Abstract
Background
Depth electroencephalography (dEEG) is a recent invasive monitoring technique used in patients with acute brain injury. This study aimed to describe in detail the clinical manifestations of nonconvulsive seizures (NCSzs) with and without a surface EEG correlate, analyze their long-standing effects, and provide data that contribute to understanding the significance of certain scalp EEG patterns observed in critically ill patients.
Methods
We prospectively enrolled a cohort of 33 adults with severe acute brain injury admitted to the neurological intensive care unit. All of them underwent multimodal invasive monitoring, including dEEG. All patients were scanned on a 3T magnetic resonance imaging scanner at 6 months after hospital discharge, and mesial temporal atrophy (MTA) was calculated using a visual scale.
Results
In 21 (65.6%) of 32 study participants, highly epileptiform intracortical patterns were observed. A total of 11 (34.3%) patients had electrographic or electroclinical seizures in the dEEG, of whom 8 had both spontaneous and stimulus-induced (SI) seizures, and 3 patients had only spontaneous intracortical seizures. An unequivocal ictal scalp correlate was observed in only 3 (27.2%) of the 11 study participants. SI-NCSzs occurred during nursing care, medical procedures, and family visits. Subtle clinical manifestations, such as restlessness, purposeless stereotyped movements of the upper limbs, ventilation disturbances, jerks, head movements, hyperextension posturing, chewing, and oroalimentary automatisms, occurred during intracortical electroclinical seizures. MTA was detected in 18 (81.8%) of the 22 patients. There were no statistically significant differences between patients with MTA with and without seizures or status epilepticus.
Conclusions
Most NCSzs in critically ill comatose patients remain undetectable on scalp EEG. SI-NCSzs frequently occur during nursing care, medical procedures, and family visits. Semiology of NCSzs included ictal minor signs and subtle symptoms, such as breathing pattern changes manifested as patient–ventilator dyssynchrony.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For decades, the use of intracerebral electrodes for recording electrical brain activity has primarily been limited to use in the presurgical evaluation of patients with refractory epilepsy. However, this scenario substantially changed with the use of depth electroencephalography (dEEG) as technique of continuous EEG monitoring (cEEGm) in comatose patients with acute brain injury (ABI) [1]. Since then, a few investigations have corroborated the safety, feasibility, and effectivity of this invasive technique to detect electrographic seizures (ESzs) and electrographic status epilepticus (ESE) among comatose patients with brain damage admitted to the neurological intensive care unit (NICU) [1,2,3,4,5,6,7,8,9,10,11,12]. Both were more often termed in the past as nonconvulsive seizures (NCSzs) and nonconvulsive status epilepticus, respectively. In addition, little is known about the enduring consequences of ESzs in individuals with ABI. Some authors have found that posttraumatic ESzs were associated with long-term disproportionate hippocampal atrophy [13]. However, many more data, including patients with ABI from different causes, are needed to confirm these findings to establish antiseizure medication (ASM) treatment protocols that may be appropriate to use in this setting, which could potentially prevent chronic damage. On the other hand, studies that apply the new concepts of the 2021 version of the American Clinical Neurophysiology Society’s (ACNS’s) Standardized Critical Care EEG Terminology [14] and focus their attention on a detailed analysis of ictal semiology and correlation between scalp EEG patterns and dEEG are absent.
We conducted a prospective study using dEEG in 33 comatose adults with ABI admitted to the NICU to comprehensively describe the clinical manifestations of NCSzs with and without a surface EEG correlate. This study also aimed to analyze long-standing effects of NCSzs and to provide data that contribute to understanding the pathophysiological mechanisms of certain scalp EEG patterns whose significance is not well understood.
Methods
Study Design and Patients
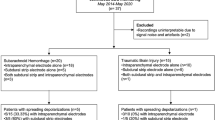
We performed a prospective descriptive study of a cohort of 33 adult patients (> 18 and < 75 years) admitted to the NICU at Marqués de Valdecilla University Hospital between September 2017 and November 2022 presenting severe ABI, such as intracerebral hemorrhage, traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and subdural hematoma, and requiring invasive monitoring devices. All of them had decreased levels of consciousness and a Glasgow Coma Scale score ≤ 9. This study was inspired by a previously published study by Waziri et al. [1]. Our NICU admits more than 500 patients annually, with around 300 being neurocritical cases. Among them, between 40 and 60 patients per year require invasive monitoring. The study period extended from September 2017 to November 2022, encompassing a duration of 5 years. Nevertheless, owing to the COVID-19 pandemic, all NICU studies at our institution were suspended for almost a period of 1 year and a half. Our initial goal was to include approximately 20 patients annually, which would have resulted in a total of 70 patients over the 3.5-year study period. Unfortunately, the COVID-19 pandemic and the availability of mini-depth electrodes limited our patient enrollment.
