Abstract
Background
Management after cerebral arteriovenous malformation (AVM) rupture aims toward preventing hemorrhagic expansion while maintaining cerebral perfusion to avoid secondary injury. We investigated associations of model-based indices of cerebral autoregulation (CA) and autonomic function (AF) with outcomes after pediatric cerebral AVM rupture.
Methods
Multimodal neurologic monitoring data from the initial 3 days after cerebral AVM rupture were retrospectively analyzed in children (< 18 years). AF indices included standard deviation of heart rate (HRsd), root-mean-square of successive differences in heart rate (HRrmssd), low–high frequency ratio (LHF), and baroreflex sensitivity (BRS). CA indices include pressure reactivity index (PRx), wavelet pressure reactivity indices (wPRx and wPRx-thr), pulse amplitude index (PAx), and correlation coefficient between intracranial pressure pulse amplitude and cerebral perfusion pressure (RAC). Percent time of cerebral perfusion pressure (CPP) below lower limits of autoregulation (LLA) was also computed for each CA index. Primary outcomes were determined using Pediatric Glasgow Outcome Score Extended-Pediatrics (GOSE-PEDs) at 12 months and acquired epilepsy. Association of biomarkers with outcomes was investigated using linear regression, Wilcoxon signed-rank, or Chi-square.
Results
Fourteen children were analyzed. Lower AF indices were associated with poor outcomes (BRS [p = 0.04], HRsd [p = 0.04], and HRrmssd [p = 0.00]; and acquired epilepsy (LHF [p = 0.027]). Higher CA indices were associated with poor outcomes (PRx [p = 0.00], wPRx [p = 0.00], and wPRx-thr [p = 0.01]), and acquired epilepsy (PRx [p = 0.02] and wPRx [p = 0.00]). Increased time below LLA was associated with poor outcome (percent time below LLA based on PRx [p = 0.00], PAx [p = 0.04], wPRx-thr [p = 0.03], and RAC [p = 0.01]; and acquired epilepsy (PRx [p = 0.00], PAx [p = 0.00], wPRx-thr [p = 0.03], and RAC [p = 0.01]).
Conclusions
After pediatric cerebral AVM rupture, poor outcomes are associated with AF and CA when applying various neurophysiologic model-based indices. Prospective work is needed to assess these indices of CA and AF in clinical decision support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral arteriovenous malformation (AVM) rupture is a common cause of intracerebral hemorrhage (ICH) in children, accounting for up to 50% of cases [1,2,3]. Given the complexity of this condition, intensive care unit management is needed in the immediate aftermath of ICH. Initial interventional treatments comprise combinations of endovascular embolization, microsurgical removal, and/or radiotherapy [4]. This initial phase of treatment is challenged by the need to prevent ICH expansion while maintaining adequate cerebral perfusion to avoid secondary brain injury.
An essential element to management after all acute brain injury (ABI), including ICH, is the maintenance of hemodynamic stability in an environment of altered cerebral autoregulation (CA). CA represents the ability of the cerebrovascular system to maintain cerebral blood flow (CBF) over varied cerebral perfusion pressures (CPP) [5, 6] and can be altered after ABI. Evidence suggests that continuous bedside monitoring of CA status following severe traumatic brain injury (TBI) can help establish optimal, individualized treatment strategies, including identification of an optimal CPP value (CPPopt) to provide clinicians with lower and upper limit targets for CPP management [7,8,9,10,11]. Continuous CPP maintenance within the range of CA may help lessen the impact of detrimental secondary insults arising from CA impairments [8]. In addition to deranged CA, autonomic impairment has been associated with mortality after ABI and physiologic indices of autonomic function (AF) may provide pathophysiologic information to guide clinical decision support [12].
Complex physiologic processes cannot be encapsulated or completely understood within a single measure of cerebral physiology. A better understanding of complex neurophysiologic processes that are occurring in injured brain could potentially lead to novel therapeutic strategies to optimize brain homeostasis. In this study, we sought to investigate the association of patient characteristics and multiple model-based indices of CA and AF with functional outcomes and acquired epilepsy after pediatric cerebral AVM rupture. We hypothesize that poor AF and poor CA are associated poor functional outcome and acquired epilepsy.
Methods
This was a retrospective study from a prospectively collected clinical database. This study was conducted at Phoenix Children’s Hospital (Phoenix Arizona, USA) and was approved by the Institutional Review Board (No: 17-899).
