Abstract
Background
Spreading depolarizations (SDs) are associated with worse outcome following subarachnoid hemorrhage (SAH) and traumatic brain injury (TBI), but gold standard detection requires electrocorticography with a subdural strip electrode. Electroencephalography (EEG) ictal–interictal continuum abnormalities are associated with poor outcomes after TBI and with both delayed cerebral ischemia (DCI) and poor outcomes after SAH. We examined rates of SD detection in patients with SAH and TBI with intraparenchymal and subdural strip electrodes and assessed which continuous EEG (cEEG) measures were associated with intracranially quantified SDs.
Methods
In this single-center cohort, we included patients with SAH and TBI undergoing ≥ 24 h of interpretable intracranial monitoring via eight-contact intraparenchymal or six-contact subdural strip platinum electrodes or both. SDs were rated according to established consensus criteria and compared with cEEG findings rated according to the American Clinical Neurophysiology Society critical care EEG monitoring consensus criteria: lateralized rhythmic delta activity, generalized rhythmic delta activity, lateralized periodic discharges, generalized periodic discharges, any ictal–interictal continuum, or a composite scalp EEG tool for seizure risk estimation: the 2HELPS2B score. Among patients with SAH, cEEG was assessed for validated DCI biomarkers: new or worsening epileptiform abnormalities and new background deterioration.
Results
Over 6 years, SDs were recorded in 5 (18%) of 28 patients recorded with intraparenchymal electrodes and 4 (40%) of 10 patients recorded with subdural strip electrodes. There was no significant association between occurrence of SDs and day 1 cEEG findings (American Clinical Neurophysiology Society main terms lateralized periodic discharges, generalized periodic discharges, lateralized rhythmic delta activity, or seizures, individually or in combination). After SAH, established cEEG DCI predictors were not associated with SDs.
Conclusions
Intraparenchymal recordings yielded low rates of SD, and documented SDs were not associated with ictal–interictal continuum abnormalities or other cEEG DCI predictors. Identifying scalp EEG correlates of SD may require training computational EEG analytics and use of gold standard subdural strip electrocorticography recordings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spreading depolarizations (SDs) are associated with poor clinical outcomes in patients with subarachnoid hemorrhage (SAH) and traumatic brain injury (TBI) [1], and SDs may be a contributing factor for in-hospital deterioration after TBI and SAH, the latter in the form of delayed cerebral ischemia (DCI) [2,3,4,5,6,7]. Additionally, SDs have been explored as a potential target for novel therapeutic approaches to improve clinical outcomes in these patients [8,9,10,11].
A barrier to implementing SD-guided therapy is the requirement of invasive neuromonitoring, such as electrocorticography (ECoG), historically requiring subdural strip electrode placement for gold standard recordings. Implementation of invasive neuromonitoring in the care of patients with SAH and TBI remains a challenge given a decreasing trend of craniotomy after SAH and TBI in SAH management [12, 13]. Therefore, a noninvasive SD detection method would enable SD monitoring and management at scale.
SDs have been detected via intraparenchymal depth electrodes inserted through a burr hole or intracranial bolt [14]. Additionally, continuous electroencephalography (cEEG) represents a potential tool for noninvasive SD recording. New or worsening epileptiform abnormalities (EAs) and cEEG background activity deterioration (BD) have both been validated as predictors of DCI after SAH [15]. Periodic discharges on the ictal–interictal continuum have also been found to be associated with focal hypermetabolism and increased cerebral blood flow [16, 17]. This increased risk for DCI may partly be due hypermetabolism, which in turn could trigger, or be triggered by, the initiation of SDs [18].
We accordingly sought to evaluate the frequency of SD detection when ECoG was recorded with either a subdural strip electrode array implanted during a clinical craniotomy or recorded using a minimally invasive intraparenchymal electrode inserted through a cranial bolt as part of clinical multimodal monitoring. We additionally sought to examine whether cEEG features documented by clinical neurophysiologists reviewing scalp cEEG monitoring were associated with the detection of SDs recorded on ECoG.
Methods
Study Design
In this prospective single-center study, we included patients admitted for aneurysmal SAH (aSAH) or TBI from May 2014 to May 2020 and who underwent ECoG following informed consent either as part of an institutional-review-board-approved protocol or as standard of care. Inclusion criteria were (1) TBI with a Glasgow Coma Scale (GCS) score of 3–8, (2) admission to the neurocritical care unit, and (3) age > 18 years old. Exclusion criteria were (1) severe coagulopathy preventing neuromonitoring and (2) comfort measures status.