Standard Protocol Approvals, Registrations, and Patient Consents
All close relatives of the participants gave written consent before any study-related procedures were performed. We obtained the approval of the Ethics Committee for Clinical Research of Cantabria (internal code: 2014.156).
EEG
Commercially available eight-contact Spencer mini-depth electrodes (Adtech 8 channel mini-depth electrode; AD-Tech Medical) designed for clinical dEEG recording were chosen for use as described elsewhere [1, 3, 5, 6, 11] (for more details, see Supplementary Data). In addition, 21 subdermal needle electrodes attached with collodion placed according the International 10–20 System were included. Both scalp EEG and dEEG monitoring were started simultaneously.
EEG analysis was performed by a senior certified clinical neurophysiologist (JLF-T). The EEG reader was not blinded to patient clinical information and analyzed the full recordings of all patients in three independent rounds. For the definitions of ESz, electroclinical seizure (ECSz), ESE, electroclinical status epilepticus (ECSE), burst suppression (BS) pattern, stimulus-induced rhythmic periodic or ictal-appearing discharges (SIRPIDs), and rhythmic and periodic patterns (RPPs) included in the ictal-interictal continuum (IIC), we used the 2021 version of the ACNS’s Standardized Critical Care EEG Terminology [14].
Recordings were reviewed for ESzs, ECSzs, ESE, ECSE, BS, and RPPs. We also pointed out the presence of interhemispheric asymmetry and triphasic generalized periodic discharges (tGPDs). In addition, we differentiated between spontaneous and stimulus-induced (SI) or reflex seizures (SI-ESzs/ECSzs). Thus, we defined an SI-ESz/ECSz as a seizure that was unequivocally and repetitively precipitated by sensory stimulation or alerting stimuli [14]. However, SIRPIDs included RPPs ≤ 2.5 Hz without spatiotemporal evolution and clinical manifestations.
When analyzing the correlation between intracortical and surface ictal recordings, we only considered a positive correlation for the scalp recording if there was an unequivocal pattern with clear evolution or epileptiform discharges (EDs) > 2.5 Hz occurring simultaneously with the intracortical seizures. Other EEG findings, such as rhythmic delta activity (RDA), RPPs ≤ 2.5 Hz, or tGPDs, without a pattern that would traditionally be considered ictal were analyzed as without a surface correlate or as “scalp-negative” seizures [15].
Multimodal Monitoring
Moreover, invasive monitoring included measurements of intracranial pressure (Integra Camino 110-4L), partial brain tissue oxygenation, and brain temperature (using a combined oxygen and temperature probe; Licox CC1.P1). These two devices were inserted through one burr hole within a double-lumen bolt (IP2 dual lumen introducer kit). In addition, another burr hole was used for the mini-depth multicontact electrode insertion. The intracortical electrode was more frequently placed on the side of the lesion. However, in some patients, it was inserted on the contralateral side. The mini-depth electrode was tunneled 5 cm under the skin and exited via a stab wound incision and was secured to the skin with interrupted stiches.
Neuroimaging
All patients were scanned on 3T magnetic resonance imaging (MRI) scanners (Philips Achieva 3T; Philips, the Netherlands) using a 32-channel head coil at 6 months after discharge. The scanning protocol included three-dimensional T1-weighted Magnetization Prepared-RApid Gradient Echo (MPRAGE), susceptibility-weighted imaging phase (SWIp), and axial T-weighted and three-dimensional T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences for anatomic definition. Mesial temporal atrophy (MTA) was calculated using the visual scale published by Scheltens et al. [16].
Data Collection
Clinical data were prospectively recorded according to a standardized protocol. Collected data included demographics (i.e., age, sex, etc.), primary admission diagnosis, time from admission to onset of cEEGm, length of cEEGm and stay, and in-hospital and out-hospital mortality. All study participants were followed up after discharge for at least 6 months, and clinical evaluation, routine EEG, and brain MRI were performed at that moment. When possible, a follow-up clinical evaluation was also conducted at 12 months after discharge. Complications, treatment, and prognosis were also recorded, and Glasgow Outcome Scale and modified Rankin scale scores were calculated.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical Analysis
Data were coded and entered into a database in SPSS, version 26.0. (IBM Corp, Armonk, NY) for statistical purposes. Continuous variables were expressed as mean and standard deviation or median and interquartile range (IQR) for nonnormally distributed variables checked by the Kolmogorov–Smirnov test. Categorical data were presented as number of events and percentage. The comparison of categorical variables between patients with and without seizures was made using the χ2 test or Fisher’s exact test, and the differences between continuous variables were obtained using a two-sided Student’s t-test or a Mann–Whitney U-test for nonnormally distributed variables. Statistical significance was set at p values ≤ 0.05.
