0.10 (20Q, 24.2 mM), 0.27 ± 0.08 (160Q, 24.2 mM). *p < 0.05, compared with the same group of cells treated with 5.6 mM glucose; Δ p < 0.05, compared with the same group of cells treated with 11.1 mM glucose; # p < 0.05, compared with control or 20Q cells treated with the same concentration of glucose. n = 3
Mutant huntingtin downregulates IRS-2 protein level by recruiting IRS-2 into aggregates in 160Q NIT-1 cells. a–d Western blotting and quantitative analysis show that mHTT downregulates the protein level of IRS-2 at 48 h or 72 h after transfection, or at different glucose concentration. Mean ± SD, 0.37 ± 0.04 (20Q, 48 h), 0.22 ± 0.03 (160Q, 48 h), 0.49 ± 0.05 (20Q, 72 h), 0.21 ± 0.03 (160Q, 72 h), 0.20 ± 0.04 (20Q, 0 mM), 0.17 ± 0.03 (160Q, 0 mM), 0.36 ± 0.04 (20Q, 5.6 mM), 0.20 ± 0.03 (160Q, 5.6 mM), 0.59 ± 0.03 (20Q, 11.1 mM), 0.44 ± 0.04 (160Q, 11.1 mM). **p < 0.01, compared with matched 20Q cells. n = 3. e Mutant huntingtin recruits IRS-2 into aggregates in 160Q NIT-1 cells. Left column shows the expression of huntingtin tagged with GFP (green). Middle columns show the expression of IRS-2 labeled by immunofluorescent stain (red) and nuclei stained with DAPI (blue). Right column shows the merged images. Arrows show aggregates of mHTT. Scale bar, 50 µm
Mutant huntingtin inhibits the activation of the glucose-stimulated PI3K/AKT/FoxO1 signaling pathway in NIT-1 cells. a Cell lysates were immunoprecipitated with an anti-IRS-2 antibody and then immunoblotted with anti-PI3K p85 or anti-IRS-2 antibody. b–e Western blotting and quantitative measurement show that mHTT inhibits the activation of glucose-stimulated AKT and FoxO1. Mean ± SD of relative level of P-AKT, 0.20 ± 0.04 (Con, 5.6 mM), 0.77 ± 0.35 (Con, 24.2 mM), 0.19 ± 0.05 (20Q, 5.6 mM), 0.81 ± 0.07 (20Q, 24.2 mM), 0.23 ± 0.03 (160Q, 5.6 mM), 0.26 ± 0.04 (160Q, 24.2 mM). Mean ± SD of relative level of P-FoxO1, 0.26 ± 0.06 (Con, 5.6 mM), 0.78 ± 0.03 (Con, 24.2 mM), 0.24 ± 0.04 (20Q, 5.6 mM), 0.78 ± 0.04 (20Q, 24.2 mM), 0.26 ± 0.03 (160Q, 5.6 mM), 0.29 ± 0.09 (160Q, 24.2 mM). **p < 0.01, compared with the same group of cells treated with 5.6 mM glucose. n = 3. P-AKT, phosphorylated AKT; P-FoxO1, phosphorylated FoxO1
Insulin can activate the PI3K/AKT signaling pathway by phosphorylating IR in pancreatic β-cells (Shirakawa and Terauchi 2020). We tested the modulation of exogenous insulin on PI3K/AKT signaling in 160Q and 20Q cells. After transfection for 48 h, NIT-1 cells were pre-incubated for 2 h with glucose-free KRB buffer, followed by insulin treatment for 30 min. As expected, co-immunoprecipitation showed that insulin dramatically upregulated the binding of PI3K p85 to IRS-2, as well as phosphorylated levels of AKT and FoxO1 in both control cells and 20Q cells (Fig. 5a–e). Surprisingly, insulin treatment considerably potentiated activation of PI3K/AKT signaling in 160Q cells as well (Fig. 5a–c). Correspondingly, the increasing phosphorylated level of FoxO1 was detected in 160Q cells (Fig. 5d and e). These results denote that exogenous addition of insulin effectively activates the PI3K/AKT signaling pathway in 160Q cells.
