Abstract
Background
The use of multimodal neuromonitoring in pediatrics is in its infancy relative to adult neurocritical care. Multimodal neuromonitoring encompasses the amalgamation of information from multiple individual neuromonitoring devices to gain a more comprehensive understanding of the condition of the brain. It allows for adaptation to the changing state of the brain throughout various stages of injury with potential to individualize and optimize therapies.
Methods
Here we provide an overview of multimodal neuromonitoring in pediatric neurocritical care and its potential application in the future.
Results
Multimodal neuromonitoring devices are key to the process of multimodal neuromonitoring, allowing for visualization of data trends over time and ideally improving the ability of clinicians to identify patterns and find meaning in the immense volume of data now encountered in the care of critically ill patients at the bedside. Clinical use in pediatrics requires more study to determine best practices and impact on patient outcomes. Potential uses include guidance for targets of physiological parameters in the setting of acute brain injury, neuroprotection for patients at high risk for brain injury, and neuroprognostication. Implementing multimodal neuromonitoring in pediatric patients involves interprofessional collaboration with the development of a simultaneous comprehensive program to support the use of multimodal neuromonitoring while maintaining the fundamental principles of the delivery of neurocritical care at the bedside.
Conclusions
The possible benefits of multimodal neuromonitoring are immense and have great potential to advance the field of pediatric neurocritical care and the health of critically ill children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a longstanding history and desire of assessing the brain by multiple invasive and noninvasive modalities in neurocritical care. The technological advances now allow us to integrate the enormous amount of time-synchronized data from these modalities into a more cohesive summary that may provide a more comprehensive insight of the injured brain. At the same time, the field of pediatric neurocritical care continues to grow and mature with increasing experience and expertise of the multidisciplinary interprofessional team in managing children with critical neurological conditions [1,2,3,4,5,6]. Together, these developments present an exciting and unprecedented opportunity to incorporate multimodal neuromonitoring as a core component of a pediatric neurocritical care program [6, 7], with the goals of expanding our knowledge of neuropathophysiology at the bedside and advancing the care of children with critical neurological conditions.
Multimodal Neuromonitoring as a Strategy for Precision Medicine in Neurocritical Care
One of the primary goals of pediatric neurocritical care is to protect the brain and minimize secondary brain injury in critically ill children. Optimizing cerebral blood flow, metabolism, and oxygen delivery while supporting cardiopulmonary physiology underlies the basic principles of neuroprotection. Currently, several published guidelines for children with neurologic disease and injury provide physiologic targets based on best available evidence and expert consensus. For example, guidelines for the care of children with traumatic brain injury (TBI) include intracranial pressure (ICP), cerebral perfusion pressure (CPP), and brain tissue oxygenation (PbtO2) goals [8, 9]. Pediatric post-cardiac-arrest management guidelines discuss targeted temperature management, oxygenation, and ventilation aims [10, 11]. Guidelines for managing acute ischemic stroke in children comment on blood pressure, temperature, and glucose targets [12]. As evidenced by these guidelines, similar physiologic target values are often applied to patients with heterogenous injuries in the contemporary model of care. This approach, while contributing to the evidence-based standardization of care, may be suboptimal for the individual patient. Therefore, implementing precision medicine with varying physiological targets over time based on the changing physiologic state of the brain has been gaining momentum in the field of pediatric neurocritical care as a potential therapeutic approach to optimizing the care of individual patients. Central to this idea is the use of multimodal neuromonitoring. Here, we provide an overview of current multimodal neuromonitoring in pediatric neurocritical care and its potential application in the future.
What is Multimodal Neuromonitoring?
Multimodal neuromonitoring refers to the use of technology from various monitoring sources to assess the physiologic state of the brain more comprehensively. Historically, the physical neurologic examination is considered the gold standard for monitoring the health and functional state of the brain. However, many factors affect the reliability of the clinical examination in children with critical neurological conditions and therefore may limit its utility to assess the status of the brain. This includes the severity of the ongoing neuropathological process and factors in the pediatric intensive care unit (PICU) that may mask the clinical examination, such as the use of sedation and neuromuscular blockade. Additionally, once the underlying neuropathological process alters the functionality of the brain, affecting the clinical examination, injury may be occurring to a critical degree, which if not quickly addressed, can lead to irreversible functional injury with significant effect on outcome. Multimodal neuromonitoring may provide early detection of pathologic changes before the impairment becomes clinically evident, thus allowing for timely intervention and preventing progression to irreversible brain injury.
Multimodal neuromonitoring encompasses time-synchronized data integration from multiple neuromonitoring devices and cardiopulmonary monitors to look beyond the physical examination for a more comprehensive understanding of the clinical state of the brain in relation to the general hemodynamic state of the patient. This may allow for individualized identification of optimal targets for physiologic variables, both within the central nervous system and systemically, during the critical phase of illness. For instance, in TBI, guideline recommendations for management are largely based on monitoring of ICP as well as targeting CPP with the use of arterial blood pressure measurements [8, 9]. Although minimum targets are suggested in the guidelines, these may not be the optimal parameters for individual patients with heterogenous injuries because cerebral autoregulatory range may differ between individuals and at different times in the same individual. Therefore, a multimodal approach combining systemic physiological parameters, such as mean arterial pressure (MAP), with cerebral physiological parameters such, as ICP, PbtO2, and microdialysis, may further refine the CPP target for each patient at a given time point [8, 9, 13,14,15,16,17,18,19,20,21,22]. When the physiologic state of the brain lies outside the target range, multimodal neuromonitoring can assist in clarifying potential causes and thus provide opportunity for more targeted intervention. Once intervention has occurred, multimodal neuromonitoring aids in detailed evaluation of the response to treatment, leading to more robust understanding of the needs of each patient and more tailored therapies [23].