Children (< 18 years of age) with ruptured cerebral AVM at a single pediatric intensive care unit who underwent continuous multimodality neurologic monitoring were retrospectively analyzed from September 2014 to January 2020. Patients were managed according to institutional standard of care, which consisted of invasive arterial blood pressure (ABP) monitoring and intracranial pressure (ICP) monitoring with an external ventricular drain (EVD) and/or intraparenchymal probe. The decision to place an intraparenchymal probe for continuous ICP monitoring in addition to an EVD was made on the basis of the risk/benefit ratio by the neurosurgeon on call. Upper and lower limits of systolic blood pressure and cerebral perfusion pressure were set daily by a multidisciplinary team consisting of an intensivist, a critical care neurologist and a neurosurgeon. All patients who presented or evolved toward a Glasgow Coma Scale (GCS) less than 8 were intubated.
The primary aim of this study was to investigate the association of model-based indices of CA and AF (Table 1) with clinical outcomes after pediatric AVM rupture. Primary outcomes measures were global functional outcome as measured by the Glasgow Outcome Score Extended-Pediatrics (GOSE-PEDs) at 12 months after injury [13] and the development of acquired epilepsy. GOSE-PEDs scores range from 1 to 8 with higher values representing worsened outcome. Acquired epilepsy was assessed by diagnosis made by a board-certified epileptologist on outpatient follow-up not earlier than 1 month after injury on the basis of a combination of recurring unprovoked seizures and/or epileptiform electroencephalographic studies. We also examined hospital length of stay (LOS) as a secondary outcome measure. The associations of outcomes with patient characteristics (age, gender, hemorrhage, location, anatomical angiographic evidence of cerebrovascular vasospasm, Spetzler-Martin grade [14]) and physiologic vital signs (heart rate [HR], ICP, CPP, ABP, respiratory rate [RR]) were also explored to evaluate other factors related to outcomes.
Physiologic Data
Patients underwent neurologic and hemodynamic monitoring that included ICP (obtained from either external ventricular drain [EVD] and/or intraparenchymal probe], invasive ABP, and electrocardiogram [EKG]). Continuous physiologic data from all monitoring devices were collected and synchronized using a multimodal monitoring device (CNS200; Moberg ICU Solutions, Philadelphia, PA). CPP was calculated as the intraparenchymal ICP subtracted from mean ABP. Calculation of CPP was limited to patients undergoing intraparenchymal ICP monitoring. ICM + software (Cambridge, UK) was used to visualize and process all multimodality monitoring data, and to calculate model-based indices of CA and AF (Table 1). Data with substantial artifact observed through visual analysis were removed.
A variety of CA indices were explored, including the pressure reactivity index [PRx] [10], pulse amplitude index [PAx] (t) [15], wavelet PRx [wPRx] [16], threshold-based wPRx [wPRx-thr], and correlation coefficient between CPP and ICP pulse amplitude (RAC) [17]. A higher value for these indices signified impaired CA. PRx was calculated as a moving Pearson correlation coefficient between ABP and ICP within a 10-min averaging window [10]. PAx was calculated similarly as a moving correlation coefficient between ABP and ICP pulse amplitude [15]. RAC was calculated similarly as a moving correlation coefficient between ICP pulse amplitude and CPP [17]. wPRx was calculated by taking the cosine of the wavelet transform phase shift between ABP and ICP [16]. wPRx-thr was calculated similarly to wPRx using a wavelet coherence threshold of 0.46, assuming that data points with coherence below 0.46 may relate to noise not related to CA.
These CA indices are continuous variables representing the underlying state of CA, and while they offer risk stratification, their immediate values are not clinically actionable. We therefore evaluated the percentage of time patients were below the lower limit of autoregulation (LLA) calculated from these indices. Optimal CPP (CPPOpt) values were identified using previously described methods [18], by plotting the CPP values versus values for all CA indices and identifying the minimum CPP value on a U-shaped curve fit to the data. CPPopt values were calculated every 1 min from CPP and each CA index value taken from the moving 4-h monitoring window (Fig. 1). CPPopt values were rejected in cases of an unreliable U-shaped fitting and corrected by using the last reliable U-shaped fitting and the last reliable CPPopt value. LLA was determined for each CA index as the CPP value at which the curve crossed a threshold value for impaired pressure reactivity (PRx, PAx, wPRx, wPRx-thr = 0.30; RAC = − 0.15) [8,9,10,11, 15,16,17,18,19]. The percentage of time below LLA for each index of CA was calculated for each day. As with the CA indices, a higher value signified impaired CA.