Insulin inhibits the aggregation of mHTT and promotes activation of the PI3K/AKT/FoxO1 signaling pathway in 160Q NIT-1 cells. a Cell lysates were immunoprecipitated with an anti-IRS-2 antibody and then immunoblotted with anti-PI3K p85 or anti-IRS-2 antibody. b–e Western blotting and quantitative measurements show that insulin upregulates the activation of AKT and FoxO1 in 160Q NIT-1 cells. Mean ± SD of relative level of P-AKT, 0.19 ± 0.04 (Con, without insulin), 0.53 ± 0.04 (Con, with insulin), 0.21 ± 0.02 (20Q, without insulin), 0.52 ± 0.08 (20Q, with insulin), 0.19 ± 0.03 (160Q, without insulin), 0.51 ± 0.06 (160Q, with insulin). Mean ± SD of relative level of P-FoxO1, 0.25 ± 0.04 (Con, without insulin), 0.68 ± 0.05 (Con, with insulin), 0.27 ± 0.07 (20Q, without insulin), 0.63 ± 0.05 (20Q, with insulin), 0.22 ± 0.03 (160Q, without insulin), 0.59 ± 0.03 (160Q, with insulin). **p < 0.01, compared with the same group of cells without insulin treatment. n = 3. f Insulin inhibits the aggregation of mHTT in 160Q NIT-1 cells. The boxed area indicates aggregates of mHTT, and is shown in the upper-right corner at a higher magnification. Scale bar, 50 µm. g The percentage of cells containing mHTT aggregates significantly decreased in 160Q cells treated with insulin. Mean ± SD, 24.33 ± 3.51 (without insulin), 5.67 ± 3.06 (with insulin). **p < 0.01, compared with 160Q cells without insulin treatment. n = 3
Given that insulin can clear mHTT insoluble aggregates (Bryan and Bowman, 2017), we speculated that insulin improved PI3K/AKT signaling by decreasing mHTT aggregation. Therefore, we investigated the effect of insulin on mHTT aggregates in 160Q NIT-1 cells. The result showed that insulin significantly decreased the aggregation of mHTT in 160Q cells (Fig. 5f and g), indicating that insulin improves the PI3K/AKT signaling pathway by inhibiting the aggregation of mHTT in β-cells.
Discussion
Endocrine abnormality is a common feature of many neurodegenerative diseases such as HD, Alzheimer’s disease, and Parkinson’s disease. These patients show a higher prevalence of diabetes, disturbed insulin sensitivity, and impaired insulin secretion, compared to the general population (Bendlin 2019; Lalić et al. 2008; Ristow 2004). HD patients are more likely to develop T2DM. Hyperglycemia and impaired β-cell function are the characters of T2DM (Garber 2012). The HD R6/2 transgenic mice exhibited the failure of β-cell mass growth with age and disrupted insulin exocytosis (Björkqvist et al. 2005), suggesting that endocrine pathology is an important feature of HD. Indeed, the pathogenetic mechanisms in the pancreatic β-cells could also reflect important cellular changes that occur in neurons. Therefore, a deeper understanding of pancreatic β-cell dysfunction in HD could ultimately lead to the discovery of novel therapeutic targets in the brain. In our studies, 160Q NIT-1 cells exhibited deficient cell proliferation and were more prone to apoptosis compared to 20Q NIT-1 cells. The present data of decreased cell proliferation were similar to the changes observed in R6/2 mice. There was a significant reduction in replication of islet cells in R6/2 mice (Björkqvist et al. 2005). Numerous studies have proved that mHTT dramatically promotes apoptosis of neurons in HD patients and transgenic mice, contributing to HD pathogenesis (Gil and Rego 2008; Yu et al. 2000). Clearly, the deficient cell proliferation and increasing apoptosis might be the key factors resulting in a decrease in pancreatic islet β-cell mass in HD.