ECoG monitoring consisted of an eight-contact intraparenchymal electrode inserted through a cranial bolt placed for multimodality monitoring, a subdural strip electrode implanted in the region of injury at the time of craniotomy, or both. Patients were all monitored in the neurosciences intensive care unit.
Data Acquisition
Multimodality monitoring included the clinical interpretation of ECoG as well as scalp EEG in all patients according to the institutional clinical guideline, which recommended cEEG monitoring for all patients with SAH with either high clinical grade (Hunt and Hess grade 4–5) or high radiologic grade (modified Fisher Scale score of 3–4) and ECoG in patients with coma.
Intraparenchymal electrodes implanted were eight-contact arrays (Depthalon, PMT Corp., Chanhassen, MN; or Ad-Tech, Racine, WI) placed through a multimodality bolt. Subdural electrodes implanted were linear six-contact (platinum) electrocorticography recording strips (Ad-Tech Medical, Cortac, or PMT Corp.) placed on cortex accessible through during a clinically performed craniotomy, as previously described [19]. ECoG recordings were acquired using a direct current (DC) or near-DC amplifier (Moberg ICU Solutions, Ambler, PA) as described below. ECoG was terminated, and the recording devices used were removed at the bedside by gentle traction when invasive neuromonitoring was no longer clinically required. All cEEG recordings were acquired using 21 electrodes applied according to the standard international 10–20 system in addition to the recorded ECoG channels.
ECoG Review for SD
ECoG was reviewed on bipolar and reference recordings of superimposed high-pass (0.5–30 Hz) and low-pass (0.005–0.5 Hz) montages using the Component Neuromonitoring System Reader software (Moberg, Ambler, PA). An SD was defined as the combination of slow potential change that corresponds to a negative slow voltage deflection and simultaneous transient suppression of faster activities in at least two adjacent channels [19, 20]. SD events were rated by consensus through visual inspection of the ECoG recordings among four readers (SS, ST, DYC, and ESR) according to established consensus criteria [19]. The peak number of SDs per 24 h of recording and peak SD days were also calculated following recommendations by the Co-Operative Studies on Brain Injury Depolarizations (COSBID) [19].
Evaluation of Scalp EEG
Two neurologists, including at least one board-certified in epilepsy or clinical neurophysiology, reviewed the raw EEG data and ECoG data prospectively as part of clinical practice [21] and generated twice-daily reports. Scalp cEEG was secondarily adjudicated by an additional epileptologist with expertise in critical care EEG monitoring (ESR) according to main terms of the American Clinical Neurophysiology Society critical care EEG monitoring consensus criteria, including lateralized rhythmic delta activity (LRDA), generalized rhythmic delta activity, lateralized periodic discharges (LPDs), and generalized periodic discharges (GPDs) [22]. Scalp cEEG was scored for the presence of findings validated as predictive of DCI: new or worsening EAs and new BD [23]. New EAs were defined as worsening in frequency, prevalence, spatial extent, or epileptiform morphology of sporadic epileptiform discharges, LRDA, LPDs, or GPDs, which were individually scored [15, 22]. New BD was defined as decreasing alpha delta ratio, relative alpha variability, or worsening focal slowing [15, 23]. A seizure risk stratification criterion, 2HELPS2B [24], which has been validated for use in acute brain injury [25], was scored through the review of clinical variables and the above-mentioned electrographic features as present on the initial 30–60 min of scalp EEG.
DCI Adjudication in Patients with SAH
The presence of DCI was established using an international consensus definition [26] as follows: (1) new focal neurologic deficits and/or a decrease in the GCS score of at least two points persisting for a minimum of 1 h and not explained by other causes by means of clinical assessment, imaging, or laboratory data or (2) cerebral infarction on computed tomography or magnetic resonance imaging of the brain that was not present on any neuroimaging done within the first 48 h following early aneurysm occlusion and not attributable to other causes, such as surgical clipping or endovascular treatment, a GCS score drop > 2 points, and radiological criteria. Radiologic infarcts attributed to DCI were confirmed by serial computed tomography/magnetic resonance imaging performed per institutional guidelines for clinical deterioration [21, 23], and this was verified by independent adjudicated medical record review by a reviewer blinded to cEEG findings (ESR).