One example of using multimodal neuromonitoring in pediatrics, described by Appavu et al. would be a child with TBI who has developed intracranial hypertension [24]. There can be many causes of increased ICP following TBI, including new or extending hemorrhage, cerebral edema, hydrocephalus, seizures, hyperemia, or plateau waves. Once conditions requiring immediate surgical procedure are excluded, options for intervention vary significantly depending on etiology, including administration of hyperosmolar therapy, rescue seizure medications, neuromuscular blockade, or adjustment of CO2 or blood pressure targets [2]. Without clear delineation of the sequence of events, it can be difficult to determine whether commonly seen complications after TBI, such as seizures, are the cause or the result of the cascade of events following increased ICP, including decreased blood flow and inadequate oxygen delivery. By reviewing synchronized neuromonitoring data trends from multiple devices, including ICP monitors, continuous electroencephalography (cEEG), transcranial Doppler (TCD), and end-tidal CO2 monitors, in conjunction with the physical examination, the cause of the increased ICP may become more readily apparent and the targeted treatment more quickly administered. This can be achieved even in the absence of a reliable clinical examination, such as with the use of neuromuscular blockade.
To date, there is no standardization of what multimodal neuromonitoring means in the field of pediatric neurocritical care, with each institution developing its own approach based on the needs of their patient population and available resources. As a result, devices used in clinical care and clinical practice vary greatly across institutions. There are many combinations of clinical devices used for monitoring the brain in the PICU depending on the diagnosis and needs of each patient. Recently, devices used for multimodal neuromonitoring in the care of pediatric patients have been described [24,25,26,27] (Table 1). The potential clinical variables frequently targeted in pediatric multimodal neuromonitoring, both neurologically and systemically, are listed in Table 2 [28,29,30].
What is a Multimodal Neuromonitoring System?
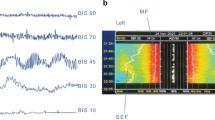
With a growing number of physiologic variables being monitored and an increasing number of devices used to collect them, the volume of data and information incorporated into the care of children in the PICU with the use of multimodal neuromonitoring is immense. A frequently cited number from 1992 is that clinicians face > 200 variables when evaluating a patient in the intensive care unit (ICU) [31]. With health care data growing exponentially, 30 years later, this number has grown substantially. It is not surprising that we have likely surpassed our human capacity to collect and interpret data at the bedside in a meaningful way with the speed required for patients who are changing so quickly in the ICU. Dedicated multimodal neuromonitoring systems may be a solution to manage this incredible volume of information from multiple sources and discover patterns and meaning, which may ultimately enhance and individualize the care of the patients. Multimodal neuromonitoring systems integrate time-synchronized data from individual neuromonitoring devices into one location in a manner that is visually helpful and ideally user-friendly. Most systems allow for review of data trends over time in which several hours of data can be visualized easily at one time (Fig. 1). Compared to the individual high-resolution waveforms in which only a few seconds can be viewed per screen, long-term simultaneous trending of multiple physiological data may improve the ability to identify important clinical patterns that may not be visible when viewing each waveform individually [32].
A 13-year-old female patient with traumatic brain injury undergoes multimodality neurologic monitoring. Increases in ICP are associated with periodic bursts of delta activity, maximal over the right hemisphere. Subsequent neuroimaging demonstrates worsened left hemispheric cerebral edema with midline shift, and the patient undergoes a left hemispheric decompressive craniectomy. ABP arterial blood pressure, CO2 carbon dioxide, CPP cerebral perfusion pressure, CSDA color dense spectral array, ECG electrocardiogram, ETCO2 end-tidal carbon dioxide, HR heart rate, ICP intracranial pressure, LH left hemisphere, RH right hemisphere, rSO2 cerebral regional oximetry. Credit to Brian Appavu, MD
There are multiple classes of multimodal neuromonitoring systems that are currently in use in pediatric institutions [26] (Fig. 2). Systems can generally be divided into two major types: a stand-alone kiosk/computer system and a distributed system [33]. The stand-alone system is a physical monitor or a computer with built-in software specific to that device that is brought to a patient’s bedside. Neuromonitoring devices are connected and input data directly into the kiosk for real-time review. The benefit of this type of system is that the kiosks are mobile and do not require dedicated data ports. Each unit allows for monitoring one patient at a time, and data may be saved on the device itself or uploaded to a hospital server. Review of the data can be done at the bedside in real-time. Additional software may allow for remote reviewing to aid in patient care, multidisciplinary collaboration, quality improvement, adherence to guidelines, or research. Newer units that are currently on the market are equipped with decision support for clinicians as well as machine learning algorithms and smart alarms. Although these are not pediatric specific, each institution can tailor the template for these kiosks and protocols to meet the needs of their specific patient populations. In contrast to the physical kiosk monitors, distributed systems involve a remote server where the data can be accessed from network computers or the Web. Data can be sent continuously to the server from multiple locations. Because there is no stand-alone unit, multiple patients can be monitored at one time, which allows for large-scale informatics without a machine for each patient. Data are viewed remotely.