Results
Overview
A total of 33 patients underwent cEEGm with dEEG. Intracortical recordings were successfully recorded in all but one (patient 3), whose data were removed from the statistical analysis. There were 11 women and 21 men ranging in age from 22 to 73 years (mean age 54 ± 11 years). Demographic and clinical data at admission can be found in Table 1 and Supplementary Table 1.
The study included 13 patients (40.6%) with TBI, 11 patients (34.4%) with spontaneous intracerebral hemorrhage, and 8 patients (25%) with SAH secondary to aneurysm (Table 1). The most frequent onset symptom was altered level of consciousness (22 of 32). Convulsive seizures occurred in 25% (8 of 32). Three (9.4%) patients had a history of epilepsy. The median delay from symptom onset to NICU admission was 2 h (IQR 1.2–3.3).
The median time from NICU admission to study inclusion was 12.1 h (IQR 3.5–25.3), the median time from admission to NICU to sensor placement was 24.0 h (IQR 12.8–39.5), and the median time from inclusion in the study to the insertion of the sensors was 4.5 h (IQR 1.6–17.2). The median time from the placement of the sensors to the onset of the cEEGm was 12.7 h (IQR 2.6–21.8). In 20 (62.5%) patients, the mini-depth multicontact electrode was placed ipsilateral to the injury, and in 12 (37.5%) patients, it was inserted on the opposite side because seven patients were undergoing decompressive craniectomy, two patients had complicated craniotomy with postsurgical pneumocephalus, two patients had extensive intraparenchymal hematoma, and one patient already had the sensors and external ventricular drainage placed on the side of the lesion. In 19 study participants, the intracortical electrode was inserted in the right hemisphere, and in 13 study participants, it was inserted in the left side. The median duration of the cEEGm was 4.9 days (IQR 3.1–6.9). There were no acute adverse events associated with device insertion, but through the cEEGm, six (18.7%) patients had minor complications, including a hypodensity at the tip or along the trajectory of the electrode (2), accidental dislodgement of the electrode (1), pneumocephalus after electrode removal (1), skin blisters without infection at the sensor entry point (1), and misplacement of the electrode in the epidural space and subsequent replacement by another one (1).
Twenty-six (81.3%) patients were treated with ASM: 12 (37.5%) received ASM for prophylaxis, 6 (18.8%) received ASM as treatment of clinical epileptic seizures, 5 (15.6%) received ASM as a consequence of scalp EEG anomalies during cEEGm, and 3 (9.4%) received ASM as chronic treatment of their previous epilepsy (Supplementary Table 1). All of them received levetiracetam, and one was treated with phenytoin. The median NICU stay was 17 days (IQR 13–22), and hospital stay until discharge was 31 days (IQR 23–41). Twenty-five (78.1%) patients survived at hospital discharge. Six (18.8%) patients died in the NICU, one (3.1%) patient died in the neurosurgery ward during the admission, and two (6.2%) patients died during follow-up. The final mortality rate at 1 year was 28.1%.
All patients were under sedation with propofol or a combination of propofol and midazolam, with 27 of 32 (84.3%) showing a BS pattern on the scalp EEG and 31 (96.8%) showing a BS pattern in the dEEG at some moment during the cEEGm. In 21 of 32 (65.6%) study participants, highly epileptiform dEEG patterns, including ESzs/ECSzs (11), ESE/ECSE (7), RPPs (17), and SIRPIDs (17), were observed (Fig. 1 and Supplementary Table 2). Seventeen patients (53.1%) had an RPP in the interictal intracortical recording, and 20 (62.5%) had an RPP on the scalp EEG. Of note, in four of five study participants with an RPP detected only on the scalp, this activity occurred contralateral to the side where the intracortical electrode was located. In 18 (56.2%) patients, RDA was observed in the dEEG, whereas unilateral or bilateral scalp RDA was detected in 25 (78.1%) study participants. Moreover, in 15 (46.8%) cases, we observed significant interhemispheric asymmetry on the scalp EEG. Scalp tGPDs were also seen in 15 patients (46.8%) (Supplementary Figs. 1 and 5).