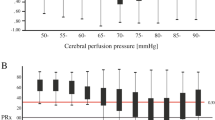
Optimal CPP Curves observed over a 4-hour window based on PRx (a), PAx (b), RAC (c), wPRx (d) and wPRx-thr (e) for a 7-year-old girl with a cerebral AVM rupture fed from the left callosomarginal artery. Distribution of CPP is also shown (f). CPP cerebral perfusion pressure, CPPopt optimal cerebral perfusion pressure, LLA lower limit of autoregulation, mmHg millimeters of mercury, PRx pressure reactivity index, PAx pulse amplitude index, RAC correlation coefficient between intracranial pressure pulse amplitude and cerebral perfusion pressure, Std Dev standard deviation, ULA upper limit of autoregulation, wPRx wavelet pressure reactivity index, wPRx-thr threshold-based wavelet pressure reactivity index
Indices of AF included baroreflex sensitivity (BRs) and three measures of heart rate variability (HRV): standard deviation of heart rate (HRsd), root-mean-square of successive differences in heart rate (HRrmssd), and heart rate low–high frequency ratio (LHF). A lower value for these indices signifies impaired autonomic function. HRV variables were computed from time- and frequency-domain analyses according to international guidelines [20]. For all HRV variables, a 30-s time series of R–R intervals was assessed from EKG that was updated every 10 s. HRsd and HRrmssd were computed from the time-domain signal. LHF was computed in the frequency domain, using Lomb–Scargle periodogram to calculate the spectral power of the RR time series in the low frequency range (0.04–0.15 Hz) and the high frequency range (0.15–0.4 Hz). BRs was calculated with a modification of the sequential cross-correlation method as previously described [21, 22]. The applied function used ABP systolic peaks to create RR interval time series by using an automated detection algorithm. The slope of the linear regression between 10-s series of RR intervals and the corresponding 10-s series of systolic blood pressure was calculated. A cross-correlation function was used to determine the maximum correlation coefficient and remove the influence of unknown time delay of the baroreceptor response. The RR window was shifted against the systolic pressure window in a stepwise manner. To ensure that correlation calculations were always performed on the same number of data points irrespective of the lag applied to the RR series, the actual data buffer was extended with each window shift. A valid BRS value was returned only if the correlation coefficient was significant at a p value less than 0.01 and if no irregular beats (ectopics) were detected by the ICM + software. To compensate for the influence of uncorrelated noise, the slope returned was adjusted to the correlation coefficient. BRS was updated every 10 s and expressed in milliseconds per millimeter of mercury (ms/mmHg).
Statistics
Descriptive data are presented as median, range and interquartile range (IQR). The relationship between quantitative outcome variables to physiologic quantitative predictor variables was assessed using linear regression, with physiologic values from the first 3 days of monitoring. All physiologic measures were assessed independently in an exploratory manner. Regression fits with p < 0.05 were considered significant. Physiologic indices investigated included median values across each day of PRx, PAx, RAC, wPRx, wPRx-thr, BRS, HRrmssd, HRsd, LHF, and the percentage of time with CPP below LLA based on PRx, PAx, RAC, wPRx and wPRx-thr. Vital signs investigated include median values across each day of ICP, HR, ABP, CPP, and RR.
We also investigated relationships of patient age, gender, hemorrhage location (middle or anterior cranial fossa vs. posterior fossa), Spetzler-Martin grade, and the presence of vasospasms) to 12-month GOSE-PEDs scores, acquired epilepsy and LOS (days) using either the Wilcoxon-ranked sum test, McNemar Chi-Square test with Yates’ correction or point biserial correlation, as appropriate depending on the variable type. Statistical analyses were performed using MATLAB or R Studio version 3.4.1.