Statistical Analysis
Because the sample size was determined only by the availability of data, the analyses were not statistically powered to address specific hypotheses. The statistical tests were performed using JMP Pro 15.0 software (SAS Institute, Inc., Cary, NC). Nonparametric statistical tests were used because measures of SDs/day burden, scalp EEG ictal–interictal continuum abnormalities, and DCI deviated significantly from normal distributions. Mann–Whitney U-tests were used for comparison of two independent samples. Fisher’s exact test was used to compare proportions with expected counts less than five. Data are reported as raw numbers (percentages) or medians (interquartile ranges), and P < 0.05 was accepted as statistically significant.
Results
From May 2014 to May 2020, there were 37 patients who met eligibility criteria of interpretable ECoG and EEG recordings of at least 24 h duration (Fig. 1). Twenty patients with SAH and 17 patients with TBI underwent intracranial EEG over this period. Of the 20 patients with SAH undergoing intracranial EEG, 19 (60%) were recorded over a 27-month period, of the 33 patients with SAH who presented with high clinical grade (Hunt and Hess grade 4–5) during that time period. An additional patient recorded during an earlier pilot phase was also included. Of 17 patients with TBI recorded with intracranial EEG, 15 (13%) were recorded over a 27-month period, of the 112 patients with TBI who presented with a GCS score ≤ 8. Two patients were excluded because of uninterpretable recordings. Among the remaining 35 patients suitable for analysis (20 with spontaneous aSAH and 15 with TBI), 4460 h of ECoG recordings were reviewed in total from the 35 patients. The median ECoG recording duration per patient (hours) was 120 (72–164). A total of 125 SD events were identified, occurring in 6 of 35 patients. The total numbers of SDs in each 24-h period were normalized to the valid recording durations for the corresponding 24-h periods to give the number of SDs per day. The maximal value for all 24-h periods was the peak SDs per day, which was 7 (3.5–14.75). No patients had a hemorrhagic or infectious complication of ECoG neuromonitoring.
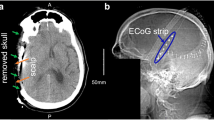
Example of spreading depolarization recording from a subdural strip electrode. a, Head computed tomography (CT) scout confirming placement of intracranial subdural strip in the left temporal position and intraparenchymal electrode in the right frontal position. b, Cortical spreading depolarizations with corresponding spreading depressions detected on intracranial subdural strip electrode acquired from the same patient with the previously described CT scout. This review was conducted on bipolar recording of superimposed high-pass (0.5–30 Hz) and low-pass (0.005–0.5 Hz) montages using the CNS Reader software. Spreading depression is observed as an abrupt large negative DC shift in ECoG recordings, with a sequential shift noted in adjacent electrodes from spread to surrounding electrically active sites. ECoG electrocorticography
Overall, SDs were identified in 4 of 10 (40%) patients with a subdural strip recording and 5 of 29 (17.74%) with an intraparenchymal recording. (Fig. 1). Among patients with aSAH (Table 1), SDs were identified in 5 of 20 (25%) patients, specifically 3 of 5 (60%) with a subdural strip and 5 of 18 (27.77%) with an intraparenchymal electrode. Among patients with TBI (Table 2), SDs were identified in 0 of 10 patients with intraparenchymal electrodes and 1 of 5 patients (20%) with a subdural strip recording. Of four patients monitored with both strip and intraparenchymal electrodes (3 SAH, 1 TBI), SDs were detected independently by the subdural strip and intraparenchymal electrodes when the recording devices used were collocated. One patient (with SAH) had bilateral recordings (subdural strip electrode, left temporal; intraparenchymal electrode, right frontal), and in this patient, SDs were detected by the subdural strip electrode alone, although there was no collocated intraparenchymal electrode (Fig. 2).
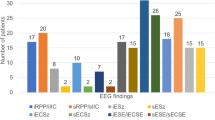
There was no significant association between occurrence of SDs and day 1 American Clinical Neurophysiology Society scalp cEEG main terms (LPDs, GPDs, LRDA, or seizure activity, individually or in combination, and the composite 2HELPS2B score) or an association with these terms for the entire monitoring duration (Table 3). This association was not examined individually for patients with TBI because of the low rate of SDs in this cohort primarily monitored with intraparenchymal electrodes.