EEG findings of the 32 patients included in the statistical analysis. Asym: interhemispheric asymmetry; iBS: intracortical burst suppression; iECSz: intracortical electroclinical seizure; iECSE: intracortical electroclinical status epilepticus; iESE: intracortical electrographic status epilepticus; iESz: intracortical electrographic seizure; iIIC: intracortical ictal-interictal continuum; iRDA: intracortical rhythmic delta activity; iRPP: intracortical rhythmic and periodic pattern; iSIRPIDs: intracortical stimulus-induced rhythmic periodic or ictal-appearing discharges; sBS: scalp burst suppression; sECSE: scalp electroclinical status epilepticus; sESE: scalp electrographic status epilepticus; sESz: scalp electrographic seizure; sIIC: scalp ictal-interictal continuum; sRDA: scalp rhythmic delta activity; sRPP: scalp rhythmic and periodic pattern; sSIRPIDs: scalp stimulus-induced rhythmic periodic or ictal-appearing discharges; stGPD: triphasic generalized periodic discharges on scalp; TDW: triphasic delta waves
Intracortical SIRPIDs occurred in 17 (53.1%) patients, and scalp SIRPIDs were seen in 15 (46.8%). Somatosensory (tactile and painful) and auditory stimuli were the most frequent triggers for SIRPIDs. However, some patients experienced self-induced SIRPIDs provoked by spontaneous cough.
In 11 (34.3%) patients, we recorded seizures in the dEEG (Figs. 2, 3, 4 and 5; Supplementary Fig. 2). Eight (72.7%) had both spontaneous and SI seizures (Table 2). Three patients had only spontaneous intracortical seizures: one with only intracortical ESzs, one with both intracortical ESzs and ECSzs, and a third with only intracortical ECSzs. SI-NCSzs occurred during nursing care, medical procedures, and family visits. All SI-NCSzs had typical spatiotemporal evolution in the dEEG. No patient had only SI-NCSzs. Ten of 11 (90%) study participants with seizures also had an RPP included in the IIC. An unequivocal ictal scalp correlate was observed in only 3 of the 11 (27.2%) study participants. Seven (63.3%) of the 11 patients with intracortical ESzs/ECSzs met criteria for the diagnosis of intracortical nonconvulsive status epilepticus, and only two on the scalp EEG.
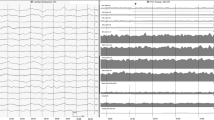
Patient 20. a–d Electrographic seizure recorded in depth electroencephalography (dEEG) derivations precipitated by manipulation during the performance of a Doppler ultrasound. Note how the absence of obvious motor signs or symptoms (nonconvulsive seizures) made the medical staff unable to recognize that the patient was having a critical event. No clinical signs were present. dEEG shows rhythmic spikes and spike-wave complexes with typical spatiotemporal evolution involving IE1-E5 derivations. Semirhythmic slowing is noted, but no ictal pattern is identified on the scalp EEG recording. Low filter: 0.53 Hz; high filter: 70 Hz; notch filter: 50 Hz; velocity: 15 mm/s. Sensitivity in the scalp recording was 15 μV/mm and 30 μV/mm in the intracortical signal. IE1-IE8 (pink): dEEG channels
Patient 23. a Rhythmic epileptiform discharges (EDs) associated with a Generalized rhythmic delta activity with associated sharp waves or spikes, or sharply contoured (GRDA + S) pattern on the scalp. Depth electroencephalography (dEEG) channels show a small spike in association with the rhythmic slow wave, which is not evident on the scalp. b During this episode, the patient experienced ventilator dyssynchrony. Low filter: 0.53 Hz; high filter: 70 Hz; notch filter: 50 Hz; velocity: 15 mm/s. Sensitivity in the scalp recording was 10 µV/mm and 15 µV/mm in the intracortical signal. IE1-IE8 (pink): dEEG channels
Patient 25. a Stimulus-induced electrographic seizure caused by a relative’s caresses and somatosensory stimulation during the visit. Depth electroencephalography (dEEG) shows rhythmic spikes and spike-wave complexes with spatiotemporal evolution involving IE5-E7 derivations. This discharge is absent on the concurrent scalp recording. b The seizure occurs and remains undetectable by the family member. c During the seizures, this patient stereotypically raised his right arm (arrow). Low filter: 0.53 Hz; high filter: 70 Hz; notch filter: 50 Hz; velocity: 15 mm/s. Sensitivity in the scalp and intracortical recording was 10 µV/mm. IE1-IE8 (pink): dEEG channels
Patient 20. a–e Stimulus-induced electrographic seizure precipitated by nursing care. Depth electroencephalography (dEEG) shows rhythmic spikes and spike-wave complexes with typical spatiotemporal evolution involving IE1-E5 derivations. Although scalp EEG is extremely affected by myogenic artifact, epileptiform discharges (EDs) are not seen on the surface. The seizure occurs and remains undetectable by health personnel. Low filter: 0.53 Hz; high filter: 70 Hz; notch filter: 50 Hz; velocity: 15 mm/s. Sensitivity in the scalp recording was 15 µV/mm and 30 µV/mm in the intracortical signal. IE1-IE8 (pink): dEEG channels
Three (cases 18, 23, and 24) patients with seizures and one without (case 28) had a stereotyped and recurrent intracortical RPP, with EDs lower than 2.5 Hz and generalized RDA (GRDA) or bilateral asymmetric lateralized RDA being observed simultaneously on the scalp EEG [17]. In 10 of 11 patients, subtle clinical manifestations, such as restlessness and purposeless stereotyped movements of the upper limbs, ventilation disturbances, jerks, head movements, hyperextension posturing, chewing, and oroalimentary automatisms, occurred, fulfilling the criteria for intracortical ECSzs (Table 2).