Overview of multimodal neuromonitoring systems (MMNM) including data flow from bedside to visualization/analysis. a Stand-alone MMNM system in which data from individual monitoring devices are input directly into the physical device brought to patient bedside. b Distributive MMNM system in which data from individual monitors are sent to a remote server where they can then be accessed from multiple locations
Current Clinical Guidelines
Currently, there is no pediatric specific guideline on the use of multimodal neuromonitoring in the PICU. In a recent survey study on the practice of multimodal neuromonitoring in PICUs in North America, only 8 of 52 institutions reported using a multimodal neuromonitoring system [6]. For those institutions using multimodal systems, device protocols included monitoring with near-infrared spectroscopy (NIRS) (8), ICP monitors (7), electroencephalography (EEG) (5), PbtO2 (3), TCD (3), brain temperature (3), pupillometry (2), and jugular venous oximetry (1). Implementation of multimodal neuromonitoring in each institution was highly variable. This study highlights the pressing need to develop evidence-based clinical guidelines for the use of multimodal neuromonitoring in pediatrics.
In the absence of dedicated pediatric guidelines, the existing consensus statement from the Neurocritical Care Society on multimodal neuromonitoring in neurocritical care, published in 2014, may serve as a guide [34]. Specifically, the statement recommends the use of time-synchronized systems that display data in a manner to “reduce cognitive load and improve judgments of clinicians,” the use of clinical decision support tools and algorithms, and the use of smart alarms. Additionally, the statement calls for device manufactures to adhere to communications standards to improve interoperability between devices.
Clinical Application of Multimodal Neuromonitoring in Pediatric Neurocritical Care Patients
The clinical experience of multimodal neuromonitoring remains limited in the pediatric population. Most studies involve the traditional physiological variables (HR, MAP, SaO2) in conjunction with one additional neuromonitoring modality [15, 16, 23, 35,36,37,38]. In contrast, there is a longstanding history of employing multiple neuromonitoring modalities in adults with critical neurological conditions [39, 40]. Nevertheless, multimodal neuromonitoring that focuses on identifying cerebral autoregulation is increasingly described in pediatric TBI, hypoxic-ischemic encephalopathy, and arteriovenous malformation rupture [15,16,17, 23, 35,36,37,38, 41]. These retrospective analyses of the prospectively acquired data revealed that impaired cerebral autoregulation was associated with unfavorable outcome [15, 16, 35, 36, 38, 41, 42]. Additionally, the amount of time and the magnitude of deviation from the derived optimal autoregulation value were associated with unfavorable outcome [15, 16, 35, 36, 38, 41, 42]. Importantly, the MAP or CPP values that yielded the optimal cerebral autoregulation may differ between patients; some may even be outside of the consensus-based MAP or CPP ranges for the specific condition [15, 17, 36]. Together, these investigations highlight the feasibility and possibly the importance of deriving individualized physiological targets for the management of children with critical neurological conditions.
Aside from evaluation of cerebral autoregulation, multimodal neuromonitoring has been applied in other pediatric patient populations with acute brain injury, including those with subarachnoid hemorrhage and acute liver failure, in an effort to optimize clinical parameters. In children with subarachnoid hemorrhage, an approach is often taken similar to neuromonitoring for adult patients, which includes cEEG with advanced analytics (alpha-delta ratio), TCD, and ICP/CPP evaluating for cerebral vasospasm and delayed cerebral ischemia while minimizing increased ICP [43,44,45]. In acute liver failure, multimodal neuromonitoring with cEEG, TCD, jugular venous oxygen saturation, and NIRS has been proposed [46,47,48]. Combining multiple noninvasive approaches in this patient population is important, particularly when the risks of invasive monitoring are amplified because of coagulopathy. More study is needed to determine optimal targets and best practice for interventions in these clinical scenarios and to determine whether applying these measures improves outcome. As clinicians gain familiarity with multimodal neuromonitoring and advance research, available modalities are likely to be applied to a growing number of conditions.
Integrating physiological data and variables derived from multimodal neuromonitoring into clinical practice in real-time is at its infancy in pediatric neurocritical care. A recent study by Appavu et al. [7] demonstrated that incorporating multimodal neuromonitoring in the management of pediatric TBI could influence clinical decision-making on the timing of neuroimaging and interventions such as neurosurgical interventions, adjustment of medications, escalation or de-escalation of therapies, adjustment of physiologic targets and neuroprognostication. In this study, multimodal neuromonitoring was associated with a reduction in duration of ICP monitoring and mechanical ventilation [7]. Continuous adjustment of the target CPP based on the cerebral autoregulation values derived from multimodal neuromonitoring is feasible and safe in adult patients with TBI [49]. Additionally, in the future, continuous refinement of the physiological targets based on the real-time multimodal data stream may provide a personalized therapeutic strategy that is adaptive to the changing clinical conditions. Ultimately, prediction analytics from real-time multimodal neuromonitoring data may forestall impending intracranial crisis, which may allow the clinicians to implement preventive interventions.