Four of the five patients with aSAH with SDs had a peak SD incidence 1–2 days from the time of ruptured aneurysms, of whom two experienced in-hospital mortality. SD events preceded scalp EEG biomarkers of DCI, as well as DCI itself, in four of five patients with SDs (Fig. 3). Peak SD events occurred concurrently with scalp EEG events in one patient. This patient also had an increased number of SD events and experienced in-hospital mortality. Among patients with aSAH, DCI occurred in all patients with SDs, with no significant difference in mortality rates in patients without DCI. Validated cEEG biomarkers of post-SAH ischemia (new or worsening EAs and new BD) were common in patients who developed DCI (Fig. 3). New or worsening EAs occurred in three of five (60%) patients with aSAH with SDs, new BD occurred in four of five (80%) patients with aSAH with SDs, and two of five (40%) patients with aSAH with SDs had both. No SDs were seen in the absence of new or worsening EAs and BDs, However, among all 20 patients with aSAH, the rate of new or worsening EAs was 65% and the rate of new BD was 70%, such that the crude sensitivity and specificity of new or worsening EAs for DCI were 83% and 43%, respectively, and the positive predictive value and negative predictive value were 77% and 42%, respectively. The crude sensitivity and specificity of new or worsening BD for DCI were 92% and 50%, respectively, and the positive predictive value and negative predictive value were 81% and 93%, respectively. A limited sample size did not allow for predictive modeling on whether presence of SD can portend DCI. Results of one-tailed binomial tests on the association of SD/ictal–interictal continuum abnormalities and DCI were not statistically significant.
Events detected by electrocorticography and electroencephalography following subarachnoid hemorrhage (SAH). a, Timeline of Scalp electroencephalography (EEG) events, monitoring duration, and clinical events in patients with SAH. All patients with spreading depressions (SDs) had delayed cerebral ischemia (DCI). Temporal alignment of SDs and DCI with use of collocated devices are demonstrated. In the absence of SDs, scalp EEG change patterns were associated with DCI. b, Early clusters of SDs preceded DCI. BD background activity deterioration, iEEG xxx, IICA ictal–interictal continuum abnormality
Discussion
In this single-center prospective study of ECoG in comatose patients with aSAH and TBI, when either intraparenchymal electrodes, subdural strip electrodes, or both, depending on the required neurosurgical management, were used, there was an overall decreased rate of SD detection with intraparenchymal electrodes (18%) compared with strip electrodes (40%). The need for craniotomy may represent a confounding by indication, which may require further exploration as to whether minimally invasive ECoG suffers low rates of SD detection because of monitoring methodology or patient severity or as a result of the craniotomy itself.
Regardless, these rates from using primarily intraparenchymal recording of ECoG in a comatose population represent lower rates of SD detection than studies in the past, which reported rates of around 70% and 60% in SAH and TBI, respectively [1, 5, 8, 27, 28].
The greater cortical surface coverage likely gives subdural strip electrodes a theoretical advantage over the intraparenchymal electrodes in detecting SDs [14]. Additionally, an intraparenchymal electrode may spatially undersample the SD detection particularly in patients with localized injuries. Another limitation is that electrodes were not always placed near the location of the aneurysm rupture. For example, there were two instances of right-sided aneurysms with left-sided probe placement and three instances of posterior circulation aneurysm rupture with a frontal probe placement. This may have decreased the effective sensitivity of SD detection. Similarly in patients with TBI, low overall rates of strip electrode placements and effects of antiseizure medications, which were not accounted for, might have impacted our rates of SD detection. We were able, however, to examine several established biomarkers in the SAH cohort.
Acknowledging a low SD detection rate, we did not find an association between clinical scalp cEEG measures and the occurrence of SD in this setting. Acknowledging limitations of spatial sampling, we noted absence of LPDs and occurrences of generalized rhythmic delta activity, GPDs, and LRDA in patients who had SDs in this cohort (Table 3). Scalp cEEG abnormalities following aSAH known to predict DCI were again demonstrated to predict DCI in this cohort but were sensitive and not specific for SDs.
Scalp ictal–interictal continuum abnormalities have been found to increase the risk of DCI, in particular when they occur within days after the SAH [23], and cEEG periodic discharges have also been found to be associated with focal hypermetabolism and increased cerebral blood flow, a pathogenic mechanism also associated with the development of SDs [16, 17]. The frequency of scalp periodic discharges has been found to be increased in patients with DCI. SDs have also been thought to trigger epileptiform discharges in the past [29]. The use of EEG measures as a surrogate to monitoring for SDs has potential for better risk stratification of poor outcomes through monitoring for both onset and progression of secondary brain injury.
We acknowledge that our rate of event detections was likely underpowered to detect a small difference in these findings. To be useful, a classifier would additionally need to differentiate patients at an early point when treatment is still meaningful, and we did not find any direct association between EAs and a seizure risk stratifying criterion (2HELPS2B score) documented in the first hour of EEG monitoring.