We have focused our statistical analysis between the group of patients without intracortical seizures (21 patients) and the group of patients with intracortical seizures (11 patients) during the cEEGm (Table 1 and Supplementary Tables 1 and 3). The total number of days on mechanical ventilation and the duration of hospitalization were higher among study participants with seizures (p = 0.02 and 0.03, respectively). The patients with intracortical seizures had a ventricular drainage more frequently (p = 0.006).
Correlation of Scalp EEG and dEEG
Well-defined intracortical rhythmic spike-wave complexes were seen as GRDA + S, arciform GRDA, or bilateral slightly asymmetric lateralized RDA on the surface (Fig. 3 and Supplementary Figs. 3 and 4). In 15 (46.8%) patients, tGPDs or triphasic delta waves were seen on the scalp EEG. In some of the patients, tGPDs unequivocally represented the scalp correlate of intracortical EDs (Supplementary Figs. 1 and 5). In our series, we found an RPP in the hemisphere contralateral to the cerebral lesion in five (15.6%) patients and RDA in three (9.3%) patients.
Follow-Up EEG and Neuroimaging Studies
We performed an EEG and brain MRI at 6 months. Additionally, the clinical follow-up included evaluations at 6 and 12 months after discharge from the NICU. Control MRIs were only performed on 22 (68.8%) patients. We missed the control MRI studies in eight cases because of death, in one case because of the lack of collaboration, and in one case because of loss to follow-up. MTA was detected in 18 (81.8%) of the 22 patients (Supplementary Table 3). There were no statistically significant differences between patients with MTA with and without intracortical seizures (p = 0.6) or status epilepticus (p = 0.54). During follow-up, only two (6.3%) of the 32 patients experienced epileptic seizures. Nine (28.1%) patients were being treated with ASM at 6 months, and 10 (31.3%) continued treatment for longer than 1 year. After 1 year of follow-up, 18 (56.3%) study participants achieved a favorable prognosis (Glasgow Outcome Scale scores of 4 and 5) (Supplementary Table 3). Of the 11 patients with seizures, only 8 had an MRI study at the 6-month follow-up. Of the remaining three patients, two died and one did not complete the study because of a lack of patient availability. In six (75%) patients we detected the existence of bilateral MTA. Seven patients had follow-up EEG studies. All but one showed some anomaly, including focal abnormalities (6), interhemispheric asymmetry (2), frank EDs (1), and encephalopathy (1) (Table 3).
Discussion
Simultaneous recording of scalp EEG and dEEG in ABI shows a relatively high prevalence of electrographic seizure activity in coma and demonstrates concerning limitations in the ability of scalp EEG to detect seizure activity, which is the main reason it is used in this clinical situation. In addition, it offers one unique opportunity to describe the clinical manifestations of “scalp-negative” NCSzs in coma. Here, we conducted a detailed analysis of the correlation between dEEG and scalp EEG and describe the semiology of ECSzs with and without a scalp EEG correlate. Our analysis applied the new ACNS terminology and definitions [14] to shed light on the origin of IIC patterns whose meaning remains unclear. Thus, our study reveals four important findings: (1) intracortical NCSzs are common in the setting of severe ABI and remain frequently undetectable on the scalp; (2) certain subtle clinical signs that may be indeterminate in terms of ictal significance based on clinical observation may actually be ictal; (3) reflex seizures are frequent among patients with ABI, and there are situations at risk of SI seizures; and (4) scalp EEG patterns that are currently considered non-ictal appear to be ictal based on dEEG.
In the first study by Waziri et al. [1] using dEEG, 71% of patients had ESzs and 86% had highly epileptiform patterns. Furthermore, of the ten patients with intracortical ESzs, six never had a scalp correlate [1]. Later on, Claassen et al. [3] found intracortical ESzs in 38% of patients with poor-grade SAH. Scalp ESzs were seen only in 8%. In a subsequent study of a cohort of 34 patients with TBI, 61.8% had ESzs or periodic discharges, with 42.9% of these seizures noted only in the dEEG [5]. In 65.6% of our patients, we detected highly epileptiform dEEG patterns. However, because the mini-depth electrode was inserted contralateral to the injury in 12 study participants, this may have affected overall detection, underestimating the rate of seizure activity.