The use of multimodal neuromonitoring has also been proposed as a means to better understand various patterns on cEEG and how both the brain’s hyperexcitability and suppression may relate to secondary brain injury [30, 50]. The use of cEEG is well established and has been expanding, with consensus statements specifically addressing its use in critically ill adults and children [43, 51]. Not only used to detect seizures, EEG is also used to identify changes in background activity, which could signify new or worsening injury, such as ischemia, new focal lesion, or increased ICP [30]. The addition of quantitative EEG has allowed for better visualization of data trends occurring on the EEG, which helps identify patterns not immediately obvious on the raw EEG [30, 52]. There are EEG patterns on the ictal-interictal continuum whose significance can be difficult to determine by EEG alone. Although not definite seizure, these hyperexcitable patterns could increase metabolic demands in the brain and contribute to secondary brain injury in certain pathological brain states. Whether to treat can be difficult to ascertain. Using a combined approach of monitoring ICP, PbtO2, and microdialysis along with NIRS and TCD could help understand whether these patterns are contributing to abnormal blood flow, increased ICP, or a cerebral metabolic crisis. If noted, treatment may be beneficial [30, 50]. Additionally, Appavu et al. [53] explored the relationship between the alpha-delta ratio on quantitative EEG and brain tissue hypoxia in pediatric patients with TBI and found that PbtO2 values < 10 mm Hg were associated with a decreased alpha-delta power ratio. This potentially identified a critical threshold for the development of brain ischemia that could impact outcome that likely would not be readily identified on the raw EEG [53]. Therapeutic interventions to mitigate this EEG suppression could lead to improved outcomes. More study is needed.
Some institutions also employ multimodal monitoring for neuroprotection in children without clear brain injury who are at high risk as a result of their overall critical illness, as exemplified by multimodal neuromonitoring protocols for patients requiring extracorporeal membrane oxygenation (ECMO) support because of the high risks of seizures, stroke, hemorrhage, and hypoxic injury as well as direct injury to major blood vessels supplying or draining the brain [29, 54,55,56,57]. Similar to children with known neurologic concerns, multimodal neuromonitoring in children at risk for neurologic complications most commonly involves a single neuromonitoring modality in conjunction with physiologic parameters. For instance, cEEG monitoring is recommended for high-risk populations on ECMO [43]. Not only can EEG identify seizures, but it can also screen for focal injury such as ischemia with changes in background activity [30]. Serial cranial ultrasounds can be used as a screening measure for injury, and certain variables can be trended as an indirect marker for autoregulation, such as resistive indices [58]. TCD allows for intermittent monitoring of blood flow in which an increase can be a marker for hemorrhage risk [59]. NIRS can be used to screen oxygen delivery and use in the brain [60]. Therefore, combining all three neuromonitoring modalities in children undergoing ECMO could theoretically lend insight into CBF and autoregulation through TCD, while determining its adequacy to support cerebral metabolism by NIRS, in the context of altered brain activities as manifested by cEEG. Although more study is needed to determine whether this type of monitoring changes outcome, it may help identify changes in the brain early, allowing for modification of treatment and ideally avoidance of injury. This concept is well established in the pediatric cardiac surgery population, in which routine use of neuromonitoring during surgery and anesthesia and perioperative cEEG monitoring have been used for neuroprotection [61,62,63]. Multimodal neuromonitoring has also been proposed for other conditions without clear brain injury, such as sepsis, acute respiratory distress syndrome, and renal disease, in which clinical examination may be confounded [64].
Finally, there is a growing interest in using multimodal neuromonitoring for guidance in neuroprognostication, particularly in the setting of cardiac arrest [10, 11]. While research is ongoing, more study is required before definitive recommendations can be made.
Challenges and Pitfalls of Multimodal Neuromonitoring
Obtaining and storing a large volume of high-resolution data, particularly when collecting information at the waveform level, can be challenging and costly. There is no standard data protocol for the disparate systems to integrate the large amount of time-synchronized data. Each multimodal system uses its own software with proprietary protocols for connectivity and for data display; therefore, there is a lack of universal compatibility across different systems and neuromonitoring devices. Many multimodal systems also obtain information from the electronic health record, and many of the same barriers exist for integration with the various electronic health record systems [65]. Ongoing governmental priority on medical device and electronic health record interoperability will undoubtedly address this fundamental and crucial issue [66].
Artifact represents a common source of error for all available modalities. Accounting for artifacts in the data algorithms proves challenging, particularly if smart alarms or decision support programs are in use [65]. Therefore, visual inspection of the data display continues to be the gold standard to ensure fidelity, which is labor-intensive and limits the potential of leveraging big data analytics for patient care.