HD patients often present with dysregulation of glucose homeostasis (Brás et al. 2019). The maintenance of glucose homeostasis largely depends on normal regulation of insulin gene transcription (Ueki et al. 2006). At the same time, glucose is the most important factor that stimulates insulin gene transcription and mRNA translation. In this work, both insulin mRNA level and content in 160Q NIT-1 cells were significantly lower than that in 20Q cells at the same glucose concentration (11.1 mM and 24.2 mM). These results were consistent with the previous report that HD transgenic mice exhibited a defect in insulin mRNA expression (Andreassen et al. 2002). Transcription dysregulation is one of the important pathogenic mechanisms in HD (Niu et al. 2020; Pogoda et al. 2020; Yildirim et al. 2019). The downregulation of insulin transcription could lead to insulin secretion reduction in 160Q NIT-1 cells. In addition, the mHTT aggregates in 160Q cells could also affect exocytosis of insulin by disrupting vesicular transport (Smith et al. 2009). Collectively, our data demonstrate that the impairment of glucose-stimulated insulin secretion is related with the inhibition of insulin gene transcription and the aggregation of mHTT in pancreatic β-cells in HD.
Glucose plays a pivotal role in improving pancreatic β-cell survival and function via the PI3K/AKT pathway of insulin signaling (Gao et al. 2019; Liao et al. 2019). Of note, the imbalance of PI3K/AKT signaling gives rise to the development of T2DM (Gao et al. 2019; Huang et al. 2018; Liao et al. 2019). The present work indicates that mHTT downregulates the expression of IRS-2 protein by recruiting IRS-2 into aggregates. Previous reports have demonstrated that mHTT induces cytotoxicity by recruiting a variety of proteins (Chiang et al. 2007). Additionally, we demonstrate that mHTT significantly prevents the activation of PI3K and phosphorylation of its downstream targets AKT and FoxO1. PI3K consists of a regulatory subunit p85 and a catalytic subunit p110. The p85 subunit plays a central role in providing stability and maintaining the activity of the PI3K complex. The functional deficiency in each subunit may result in glucose metabolism disorder (Fernández et al. 2007). Here, the binding of p85 to IRS-2 was significantly inhibited by mHTT in 160Q NIT-1 cells, causing the failure of the activation of the PI3K signaling pathway. FoxO proteins are important transcription factors regulating proliferation and metabolism. FoxO1, the most abundant family member in pancreatic β-cells, is negatively regulated by AKT activation. Glucose treatment inhibits FoxO1 activity through AKT-mediated phosphorylation and nuclear exclusion in β-cells (Martinez et al. 2006). FoxO1 in a nucleus is a negative regulator of Pdx1 mRNA expression (Kawamori et al. 2006). Phosphorylated FoxO1 is translocated from the nucleus to the cytoplasm, which relieves the inhibition of Pdx1 gene transcription. In our studies, the failure of FoxO1 phosphorylation caused by mHTT can inhibit Pdx1 expression. It could provide an explanation for the decrease in Pdx1 expression found in β-cells in HD R6/2 transgenic mice (Andreassen et al. 2002). Moreover, Pdx-1 is a key transcription factor of the glucose-responsive insulin gene (Andreassen et al. 2002; Kaneto et al. 2008). Small changes in Pdx-1 expression levels can result in major metabolic consequences (Fu et al. 2013; Thomas et al. 2001). Thus, we conclude that the deficient insulin expression induced by mHTT may result from the impairment of the PI3K/AKT/FoxO1 signaling pathway in β-cells.
On the other hand, Pdx-1 is essential for the genes involved in glucose sensing and metabolism including GLUT2 and glucokinase (Andreassen et al. 2002; Kaneto et al. 2008). In our studies, glucose treatment failed to upregulate FoxO1 phosphorylation in 160Q cells, which in turn could reduce GLUT2 expression by inhibiting Pdx-1 transcription. GLUT2 constitutively expressed in β-cells is the first glucose sensor encountered in β-cells (Fu et al. 2013). Abnormal trafficking and expression of GLUT have been described in HD, suggesting that altered GLUT expression is relevant to HD pathology (Besson et al. 2015; Gamberino et al. 1994; Kita et al. 2002; McClory et al. 2014; Ravikumar et al. 2003). For instance, in HD transgenic mice, reduced transport of GLUT3 to the cell surface was observed in neurons, underlying the glucose hypometabolism (McClory et al. 2014). Moreover, post-mortem analysis indicated that glucose transporter levels decreased in the caudate of HD brains (Gamberino et al. 1994). Consequently, we reason that increasing FoxO1 in the nucleus could also reduce the expression of GLUT2, which interrupts glucose uptake and metabolism in β-cells. In brief, our data imply that the reduction of Pdx-1 in HD might be associated with a disturbed PI3K/AKT/FoxO1 signaling pathway. Downregulated Pdx-1 could weaken insulin and GLUT2 expression, leading to decreased insulin secretion and glucose sensitivity in pancreatic β-cells in HD.