Our study corroborates previous results confirming the observation that a great majority of NCSzs in study participants with ABI remain undetectable on scalp EEG [1, 3, 5, 11]. An unequivocal ictal scalp correlate was observed in only 3 of the 11 (27.2%) patients with seizures in the dEEG. Although certainly a high percentage of NCSzs were invisible on the scalp, some patients exhibited both, with a minority detectable on the surface. This is not surprising because past investigations on focal epilepsy demonstrated that often scalp-negative and scalp-positive seizures coexist in the same patient [15]. Obviously, for intracortical seizures, the difficulty of recording the electrical activity of neurons located in deeper regions seems to be minimized. Therefore, the existence of a critical volume of brain tissue for the signal to be recorded on the surface takes more relevance.
There are few studies that simultaneously compare intracranial EEG patterns with those seen on the scalp [18,19,20,21,22,23]. These investigations come mainly from patients with refractory epilepsy and were performed with depth or subdural electrodes. Recent research found that 42% of seizures were detected exclusively on stereo electrodes [23]. Seizures recorded in scalp EEG were more likely to be clinically apparent and longer. Thus, only 21% of scalp-negative seizures were clinical. They determined a poor detection rate (33%) of scalp recordings for subclinical and focal aware symptoms, probably reflecting that these NCSzs involve smaller regions of cortical activation. Of note, in this study lesional cases were less likely to have a scalp correlate. Because most seizures in patients with ABI are NCSzs, one possible explanation would be that they involve smaller cortical areas and that brain damage could limit their spread. This hypothesis could be complementary to the concept of microdischarges and microseizures [24,25,26]. Thus, differences in connectivity, synchronization, or inhibition between neuronal populations would determine the rate of propensity of microdischarges or microseizures to spread into large-scale seizures [25, 26]. Similarly, there could be a hierarchy that would encompass clinically silent limited intracortical seizures or more extensive ones with subtle or obvious clinical signs.
Our patients with NCSzs had both ESzs and ECSzs. The use of dEEG allowed us to characterize ictal minor signs and symptoms that would otherwise have gone unnoticed. Face twitching, eye deviation, and nystagmus are most frequently recognized as subtle clinical manifestation [14]. Our patients exhibited restlessness, changes in breathing pattern manifested as patient–ventilator dyssynchrony, purposeless stereotyped movements of the upper limbs, jerks, head movements, hyperextension posturing, chewing, and oroalimentary automatisms (Fig. 4 and Supplementary Fig. 2). Florea et al. [27] found subtle motor phenomena, such as myoclonus, tonic muscle activation, automatisms, and eye deviation, in 23% of study participants. They mixed adult and pediatric cases, employed scalp recordings, and did not perform cEEGm. Patient–ventilator dyssynchrony or asynchrony occurs when, for any parameter of respiration, discordance exists between the patient’s spontaneous effort and the ventilator’s provided support [28]. To the best of our knowledge, a possible epileptic cause of this sign has not been previously emphasized.
SI-ESzs/ECSzs were detected in 8 of 32 (25%) patients. This is one of the most relevant findings of our research, suggesting that there are situations at risk of SI seizures, such as nursing care, medical procedures, and family visits, during which reflex NCSzs (ESz or ECSz) often occur. Of note, these events can happen completely unnoticed by health care personnel and relatives (Figs. 2, 4 and 5). We hypothesized that the existence of reflex nonpurely rhythmic motor seizures could be more frequent than expected and that the majority could be clinically undetectable with scalp EEG.
In the same way as our study, Casale et al. [23] also observed how intracranial seizures or interictal EDs could originate slow wave patterns, such as diffuse or focal polymorphic slowing on the scalp (Fig. 3 and Supplementary Figs. 3 and 4). Paradoxically, although GRDA, even with higher frequency and plus modifiers, does not seem to increase risk for seizures [29, 30], in rare occasions, as showed our study, it may constitute a true ictal scalp EEG pattern. Most recently, GRDA has been identified as a scalp epileptogenic phenomenon and possible marker for latent refractory mesial epilepsy [31].
tGPDs can be seen in study participants with ABI [32]. It is also important to highlight that intracortical EDs may be seen on the surface adopting the morphology of tGPDs (Supplementary Figs. 1 and 5). This could be of importance in patients with ABI without metabolic or toxic alterations, in whom the initiation of ASM could be suitable.
In some patients, an RPP was only seen on the scalp EEG. In four of five of these study participants, this activity occurred contralateral to insertion of an intracortical electrode, suggesting that the presence of midline shift and depressed neuronal activity due to transient neuronal block at the damaged hemisphere could have favored this phenomenon [33]. This finding in patients with ABI has already been commented on by other authors [34].