Each monitoring modality has inherent assumptions and limitations. Therefore, it is possible that multiple monitoring modalities may lead to differing conclusions regarding the condition of the brain. Recognizing the inherent limitations of each monitoring device and when the assumptions are violated is paramount in order to minimize the potential misinterpretation of the data. For instance, cerebral autoregulation is evaluated by examining the slow ICP changes (a proxy for CBF) in response to systemic blood pressure fluctuations, expressed as the pressure reactivity index. Although the magnitude of the blood pressure changes is important, the rate at which it occurs also influences the brain’s ability to compensate to maintain appropriate CBF [67]. Therefore, administration of vasoactive medications represent an exogenous factor that may interfere with the physiological correlation between CBF and MAP, a potential violation of the basic assumption for the derivation of cerebral autoregulation curves through the pressure reactivity index [68].
Additionally, whether proposed physiologic targets using multimodal neuromonitoring in various disease processes are indeed the optimal targets remains unclear. At the same time, indices that are derived from different monitoring devices may differ from each other, as exemplified by the lack of agreement on the derived optimal MAP and upper and lower limits of cerebral autoregulation values between the pressure reactivity index and the cerebral oximetry index methods in adults with hypoxic-ischemic encephalopathy [69]. Similarly, the pressure reactivity index and cerebral oximetry index are also poorly correlated in children with TBI [17]. Together, these studies highlight the difficulty of deciphering data and determining their validity from multimodal neuromonitoring. However, combining multiple monitoring modalities may leverage the strength of each modality while mitigating the inherent limitations. For instance, cerebral blood flow velocity estimation by TCD varies based on the angle of insonation, possibly resulting in intraoperator and interoperator variability. The Doppler-derived flow velocity provides limited insight regarding the actual and the adequacy of CBF. These limitations may be addressed by NIRS with operator-independent regional cerebral oxygenation measurements based on which the adequacy of CBF is inferred. Conversely, TCD can potentially mitigate the technical challenges of NIRS infrared beam due to the thickness of the skull and scalp edema and the inherent spatial resolution limitation by providing direct Doppler measurement of each major cerebral vessel.
When interpreting data collected in the ICU, clinicians rely on additional contextual information, such as treatments or medications, ongoing interventions, ventilator settings, laboratory values, and nursing reports, which may be located in different electronic health databases with varying difficulty to access and correlate temporally with other data. Therefore, identifying the data sources and seamlessly integrating them with the data from the multimodal system remain a significant challenge [65]. Moreover, it is important to recognize that simply connecting neuromonitoring devices to patients is unlikely to result in improved outcome. It is the manner in which the data are collected, analyzed, and interpreted and in which change is implemented at both the individual patient level and the programmatic level where the ultimate benefits are likely to be seen.
Finally, data supporting the positive influence of multimodal neuromonitoring on patient outcome, as well as the optimal target values in pediatrics, are limited. However, a recent single-center retrospective analysis of a prospectively implemented standardized multimodal neuromonitoring program did identify an association between the use of multimodal neuromonitoring in children with TBI and decreased duration of mechanical ventilation and ICP monitoring [7]. More study is needed to better characterize the effect of multimodal neuromonitoring in children.
Despite these challenges, the potential benefits can be immense. Collection of large-volume high-resolution waveform data, combined with computer analytics, has the potential to allow for identification of new patterns and connections otherwise not recognizable to the clinicians [65]. This can potentially lead to better individualized care at the bedside and better evaluation of response to treatment for each patient. More importantly for the field as a whole, it may lead to a better understanding of the pathophysiology of various brain injuries, which may inform future research on a greater scale. Standardization of neurocritical care informatics and the rapidly growing field of big data and predictive analytics for both adult and pediatric patients have the potential to advance the field greatly in a relatively short period of time. As more innovative technology is incorporated at the bedside, the potential to revolutionize care will become even greater, and the importance of integrating data in a meaningful way will become even more necessary. Already, quantitative EEG, three-dimensional color plots illustrating time and magnitude of ICP elevations, and three-dimensional computational models of CSF dynamics are elevating the approach to understanding the physiologic state of the brain for patients with neurologic conditions [70,71,72]. These and other novel approaches to data visualization and identification of important signals and trends in the critically ill brain are likely to provide important clues necessary to transform therapeutic options and positively influence patient outcomes.
Building Multimodal Neuromonitoring Programs in Pediatrics
Few pediatric institutions have published their experiences on the design and implementation of a pediatric neuromonitoring program. Appavu et al. described their experience of implementing a multimodal neuromonitoring program in pediatric TBI at a pediatric hospital [7]. Physiologic recordings were reviewed at the bedside and remotely. With the implementation of remote review, additional monitoring capabilities included cerebral autoregulation based on the pressure reactivity and pulse amplitude indices and calculation of brain compliance. Following review of the data, a multidisciplinary team discussed physiologic targets and goals of care for the day. A formal daily report for multimodal neuromonitoring was placed in the chart outlining details of monitoring to assist targeted individualized care.
This study highlights several factors to consider for the implementation of a multimodal neuromonitoring program (Table 3). A clearly defined criterion will facilitate the implementation on the appropriate patient cohort. Integrating information from multimodal neuromonitoring into clinical practice with respect to the frequency of data review, criteria for intervention, and assessment of the therapeutic response requires careful deliberations and discussions among the stakeholders because it could lead to a paradigm shift in the overall management strategy. Revisions of the guidelines and protocols to include measurements and derived values, such as optimal MAP, from multimodal neuromonitoring will provide guidance to the providers, as exemplified by the recent incorporation of a treatment algorithm for brain tissue hypoxia by PbtO2 in the pediatric TBI guideline [9]. It is also essential to establish an internal performance review on the impact of multimodal neuromonitoring on the therapeutic intensity and outcome because there is a paucity of published data.