Additionally, the PI3K/AKT/FoxO1 signaling pathway can affect pancreatic β-cell viability. Previous reports have described that the overexpression of AKT improves β-cell proliferation through the downstream signaling of FoxO1 (Bernal-Mizrachi et al. 2001). Glucose treatment protects β-cells against free fatty-induced apoptosis by increasing phosphorylation of both AKT and FoxO1 (Wrede et al. 2002). Hence, the failure of the activation of glucose-induced PI3K/AKT signaling could also cause deficient cell proliferation and increased apoptosis in HD pancreatic β-cells.
Glucose cannot activate PI3K/AKT signaling in pancreatic β-cells expressing small interfering RNA toward IR. AKT and FoxO1 phosphorylation induced by glucose is dependent of an autocrine/paracrine effect of released insulin on its receptor (Martinez et al. 2006). In this work, we further determined the effect of exogenous insulin treatment on the PI3K/AKT signaling pathway in 160Q NIT-1 cells. As we expected, insulin treatment effectively activated PI3K and upregulated the phosphorylation of both AKT and FoxO1 in 160Q cells, just like 20Q cells. Importantly, insulin promotes the clearance of mHTT aggregates, indicating that impairment of the PI3K/AKT signaling pathway might result from mHTT aggregates and its recruitment of IRS-2 in pancreatic β-cells.
A schematic illustration of pancreatic β-cell dysfunction induced by mHTT is shown in Fig. 6. In healthy subjects, β-cells can quickly sense the change of plasma glucose concentration and response by releasing corresponding amounts of insulin (Schmitz et al. 2008). Subsequently, released insulin activates the PI3K/AKT signaling pathway by binding to IR and IRS-2, which furthers the phosphorylation of FoxO1 and its translocation into cytoplasm from the nucleus. Expression of both insulin and GLUT2 is upregulated, enhancing insulin production, glucose uptake, and insulin secretion. Thus, the levels of blood glucose and insulin are kept in balance (Fig. 6a). However, in HD patients, mHTT recruits IRS-2 into its aggregates and inhibits the upregulation of PI3K/AKT activity. When the blood glucose concentration increases, insulin signaling via IRS-2 is blocked. Following this, β-cells fail to phosphorylate downstream targets including AKT and FoxO1, which results in increasing FoxO1 located in the nucleus. Then, the expression of insulin and GLUT2 is inhibited by FoxO1, leading to decreased insulin secretion and glucose uptake. In turn, deficient insulin release deteriorates the impairment of the PI3K/AKT signaling pathway and the downregulation of glucose sensing, reinforcing a vicious cycle to disrupt glucose homeostasis (Fig. 6b).
Schematic illustration of pancreatic β-cell impairment in HD. a In the healthy group, glucose induces a PI3K/AKT-dependent nuclear to cytoplasmic FoxO1 translocation in β-cells, which further promotes insulin expression, glucose uptake, and cell survival of pancreatic β-cells. b In HD, mHTT recruits IRS-2 into the aggregates and inhibits activation of the glucose-stimulated PI3K/AKT signaling pathway. Increasing FoxO1 in nuclear localization promotes β-cell apoptosis and depresses the expression of insulin and GLUT2 genes, which downregulates insulin secretion and glucose sensing
In general, this work has illuminated that mHTT impairs β-cells by recruiting IRS-2 and disturbing the PI3K/AKT/FoxO1 signaling pathway in HD. These findings provide novel insights into the pathogenesis of diabetes in HD.