DCI in this study always occurred when SDs were present but also occurred when SDs were absent, and in these situations, scalp cEEG biomarkers of post-SAH DCI were evident in advance of DCI. Given the only partial overlap of SDs and cEEG biomarkers of DCI, we interpret that either there are multiple pathways to DCI or, alternatively, that SDs are not always detected by intraparenchymal and subdural strip electrodes. A few studies have explored the use of scalp EEG correlates for noninvasive detection of SDs [30]. These are currently insufficient when used alone to reliably detect SDs. The automated EEG-based algorithms for SD detection have been explored and are currently not primed for clinical use [31].
Although these clinically defined scalp cEEG measures were not associated with SD events or quantity, it is possible that nonobvious scalp cEEG measures may be associated with patients with SDs or with SD events themselves. Additionally, going forward, there is sufficient evidence to recommend intraparenchymal depth electrode placement in standard positioning for the purpose of SD detection, and further studies examining scalp EEG associations with SD would need to be performed in patients with subdural strip electrodes or equivalent. Noninvasive approaches to predict which patient will have SDs or to monitor for SD events themselves will enable more widespread practice of SD monitoring and differentiation between other ictal and inter-ictal phenomena to guide future neuroprotective therapy in patients with SAH and TBI.
Conclusions
In this small single-center study in which monitoring methodology was likely influenced by indication, intraparenchymal electrodes experienced an overall low rate of SD detection. Additionally, there was no significant association between noninvasive cEEG measures and cortical SDs. It is uncertain whether cEEG biomarkers of post-SAH DCI represent a contributor to DCI independent of SDs or whether clinically interpreted scalp EEG is insufficient to detect SD. Further studies examining EEG correlates of SD may benefit from computational approaches with composite and quantitative features conducted in populations of suitable sample size.
References
Hartings JA, Strong AJ, Fabricius M, et al. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26(11):1857–66.
Roos YBWEM, De Haan RJ, Beenen LFM, et al. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in The Netherlands. J Neurol Neurosurg Psychiat. 2000;68(3):337–41.
Lantigua H, Ortega-Gutierrez S, Schmidt JM, et al. Subarachnoid hemorrhage: who dies, and why? Crit Care. 2015;19(1):309.
Maher M, Schweizer TA, Macdonald RL. Treatment of spontaneous subarachnoid hemorrhage: guidelines and gaps. Stroke. 2020;51(4):1326–32.
Sugimoto K, Chung DY. spreading depolarizations and subarachnoid hemorrhage. Neurotherapeutics. 2020;17(2):497–510.
Woitzik J, Dreier JP, Hecht N, et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2012;32(2):203–12.
Sánchez-Porras R, Zheng Z, Santos E, et al. The role of spreading depolarization in subarachnoid hemorrhage. Eur J Neurol. 2013;20(8):1121–7.
Chung DY, Oka F, Ayata C. Spreading depolarizations: a therapeutic target against delayed cerebral ischemia after subarachnoid hemorrhage. J Clin Neurophysiol. 2016;33(3):196–202.
Bosche B, Graf R, Ernestus RI, et al. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann Neurol. 2010;67(5):607–17.
Kramer DR, Fujii T, Ohiorhenuan I, Liu CY. Cortical spreading depolarization: pathophysiology, implications, and future directions. J Clin Neurosci. 2016;24:22–7.
Carlson, A.P., Abbas, M., Alunday, R.L., Qeadan, F.Shuttleworth, C.W. 2018 Spreading depolarization in acute brain injury inhibited by ketamine: a prospective, randomized, multiple crossover trial. J Neurosurg 1–7.
Sinha S, Hudgins E, Schuster J, Balu R. Unraveling the complexities of invasive multimodality neuromonitoring. Neurosurg Focus FOC. 2017;43(5):E4.
Brinjikji W, Lanzino G, Rabinstein AA, Kallmes DF, Cloft HJ. Age-related trends in the treatment and outcomes of ruptured cerebral aneurysms: a study of the nationwide inpatient sample 2001–2009. AJNR Am J Neuroradiol. 2013;34(5):1022–7.
Jeffcote, T., Hinzman, J.M., Jewell, S.L., et al. Detection of Spreading Depolarization with Intraparenchymal Electrodes in the Injured Human Brain. 2013.