Ideally, the implementation of a pediatric multimodal neuromonitoring program includes the development of a comprehensive multidisciplinary interprofessional team with expertise in neurocritical care. Experience and competence in the use of each specific neuromonitoring modality as well as in the interpretation, integration, and application of the acquired data is required. Creating this community of practice surrounding the neuromonitoring program supports a more standardized approach, allowing for ongoing improvement initiatives as experience is gained. Developing an educational program for members of the team caring for patients undergoing multimodal neuromonitoring is critical and allows for a broader reach of expertise throughout each institution [6, 30].
How multimodal neuromonitoring is used in clinical practice and the interoperability of the devices and systems will greatly influence the cost and infrastructure considerations and vice versa. Therefore, significant efforts should be devoted to specifying the requisite physiological parameters to monitor and the equipment needed for each neurological condition. A thorough familiarity with the capability, and perhaps more importantly the limitation, of different multimodal systems should guide the decision on a suitable platform. Because of the rapid advances in medical technology that may quickly render the devices and platforms obsolete, long-term functionality and adaptability need to be considered.
Conclusions
Multimodal neuromonitoring provides innovative approaches to investigate the physiology and pathophysiology of the brain at the bedside and holds great promise to transform the management of children with critical neurological conditions. It must be noted, however, that multimodal neuromonitoring alone is unlikely to substantially improve outcomes for patients with critical neurologic illness. It must be incorporated into a model of care with strong focus on neurocritical care fundamentals, including standardized physical neurologic examinations, proper patient positioning, and education of all providers caring for neurocritical care patients at the bedside [73]. Comprehensive programs to support the implementation of multimodal neuromonitoring with multidisciplinary involvement must be developed alongside the employment of the technology to realize its greatest potential.
References
LaRovere KL, Riviello JJ. Emerging subspecialties in neurology: building a career and a field: pediatric neurocritical care. Neurology. 2008;70(22):e89-91.
Bell MJ, Carpenter J, Au AK, et al. Development of a pediatric neurocritical care service. Neurocrit Care. 2009;10(1):4–10.
Pineda JA, Leonard JR, Mazotas IG, et al. Effect of implementation of a paediatric neurocritical care programme on outcomes after severe traumatic brain injury: a retrospective cohort study. Lancet Neurol. 2013;12(1):45–52.
Wainwright MS, Grimason M, Goldstein J, et al. Building a pediatric neurocritical care program: a multidisciplinary approach to clinical practice and education from the intensive care unit to the outpatient clinic. Semin Pediatr Neurol. 2014;21(4):248–54.
LaRovere KL, Murphy SA, Horak R, et al. Pediatric neurocritical care: evolution of a new clinical service in PICUs across the United States. Pediatr Crit Care Med. 2018;19(11):1039–45.
Erklauer JC, Thammasitboon S, Shekerdemian LS, Riviello JJ, Lai Y-C. Creating a robust community of practice as a foundation for the successful development of a pediatric neurocritical care program. Pediatr Neurol. 2022;136:1–7.
Appavu B, Burrows BT, Nickoles T, et al. Implementation of multimodality neurologic monitoring reporting in pediatric traumatic brain injury management. Neurocrit Care. 2021;35(1):3–15.
Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the brain trauma foundation guidelines. Exec Summ Pediatr Crit Care Med. 2019;20(3):280–9.
Kochanek PM, Tasker RC, Bell MJ, et al. Management of pediatric severe traumatic brain injury: 2019 consensus and guidelines-based algorithm for first and second tier therapies. Pediatr Crit Care Med. 2019;20(3):269–79.
Topjian AA, de Caen A, Wainwright MS, et al. Pediatric post-cardiac arrest care: a scientific statement from the American Heart Association. Circulation. 2019;140(6):e194-233.
Topjian AA, Raymond TT, Atkins D, et al. Part 4: pediatric basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S469-523.
Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51-96.
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41(1):11–7 (discussion 17–19).
Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–8.
Brady KM, Shaffner DH, Lee JK, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124(6):e1205-1212.
Lewis PM, Czosnyka M, Carter BG, et al. Cerebrovascular pressure reactivity in children with traumatic brain injury. Pediatr Crit Care Med. 2015;16(8):739–49.
Abecasis F, Dias C, Zakrzewska A, Oliveira V, Czosnyka M. Monitoring cerebrovascular reactivity in pediatric traumatic brain injury: comparison of three methods. Childs Nerv Syst. 2021;37(10):3057–65.
Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: relationship with outcome. Childs Nerv Syst. 2009;25(10):1325–33.
Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 2: relationship with clinical, physiological, and treatment factors. Childs Nerv Syst. 2009;25(10):1335–43.
Tolias C, Richards D, Bowery N, Sgouros S. Investigation of extracellular amino acid release in children with severe head injury using microdialysis. A pilot study. Acta Neurochir Suppl. 2002;81:377–9.