Availability of Data and Materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- HD::
-
Huntington’s disease
- HTT::
-
Huntingtin
- mHTT::
-
Mutant huntingtin
- NIT-1 cells::
-
Mouse pancreatic insulinoma cells
- PI3K::
-
Phosphatidylinositol-3-kinase
- AKT::
-
Serine–threonine protein kinase
- FoxO1::
-
Forkhead box protein O1
- T2DM::
-
Type 2 diabetes mellitus
- GLUT2::
-
Glucose transporter 2
- Pdx-1::
-
Pancreatic and duodenal homeobox-1
- IR::
-
Insulin receptor
- IRS-2::
-
Insulin receptor substrate 2
References
Andreassen OA, Dedeoglu A, Stanojevic V, Hughes DB, Browne SE, Leech CA, Ferrante RJ, Habener JF, Beal MF, Thomas MK (2002) Huntington’s disease of the endocrine pancreas: Insulin deficiency and diabetes mellitus due to impaired insulin gene expression. Neurobiol Dis 11(3):410–424. https://doi.org/10.1006/nbdi.2002.0562
Bendlin BB (2019) Antidiabetic therapies and Alzheimer disease. Dialogues Clin Neurosci 21(1):83–91. https://doi.org/10.31887/DCNS.2019.21.1/bblendin
Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA (2001) Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 108(11):1631–1638. https://doi.org/10.1172/JCI13785
Besson MT, Alegría K, Garrido-Gerter P, Barros LF, Liévens JC (2015) Enhanced neuronal glucose transporter expression reveals metabolic choice in a HD Drosophila model. PLoS ONE 10(3):e0118765. https://doi.org/10.1371/journal.pone.0118765
Björkqvist M, Fex M, Renström E, Wierup N, Peters´en A, Gil J, Bacos K, Popovic N, Li JY, Sundler F, Brundin P, Mulder H, (2005) The R6/2 transgenic mouse model of Huntington’s disease develops diabetes due to deficient beta-cell mass and exocytosis. Hum Mol Genet 14(5):565–574. https://doi.org/10.1093/hmg/ddi053
Brás IC, König A, Outeiro TF (2019) Glycation in Huntington’s Disease: A Possible Modifier and Target for Intervention. J Huntingtons Dis 8(3):245–256. https://doi.org/10.3233/JHD-190366
Bryan MR, Bowman AB (2017) Manganese and the Insulin-IGF Signaling Network in Huntington’s Disease and Other Neurodegenerative Disorders. Adv Neurobiol 18:113–142. https://doi.org/10.1007/978-3-319-60189-2_6
Chiang MC, Chen HM, Lee YH, Chang HH, Wu YC, Soong BW, Chen CM, Wu YR, Liu CS, Niu DM, Wu JY, Chen YT, Chern Y (2007) Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington’s disease. Hum Mol Genet 16(5):483–498. https://doi.org/10.1093/hmg/ddl481
Chong ZZ, Shang YC, Wang S, Maiese K (2012) A Critical Kinase Cascade in Neurological Disorders: PI 3-K, Akt, and mTOR. Future Neurol 7(6):733–748. https://doi.org/10.2217/fnl.12.72
Craft S, Watson GS (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3(3):169–178. https://doi.org/10.1016/S1474-4422(04)00681-7
Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV (2007) Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle 6(6):705–713. https://doi.org/10.4161/cc.6.6.4035
Du LJ, Pang B, Tan YM, Yang YN, Zhang MZ, Pang Q, Sun M, Ni Q (2020) Banxia Xiexin Decoction Ameliorates t-BHP-Induced Apoptosis in Pancreatic Beta Cells by Activating the PI3K/AKT/FOXO1 Signaling Pathway. J Diabetes Res 2020:3695689. https://doi.org/10.1155/2020/3695689
Farrer LA (1985) Diabetes mellitus in Huntington disease. Clin Genet 27:62–67. https://doi.org/10.1111/j.1399-0004.1985.tb00185.x
Fernández E, Martín MA, Fajardo S, Escrivá F, Alvarez C (2007) Increased IRS-2 content and activation of IGF-I pathway contribute to enhance beta-cell mass in fetuses from undernourished pregnant rats. Am J Physiol Endocrinol Metab 292(1):E187–E195. https://doi.org/10.1152/ajpendo.00283.2006
Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev 9(1):25–53
Gamberino WC, Brennan WA Jr (1994) Glucose transporter isoform expression in Huntington’s disease brain. J Neurochem 63:1392–1397. https://doi.org/10.1046/j.1471-4159.1994.63041392.x
Gao JR, Qin XJ, Fang ZH, Li-Shan HLP, Hui-Jiang GMF, Jiang NN (2019) To Explore the Pathogenesis of Vascular Lesion of Type 2 Diabetes Mellitus Based on the PI3K/Akt Signaling Pathway. J Diabetes Res 2019:4650906. https://doi.org/10.1155/2019/4650906
Garber AJ (2012) Obesity and type 2 diabetes: which patients are at risk? Diabetes Obes Metab 14(5):399–408. https://doi.org/10.1111/j.1463-1326.2011.01536.x
Gil JM, Rego AC (2008) Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci 27(11):2803–2820. https://doi.org/10.1111/j.1460-9568.2008.06310.x
Huang X, Liu G, Guo J, Su Z (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14(11):1483–1496. https://doi.org/10.7150/ijbs.27173
Hunt MJ, Morton AJ (2005) Atypical diabetes associated with inclusion formation in the R6/2 mouse model of Huntington’s disease is not improved by treatment with hypoglycaemic agents. Exp Brain Res 166(2):220–229. https://doi.org/10.1007/s00221-005-2357-z
Hurlbert MS, ZhouW WC, Kaddis FG, Hutton JC, Freed CR (1999) Mice transgenic for an expanded CAG repeat in the Huntington’s disease gene develop diabetes. Diabetes 48:649–651. https://doi.org/10.2337/diabetes.48.3.649
Hu Y, Liang J, Yu S (2014) High prevalence of diabetes mellitus in a five-generation Chinese family with Huntington’s disease. J Alzheimers Dis 40(4):863–868. https://doi.org/10.3233/JAD-131847
Josefsen K, Nielsen MD, Jørgensen KH, Bock T, Nørremølle A, Sørensen SA, Naver B, Hasholt L (2008) Impaired glucose tolerance in the R6/1 transgenic mouse model of Huntington’s disease. J Neuroendocrinol 20(2):165–172. https://doi.org/10.1111/j.1365-2826.2007.01629.x
Kaneto H, Miyatsuka T, Kawamori D, Yamamoto K, Kato K, Shiraiwa T, Katakami N, Yamasaki Y, Matsuhisa M, Matsuoka TA (2008) PDX-1 and MafA play a crucial role in pancreatic beta-cell differentiation and maintenance of mature beta-cell function. Endocr J 55(2):235–252. https://doi.org/10.1507/endocrj.k07e-041
Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y (2006) The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem 281(2):1091–1098. https://doi.org/10.1074/jbc.M508510200
Kita H, Carmichael J, Swartz J, Muro S, Wyttenbach A, Matsubara K, Rubinsztein DC, Kato K (2002) Modulation of polyglutamine-induced cell death by genes identified by expression profiling. Hum Mol Genet 11(19):2279–2287. https://doi.org/10.1093/hmg/11.19.2279
Lalić NM, Marić J, Svetel M, Jotić A, Stefanova E, Lalić K, Dragasević N, Milicić T, Lukić L, Kostić VS (2008) Glucose homeostasis in Huntington disease: abnormalities in insulin sensitivity and early-phase insulin secretion. Arch Neurol 65(4):476–480. https://doi.org/10.1001/archneur.65.4.476
Liao Z, Zhang J, Liu B, Yan T, Xu F, Xiao F, Wu B, Bi K, Jia Y (2019) Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 24(10):1906. https://doi.org/10.3390/molecules24101906
Lingohr MK, Briaud I, Dickson LM, McCuaig JF, Alárcon C, Wicksteed BL, Rhodes CJ (2006) Specific regulation of IRS-2 expression by glucose in rat primary pancreatic islet beta-cells. J Biol Chem 281(23):15884–15892. https://doi.org/10.1074/jbc.M600356200.Erratum.In:JBiolChem.2006Aug11;281(32):23296