There is growing evidence indicating that NCSzs cause neuronal damage leading to secondary brain injury [5, 13, 29, 30, 35, 36]. Although patients with intracortical seizures during cEEGm showed significant chronic brain lesions, we could not show that they were associated with MTA. Of note, in six of our eight study participants with seizures during cEEGm and MRI follow-up, we found bilateral MTA. In a previous study on TBI, Vespa et al. [13] found disproportionate long-term ipsilateral hippocampal atrophy in six study participants with posttraumatic NCSzs. They performed volumetric analysis of brain atrophy [13]. We calculated the existence of MTA using a universally accepted visual scale [17], and although in six of our patients, we demonstrated bilateral chronic damage, in the three study participants with asymmetric MTA, the greatest degree of atrophy was ipsilateral to the ESzs.
Our study had several limitations. First, this was a single-center study with a limited sample size, which weakens statistical analysis. Second, EEG assessment was based on analysis from one reviewer who was not blinded to clinical data. This drawback could be minimized by including three independent reviews of all EEG recordings. Indeed, it would have been desirable to include more reviewers, although the results likely would not have varied significantly considering the high interrater agreement demonstrated in the past for the majority of patterns, as well as ACNS’s Standardized Critical Care EEG Terminology [37]. Third, a depth electrode covers a limited sample of cerebral tissue, and therefore some intracortical seizures could be missed, contributing to the lack of correlation with scalp events. Fourth, ED burden was not calculated during the interictal state, and we did not quantify the total duration of ictal and IIC periods. Fifth, the rate of mortality was significantly high, and therefore the number of study participants did not permit us to obtain statistical conclusions.
Conclusions
The correlation between intracortical and scalp recordings in comatose patients with ABI, combined with invasive brain multimodality monitoring, can be a relevant source of data that will allow us to understand better surface EEG patterns and identify ictal semiology in critically ill patients to optimize ASM.
Electronic Supplementary Material
The online version of this article (https://doi.org/10.1007/s12028-024-02016-z) contains supplementary material.
References
Waziri A, Claassen J, Stuart RM, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66:366–77.
Stuart RM, Waziri A, Weintraub D, et al. Intracortical EEG for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2010;13:355–8.
Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64.
Jeffcote T, Hinzman JM, Jewell SL, et al. Detection of spreading depolarization with intraparenchymal electrodes in the injured human brain. Neurocrit Care. 2014;20:21–31.
Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579–90.
Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74:301–9.
Foreman B, Albers D, Schmidt JM, et al. Intracortical electrophysiological correlates of blood flow after severe SAH: a multimodality monitoring study. J Cereb Blood Flow Metab. 2018;38:506–17.
Foreman B, Ngwenya LB, Stoddard E, Hinzman JM, Andaluz N, Hartings JA. Safety and reliability of bedside, single burr hole technique for intracranial multimodality monitoring in severe traumatic brain injury. Neurocrit Care. 2018;29:469–80.
Marcellino CR, Lapalme-Remis S, Rabinstein AA, et al. Cortical surface intracranial electrodes identify clinically relevant seizures missed on scalp EEG after traumatic intracranial hemorrhage. Epileptic Disord. 2018;20:551–6.
Appavu B, Foldes S, Temkit M, et al. Intracranial electroencephalography in pediatric severe traumatic brain injury. Pediatr Crit Care Med. 2020;21:240–7.
Fernández-Torre JL, Hernández-Hernández MA, Mato-Mañas D, de Lucas EM, Gómez-Ruiz E, Martín-Láez R. Intracortical focal non-convulsive status epilepticus causing cerebral hypoxia and intracranial hypertension. Epileptic Disord. 2021;23:911–6.
Al Barajraji M, Bogossian E, Dewitte O, et al. Safety profile of an intracranial multimodal monitoring bolt system for neurocritical care: a single-center experience. Acta Neurochir (Wien). 2021;163:3259–66.
Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–8.
Hirsch LJ, Fong MWK, Leitinger M, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38:1–29.
Lam AD, Zepeda R, Cole AJ, Cash SS. Widespread changes in network activity allow non-invasive detection of mesial temporal lobe seizures. Brain. 2016;139:2679–93.
Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72.
Fong MWK, Jadav R, Alzawahmah M, Hussein OM, Gilmore EJ, Hirsch LJ. The significance of LRDA with bilateral involvement compared with GRDA on EEG in critically ill patients. J Clin Neurophysiol. 2023;40:434–42.
Lieb JP, Walsh GO, Babb TL, Walter RD, Crandall PH. A comparison of EEG seizure patterns recorded with surface and depth electrodes in patients with temporal lobe epilepsy. Epilepsia. 1976;17:137–60.
Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia. 1997;38:642–54.
Fernández Torre JL, Alarcón G, Binnie CD, et al. Generation of scalp discharges in temporal lobe epilepsy as suggested by intraoperative electrocorticographic recordings. J Neurol Neurosurg Psychiatry. 1999;67:51–8.
Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–76.
Tao JX, Baldwin M, Ray A, Hawes-Ebersole S, Ebersole JS. The impact of cerebral source area and synchrony on recording scalp electroencephalography ictal patterns. Epilepsia. 2007;48:2167–76.
Casale MJ, Marcuse LV, Young JJ, et al. The sensitivity of scalp EEG at detecting seizures—a simultaneous scalp and stereo EEG study. J Clin Neurophysiol. 2022;39:78–84.
Schevon CA, Ng SK, Cappell J, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–30.
Stead M, Bower M, Brinkmann BH, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–97.
Sun J, Barth K, Qiao S, et al. Intraoperative microseizure detection using a high-density micro-electrocorticography electrode array. Brain Commun. 2022;4:fcac122.
Florea B, Beniczky SA, Demény H, Beniczky S. Semiology of subtle motor phenomena in critically ill patients. Seizure. 2017;48:33–5.
Oto B, Annesi J, Foley RJ. Patient-ventilator dyssynchrony in the intensive care unit: a practical approach to diagnosis and management. Anaesth Intensive Care. 2021;49:86–97.
Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181–8.
Holla SK, Krishnamurthy PV, Subramaniam T, Dhakar MB, Struck AF. Electrographic seizures in the critically ill. Neurol Clin. 2022;40:907–25.
Getter N, Sepkuty J, Weinstein M, Norman Y, Fried I, Lockman J, Lorberboim M, Heyman E, Levy M. FIRDA, refractory epilepsy, and SEEG-guided RF: a case report. Epilepsia Open. 2023;8:298–306.
Fernández-Torre JL, Kaplan PW. Atypical or typical triphasic waves-is there a difference? A Review. J Clin Neurophysiol. 2021;38:384–98.
Fernández-Torre JL, Kaplan PW, Hernández-Hernández MA. New understanding of nonconvulsive status epilepticus in adults: treatments and challenges. Expert Rev Neurother. 2015;15:1455–73.
Ghearing GR, Abramovici S, Popescu A, Baldwin ME. Misleading EEG lateralization associated with midline shift. J Clin Neurophysiol. 2017;34:542–5.
Fernández-Torre JL, Figols J, Martínez-Martínez M, González-Rato J, Calleja J. Localisation-related nonconvulsive status epilepticus: further evidence of permanent cerebral damage. J Neurol. 2006;253:392–5.
Hocker S, Nagarajan E, Rabinstein AA, Hanson D, Britton JW. Progressive brain atrophy in super-refractory status epilepticus. JAMA Neurol. 2016;73:1201–7.
Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB. Critical care EEG monitoring research consortium. Interrater agreement for critical care EEG terminology. Epilepsia. 2014;55:1366–73.
Acknowledgements
The authors would like to thank all staff of the Departments of Clinical Neurophysiology, Intensive Medicine, Radiology, and Neurosurgery of Marqués de Valdecilla University Hospital for their collaboration in this study. This study received the award for the best oral presentation at the Congress of the Spanish Society of Epilepsy, held in Santiago de Compostela in October 2023.
Funding
This study was supported by the Instituto de Salud Carlos III, Acción Estratégica en Salud, expedient PI17/00156.
Author information
Authors and Affiliations
Contributions
JLF-T conceived and designed the study, acquired data, analyzed EEG recordings and videos, made the figures, and wrote the manuscript. MAH-H conceived and designed the study, acquired data, performed statistical analysis, made the tables, and critically revised the manuscript. MSC acquired data and critically revised the manuscript. DM-M acquired data and critically revised the manuscript. EMdL acquired data and critically revised the manuscript. EG-R acquired data and critically revised the manuscript. JLV-H acquired data and critically revised the manuscript. FF-V acquired data and critically revised the manuscript. JLA-D acquired data and critically revised the manuscript. RM-L acquired data and critically revised the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Ethical approval/informed consent
This study involves human participants and was approved by the Ethics Committee for Clinical Research of Cantabria (internal code: 2014.156). Family members of the patients gave informed consent to participate in the study before taking part.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández-Torre, J.L., Hernández-Hernández, M.A., Cherchi, M.S. et al. Comparison of Continuous Intracortical and Scalp Electroencephalography in Comatose Patients with Acute Brain Injury. Neurocrit Care (2024). https://doi.org/10.1007/s12028-024-02016-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12028-024-02016-z