Richards DA, Tolias CM, Sgouros S, Bowery NG. Extracellular glutamine to glutamate ratio may predict outcome in the injured brain: a clinical microdialysis study in children. Pharmacol Res. 2003;48(1):101–9.
Thango NS, Rohlwink UK, Dlamini L, et al. Brain interstitial glycerol correlates with evolving brain injury in paediatric traumatic brain injury. Childs Nerv Syst. 2021;37(5):1713–21.
Wellard J, Kuwabara M, Adelson PD, Appavu B. Physiologic characteristics of hyperosmolar therapy after pediatric traumatic brain injury. Front Neurol. 2021;12:662089.
Appavu B, Burrows BT, Foldes S, Adelson PD. Approaches to multimodality monitoring in pediatric traumatic brain injury. Front Neurol. 2019;10:1261.
Young AMH, Guilfoyle MR, Donnelly J, et al. Multimodality neuromonitoring in severe pediatric traumatic brain injury. Pediatr Res. 2018;83(1–1):41–9.
Kirschen MP, LaRovere K, Balakrishnan B, et al. A survey of neuromonitoring practices in North American pediatric intensive care units. Pediatr Neurol. 2022;126:125–30.
Laws JC, Jordan LC, Pagano LM, Wellons JC, Wolf MS. Multimodal neurologic monitoring in children with acute brain injury. Pediatr Neurol. 2022;129:62–71.
Monitoring MNC. Conceptual approach and indications. J Neurocrit Care. 2008;1(2):117–27.
Said AS, Guilliams KP, Bembea MM. Neurological monitoring and complications of pediatric extracorporeal membrane oxygenation support. Pediatr Neurol. 2020;108:31–9.
Riviello JJ, Erklauer J. Neurocritical care and brain monitoring. Neurol Clin. 2021;39(3):847–66.
Morris G, Gardner R. Computer Applications. In: Principles of critical care. New York: McGraw-Hill; 1992. pp. 500–14.
Sinha S, Hudgins E, Schuster J, Balu R. Unraveling the complexities of invasive multimodality neuromonitoring. Neurosurg Focus. 2017;43(5):E4.
Hemphill JC, Andrews P, De Georgia M. Multimodal monitoring and neurocritical care bioinformatics. Nat Rev Neurol. 2011;7(8):451–60.
Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S1-26.
Appavu B, Foldes S, Burrows BT, et al. Multimodal assessment of cerebral autoregulation and autonomic function after pediatric cerebral arteriovenous malformation rupture. Neurocrit Care. 2021;34(2):537–46.
Lee JK, Brady KM, Chung S-E, et al. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation. 2014;85(10):1387–93.
Topjian AA, Zhang B, Xiao R, et al. Multimodal monitoring including early EEG improves stratification of brain injury severity after pediatric cardiac arrest. Resuscitation. 2021;167:282–8.
Young AMH, Donnelly J, Czosnyka M, et al. Continuous multimodality monitoring in children after traumatic brain injury-preliminary experience. PLoS ONE. 2016;11(3):e0148817.
Steinmeier R, Bauhuf C, Hübner U, et al. Slow rhythmic oscillations of blood pressure, intracranial pressure, microcirculation, and cerebral oxygenation. Dynamic interrelation and time course in humans. Stroke. 1996;27(12):2236–43.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830–6.
Kirschen MP, Majmudar T, Beaulieu F, et al. Deviations from NIRS-derived optimal blood pressure are associated with worse outcomes after pediatric cardiac arrest. Resuscitation. 2021;168:110–8.
Appavu B, Temkit MH, Foldes S, et al. Association of outcomes with model-based indices of cerebral autoregulation after pediatric traumatic brain injury. Neurocrit Care. 2021;35(3):640–50.
Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87–95.
Moftakhar P, Cooke DL, Fullerton HJ, et al. Extent of collateralization predicting symptomatic cerebral vasospasm among pediatric patients: correlations among angiography, transcranial Doppler ultrasonography, and clinical findings. J Neurosurg Pediatr. 2015;15(3):282–90.
Song Y, Qian SY, Li Y, et al. Effectiveness and safety of nimodipine in preventing cerebral vasospasm after subarachnoid hemorrhage in children. Zhonghua Er Ke Za Zhi. 2019;57(5):338–43.
Hunt A, Tasker RC, Deep A. Neurocritical care monitoring of encephalopathic children with acute liver failure: a systematic review. Pediatr Transpl. 2019;23(7):e13556.
Hussain E, Grimason M, Goldstein J, et al. EEG abnormalities are associated with increased risk of transplant or poor outcome in children with acute liver failure. J Pediatr Gastroenterol Nutr. 2014;58(4):449–56.
Press CA, Morgan L, Mills M, et al. Spectral electroencephalogram analysis for the evaluation of encephalopathy grade in children with acute liver failure. Pediatr Crit Care Med. 2017;18(1):64–72.
Tas J, Beqiri E, van Kaam RC, et al. Targeting autoregulation-guided cerebral perfusion pressure after traumatic brain injury (COGiTATE): a feasibility randomized controlled clinical trial. J Neurotrauma. 2021;38(20):2790–800.
Appavu B, Riviello JJ. Electroencephalographic patterns in neurocritical care: pathologic contributors or epiphenomena? Neurocrit Care. 2018;29(1):9–19.
Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32(2):96–108.
Scheuer ML, Wilson SB. Data analysis for continuous EEG monitoring in the ICU: seeing the forest and the trees. J Clin Neurophysiol. 2004;21(5):353–78.
Appavu BL, Temkit MH, Hanalioglu D, Burrows BT, Adelson PD. Quantitative electroencephalographic changes associated with brain tissue hypoxia after pediatric traumatic brain injury: a retrospective exploratory analysis. J Clin Neurophysiol. 2023.
Yuliati A, Federman M, Rao LM, Chen L, Sim MS, Matsumoto JH. Prevalence of seizures and risk factors for mortality in a continuous cohort of pediatric extracorporeal membrane oxygenation patients. Pediatr Crit Care Med. 2020;21(11):949–58.
Okochi S, Shakoor A, Barton S, et al. Prevalence of seizures in pediatric extracorporeal membrane oxygenation patients as measured by continuous electroencephalography. Pediatr Crit Care Med. 2018;19(12):1162–7.
Pinto VL, Pruthi S, Westrick AC, Shannon CN, Bridges BC, Le TM. Brain magnetic resonance imaging findings in pediatric patients post extracorporeal membrane oxygenation. ASAIO J. 2017;63(6):810–4.
Caturegli G, Cho S-M, White B, Chen LL. Acute brain injury in infant venoarterial extracorporeal membrane oxygenation: an autopsy study. Pediatr Crit Care Med. 2021;22(3):297–302.
Zamora CA, Oshmyansky A, Bembea M, et al. Resistive index variability in anterior cerebral artery measurements during daily transcranial duplex sonography: a predictor of cerebrovascular complications in infants undergoing extracorporeal membrane oxygenation? J Ultrasound Med. 2016;35(11):2459–65.
O’Brien NF, Hall MW. Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Pediatr Crit Care Med. 2013;14(3):e126-134.
Tsou P-Y, Garcia AV, Yiu A, Vaidya DM, Bembea MM. Association of cerebral oximetry with outcomes after extracorporeal membrane oxygenation. Neurocrit Care. 2020;33(2):429–37.
Finucane E, Jooste E, Machovec KA. Neuromonitoring modalities in pediatric cardiac anesthesia: a review of the literature. J Cardiothorac Vasc Anesth. 2020;34(12):3420–8.
Jalali A, Simpao AF, Gálvez JA, Licht DJ, Nataraj C. Prediction of periventricular leukomalacia in neonates after cardiac surgery using machine learning algorithms. J Med Syst. 2018;42(10):177.
Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150(1):169–78 (discussion 178–180).
Battaglini D, Pelosi P, Robba C. The importance of neuromonitoring in non brain injured patients. Crit Care. 2022;26(1):78.
Foreman B, Lissak IA, Kamireddi N, Moberg D, Rosenthal ES. Challenges and opportunities in multimodal monitoring and data analytics in traumatic brain injury. Curr Neurol Neurosci Rep. 2021;21(3):6.
Gowda V, Schulzrinne H, Miller BJ. The case for medical device interoperability. JAMA Health Forum. 2022;3(1):e214313–e214313.
Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487–559.
Weersink CSA, Aries MJH, Dias C, et al. Clinical and physiological events that contribute to the success rate of finding “optimal” cerebral perfusion pressure in severe brain trauma patients. Crit Care Med. 2015;43(9):1952–63.
Hoiland RL, Sekhon MS, Cardim D, et al. Lack of agreement between optimal mean arterial pressure determination using pressure reactivity index versus cerebral oximetry index in hypoxic ischemic brain injury after cardiac arrest. Resuscitation. 2020;152:184–91.
Güiza F, Depreitere B, Piper I, et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015;41(6):1067–76.
Weisenberg SH, TerMaath SC, Barbier CN, Hill JC, Killeffer JA. A computational fluid dynamics simulation framework for ventricular catheter design optimization. J Neurosurg. 2018;129(4):1067–77.
Vandenbulcke S, De Pauw T, Dewaele F, Degroote J, Segers P. Computational fluid dynamics model to predict the dynamical behavior of the cerebrospinal fluid through implementation of physiological boundary conditions. Front Bioeng Biotechnol. 2022;10:1040517.
Patel A, Bell MJ. Pediatric neurocritical care and neuromonitoring in 2018-maybe we need to go back to the basics? Pediatr Crit Care Med. 2018;19(4):379–80.
Funding
None.
Author information
Authors and Affiliations
Contributions
Both authors contributed substantially to the conception and design of the manuscript. Both authors contributed to the drafting and revising of the manuscript. The final manuscript was approved by both authors.
Corresponding author
Ethics declarations
Conflict of interest
Jennifer Erklauer is the co-chair for the Subcommittee on Educational Products of the Pediatric Neurocritical Care Research Group Education Committee. Jennifer Erklauer received support to attend the P-ICECAP investigators orientation meeting and training sessions. Yi-Chen Lai has no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erklauer, J.C., Lai, YC. The State of the Field of Pediatric Multimodality Neuromonitoring. Neurocrit Care 40, 1160–1170 (2024). https://doi.org/10.1007/s12028-023-01858-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01858-3