Martinez SC, Cras-Méneur C, Bernal-Mizrachi E, Permutt MA (2006) Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet beta-cell. Diabetes 55(6):1581–1591. https://doi.org/10.2337/db05-0678
Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA (2008) Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 57(4):846–859. https://doi.org/10.2337/db07-0595
McClory H, Williams D, Sapp E, Gatune LW, Wang P, DiFiglia M, Li X (2014) Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington’s disease mice. Acta Neuropathol Commun 2:179. https://doi.org/10.1186/s40478-014-0178-7
Miller TW, Shirley TL, Wolfgang WJ, Kang X, Messer A (2003) DNA vaccination against mutant huntingtin ameliorates the HDR6/2 diabetic phenotype. Mol Ther 7(5 Pt 1):572–579. https://doi.org/10.1016/s1525-0016(03)00063-7
Moffitt H, McPhail GD, Woodman B, Hobbs C, Bates GP (2009) Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of Huntington’s disease. PLoS ONE 4(11):e8025. https://doi.org/10.1371/journal.pone.0008025
Montojo MT, Aganzo M, González N (2017) Huntington’s Disease and Diabetes: Chronological Sequence of its Association. J Huntingtons Dis 6(3):179–188. https://doi.org/10.3233/JHD-170253
Morea V, Bidollari E, Colotti G, Fiorillo A, Rosati J, De Filippis L, Squitieri F, Ilari A (2017) Glucose transportation in the brain and its impairment in Huntington disease: one more shade of the energetic metabolism failure? Amino Acids 49(7):1147–1157. https://doi.org/10.1007/s00726-017-2417-2
Niu L, Li L, Yang S, Wang W, Ye C, Li H (2020) Disruption of zinc transporter ZnT3 transcriptional activity and synaptic vesicular zinc in the brain of Huntington’s disease transgenic mouse. Cell Biosci 10:106. https://doi.org/10.1186/s13578-020-00459-3
Pogoda A, Chmielewska N, Maciejak P, Szyndler J (2020) Transcriptional dysregulation in Huntington's Disease. The role in pathogenesis and potency for pharmacological targeting. Curr Med Chem. https://doi.org/10.2174/0929867327666200705225821
Ravikumar B, Stewart A, Kita H, Kato K, Duden R, Rubinsztein DC (2003) Raised intracellular glucose concentrations reduce aggregation and cell death caused by mutant huntingtin exon 1 by decreasing mTOR phosphorylation and inducing autophagy. Hum Mol Genet 12(9):985–994. https://doi.org/10.1093/hmg/ddg109
Ristow M (2004) Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (berl) 82(8):510–529. https://doi.org/10.1007/s00109-004-0552-1
Sathasivam K, Hobbs C, Turmaine M, Mangiarini L, Mahal A, Bertaux F, Wanker EE, Doherty P, Davies SW, Bates GP (1999) Formation of polyglutamine inclusions in non-CNS tissue. Hum Mol Genet 8(5):813–822. https://doi.org/10.1093/hmg/8.5.813
Sayed NH, Fathy N, Kortam MA, Rabie MA, Mohamed AF, Kamel AS (2020) Vildagliptin Attenuates Huntington’s Disease through Activation of GLP-1 Receptor/PI3K/Akt/BDNF Pathway in 3-Nitropropionic Acid Rat Model. Neurotherapeutics 17(1):252–268. https://doi.org/10.1007/s13311-019-00805-5
Schmitz O, Rungby J, Edge L, Juhl CB (2008) On high-frequency insulin oscillations. Ageing Res Rev 7(4):301–305. https://doi.org/10.1016/j.arr.2008.04.002
Shirakawa J, Fernandez M, Takatani T, El Ouaamari A, Jungtrakoon P, Okawa ER, Zhang W, Yi P, Doria A, Kulkarni RN (2017) Insulin Signaling Regulates the FoxM1/PLK1/CENP-A Pathway to Promote Adaptive Pancreatic β Cell Proliferation. Cell Metab 25(4):868-882.e5. https://doi.org/10.1016/j.cmet
Shirakawa J, Terauchi Y (2020) Newer perspective on the coupling between glucose-mediated signaling and β-cell functionality. Endocr J 67(1):1–8. https://doi.org/10.1507/endocrj.EJ19-0335