Kim JA, Rosenthal ES, Biswal S, et al. Epileptiform abnormalities predict delayed cerebral ischemia in subarachnoid hemorrhage. Clin Neurophysiol. 2017;128(6):1091–9.
Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79(4):579–90.
Dreier JP, Isele T, Reiffurth C, et al. Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? Neuroscientist. 2013;19(1):25–42.
Struck AF, Westover MB, Hall LT, et al. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit Care. 2016;24(3):324–31.
Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: Review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37(5):1595–625.
Dreier JP, Major S, Pannek HW, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135(Pt 1):259–75.
Muniz CF, Shenoy VA, O’Connor KL, et al. Clinical development and implementation of an institutional guideline for prospective EEG monitoring and reporting of delayed cerebral ischemia. J Clin Neurophysiol. 2016;33(3):217–26.
Hirsch LJ, Fong MWK, Leitinger M, et al. American clinical neurophysiology society’s standardized critical Care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38(1):1–29.
Rosenthal ES, Biswal S, Zafar SF, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol. 2018;83(5):958–69.
Struck AF, Ustun B, Ruiz AR, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol. 2017;74(12):1419–24.
Moffet, E.W., Subramaniam, T., Hirsch, L.J., et al. Validation of the 2HELPS2B Seizure Risk Score in Acute Brain Injury Patients. Neurocritical Care 2020.
Vergouwen MDI, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke A J Cerebral Circulat. 2010;41(10):2391–5.
Hartings, J.A., Andaluz, N., Bullock, M.R., et al. Prognostic Value of Spreading Depolarizations in Patients with Severe Traumatic Brain Injury. JAMA Neurology 2019.
Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129(12):3224–37.
Eickhoff M, Kovac S, Shahabi P, et al. Spreading depression triggers ictaform activity in partially disinhibited neuronal tissues. Exp Neurol. 2014;253:1–15.
Drenckhahn C, Winkler MK, Major S, et al. Correlates of spreading depolarization in human scalp electroencephalography. Brain. 2012;135(Pt 3):853–68.
Chamanzar, A., George, S., Venkatesh, P., et al. An algorithm for automated, noninvasive detection of cortical spreading depolarizations based on EEG simulations. IEEE Transactions on Biomedical Engineering 2019 66(4).
Funding
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke Grants 1K23NS105950 (ESR), KL2TR002542 (DYC), and K08NS112601 (DYC); United States Army Grant W81XWH-BAA-15-1 (ESR); the American Heart Association (18POST34030369; DYC); the Andrew David Heitman Foundation (ESR, ABP, DYC); the Aneurysm and AVM Foundation (DYC); and the Brain Aneurysm Foundation’s Timothy P. Susco and Andrew David Heitman Foundation Chairs of Research (DYC).
Author information
Authors and Affiliations
Contributions
SS conceptualized the study design; conducted the data acquisition, analysis, and interpretation; and drafted and revised the manuscript. ABP, CJS, BLG, and JSS conceptualized the study design, performed intracranial monitoring, and contributed to drafting the manuscript. DYC contributed to the study design and Fig. modeling and conducted the data review, analysis, and interpretation, as well as manuscript revisions. ST contributed to data review and manuscript revisions. ESR conceptualized the study design and performed data analysis and interpretation, as well as manuscript drafting and revisions. DYC and ESR contributed equally to this work.
Corresponding author
Ethics declarations
Conflicts of interest
SS has nothing to disclose. ST has nothing to disclose. DYC has nothing to disclose. BLG has nothing to disclose. ABP has nothing to disclose. CJS has nothing to disclose. JSS has nothing to disclose. ESR reports consulting fees from UCB Pharma, Inc. and Ceribell, Inc.; medicolegal consultation; funding from National Institutes of Health National Institute of Neurological Disorders and Stroke grant 1K23NS105950 and United States Army grant W81XWH-BAA-15–1. ESR’s institution is a subcontract to Moberg ICU Solutions, whose clinical monitoring equipment was used in clinical practice in this patient population.
Ethical approval
The authors adhered to ethical guidelines, and this study was approved by the institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the collection “Spreading Cortical Depolarization”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sivakumar, S., Tsetsou, S., Patel, A.B. et al. Cortical Spreading Depolarizations and Clinically Measured Scalp EEG Activity After Aneurysmal Subarachnoid Hemorrhage and Traumatic Brain Injury. Neurocrit Care 37 (Suppl 1), 49–59 (2022). https://doi.org/10.1007/s12028-021-01418-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01418-7