Smith R, Bacos K, Fedele V, Soulet D, Walz HA, Obermüller S, Lindqvist A, Björkqvist M, Klein P, Onnerfjord P, Brundin P, Mulder H, Li JY (2009) Mutant huntingtin interacts with {beta}-tubulin and disrupts vesicular transport and insulin secretion. Hum Mol Genet 18(20):3942–3954. https://doi.org/10.1093/hmg/ddp336
Thomas MK, Devon ON, Lee JH, Peter A, Schlosser DA, Tenser MS, Habener JF (2001) Development of diabetes mellitus in aging transgenic mice following suppression of pancreatic homeoprotein IDX-1. J Clin Invest 108(2):319–329. https://doi.org/10.1172/JCI12029
Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN (2006) Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet 38(5):583–588. https://doi.org/10.1038/ng1787
van der Burg JM, Bjorkqvist M, Brundin P (2009) Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol 8(8):765–774. https://doi.org/10.1016/S1474-4422(09)70178-4
Walker FO (2007) Huntington’s disease. Lancet 369:218–228. https://doi.org/10.1016/S0140-6736(07)60111-1
White JA 2nd, Krzystek TJ, Hoffmar-Glennon H, Thant C, Zimmerman K, Iacobucci G, Vail J, Thurston L, Rahman S, Gunawardena S (2020) Excess Rab4 rescues synaptic and behavioral dysfunction caused by defective HTT-Rab4 axonal transport in Huntington’s disease. Acta Neuropathol Commun 8(1):97. https://doi.org/10.1186/s40478-020-00964-z
Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ (2002) Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J Biol Chem 277(51):49676–49684. https://doi.org/10.1074/jbc.M208756200
Ye CF, Li H (2009) HSP40 ameliorates impairment of insulin secretion by inhibiting huntingtin aggregation in a HD pancreatic beta cell model. Biosci Biotechnol Biochem 73(8):1787–1792. https://doi.org/10.1271/bbb.90147
Yildirim F, Ng CW, Kappes V, Ehrenberger T, Rigby SK, Stivanello V, Gipson TA, Soltis AR, Vanhoutte P, Caboche J, Housman DE, Fraenkel E (2019) Early epigenomic and transcriptional changes reveal Elk-1 transcription factor as a therapeutic target in Huntington’s disease. Proc Natl Acad Sci U S A 116(49):24840–24851. https://doi.org/10.1073/pnas.1908113116
Yu Z, Zhou D, Cheng G, Mattson MP (2000) Neuroprotective role for the p50 subunit of NF-kappaB in an experimental model of Huntington’s disease. J Mol Neurosci 15(1):31–44. https://doi.org/10.1385/JMN:15:1:31
Acknowledgements
We thank Dr. Xiao-jiang Li (Jinan University, Guangzhou, China) and Professor Guan-xin Shen (Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) for providing anti-huntingtin antibody (EM48) and mouse pancreatic insulinoma cell line NIT-1. We are grateful to Professor He Li (Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) for his help in writing the paper.
Funding
This work was supported by the National Natural Science Foundation of China [81100947].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by YS, LL, YZ, and WW. The first draft of the manuscript was written by CY and LL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with animals or human participants performed by any of the authors.
Competing Interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Sun, Y., Zhang, Y. et al. Mutant Huntingtin Impairs Pancreatic β-cells by Recruiting IRS-2 and Disturbing the PI3K/AKT/FoxO1 Signaling Pathway in Huntington’s Disease. J Mol Neurosci 71, 2646–2658 (2021). https://doi.org/10.1007/s12031-021-01869-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01869-9