Results
Population
Fourteen children were included in the study, treated between 2014 and 2020. Data are summarized in Table 2. Eight of the 14 (57.1%) patients underwent intraparenchymal pressure monitoring in addition to EVD monitoring and were induced in the analysis of CA indices. EKG was collected for all children allowing for calculation of AF indices. The median age of the patients was 11 years (range 5–14; IQR 1.5), eight (57.1%) were male, median Glasgow Coma Score upon presentation was 5 (range 3–14; IQR 4), median Spetzler-Martin grade was 2 (range 1–5; IQR 1), and median GOSE-PEDs at 12 months was 3.5 (range 2–8; IQR 2). Median length of monitoring was 260.8 h (range 49.3–487.8; IQR 155.4). Median percent artifact removed was 17.9% (range 0.1–77.0; IQR 17.9). All monitoring periods between artifacts removed were continuous and at least 2 h in duration. Seven patients (50%) experienced AVM rupture within the middle or anterior cranial fossa, and seven (50%) experienced rupture within the posterior cranial fossa. Three (21.4%) experienced cerebrovascular vasospasms after AVM rupture. Two patients died (17%). Two of the 12 children alive at 12 months were identified to have developed epilepsy (17%).
Association of Patient Characteristics with Clinical Outcomes
The relationship of outcomes (12-month GOSE-PEDs scores, acquired epilepsy, and LOS) with patient characteristics (gender, age, hemorrhage location, Spetzler-Martin grade, and occurrence of vasospasms) is summarized in Table 3. Hemorrhage in the posterior fossa was associated with worse outcome of global function (p = 0.03). Higher Spetzler-Martin grade was associated with increased length of hospitalization (p = 0.02). No association was found between epilepsy with gender, hemorrhage location, vasospasm, age, or Spetzler-Martin grade.
Association of Vital Signs with Clinical Outcomes and Patient Characteristics
The relationship of vital signs with outcomes is summarized in Table 4. Lower CPP was associated with worse global functional outcome based on GOSE-PEDs scores (R = − 0.62; p = 0.00), acquired epilepsy (R = − 0.50; p = 0.02), and increased LOS (R = − 0.56; p = 0.00). Higher ICP was associated with worse global functional outcome (GOSE-PEDs scores [R = 0.47; p = 0.01]). Lower HR was associated with acquired epilepsy (R = − 0.42; p = 0.01).
Association of Physiologic Indices of AF and CA with Clinical Outcomes
The relationship of physiologic indices with clinical outcomes is summarized in Table 5. Lower autonomic function indices were significantly related to worse outcomes [12-month GOSE-PEDs scores vs. BRS (R = − 0.33; p = 0.04), HRsd (R = − 0.37; p = 0.02), and HRrmssd (R = − 0.46; p = 0.00); the development of epilepsy vs. LHF (R = − 0.49; p = 0.00); and LOS vs. HRsd (R = − 0.32; p = 0.05)]. Higher CA indices were also significantly related to worse outcomes [12-month GOSE-PEDs scores vs. PRx (R = 0.70; p = 0.00), wPRx (R = 0.67; p = 0.00), wPRx-thr (R = 0.47; p = 0.01), and percentage of time below the LLA based on PRx (R = 0.57; p = 0.00), PAx (R = 0.40; p = 0.04), wPRx-thr (R = 0.42; p = 0.03), and RAC (R = 0.47; p = 0.01); and the development of epilepsy vs. PRx (R = 0.47; p = 0.02) and wPRx (R = 0.46; p = 0.03), and percentage of time below the LLA based on PRx (R = 0.59; p = 0.00), PAx (R = 0.59; p = 0.00), wPRx-thr (R = 0.49; p = 0.02), and RAC (R = 0.52; p = 0.01)].
Discussion
We explored the relationships between outcomes with patient characteristics, vital signs, and indices of AF and CA in children with cerebral AVM rupture. Worse outcomes were associated with cerebral AVM rupture within the posterior fossa, depressed AF, impaired CA, and depressed hemodynamics. Our study is the first to explore model-based indices of CA and AF after cerebral AVM rupture.
Our findings are consistent with emerging literature for multimodal physiologic monitoring of CA after ABI. PRx, the most well described index of CA, was significantly associated with GOSE-PEDs and acquired epilepsy. The relationship of PRx with outcomes has been described previously after TBI [8,9,10,11], but not after pediatric hemorrhagic stroke. A limitation of PRx is that it assumes that ICP changes are secondary to changes in intracranial arterial blood volume, which may not apply when changes are related to impaired CSF outflow or cerebral edema. To address this, variant ICP-derived CA indices have emerged including PAx, RAC, and wPRx, each also displaying critical thresholds associated with global functional outcome [15,16,17]. These indices utilize ICP waveform changes or waveform coherence characteristics to inspect for fluctuations in intracranial arterial blood volume. Unsurprisingly, we found some of these variant ICP-derived CA indices were significantly related to outcomes (wPRx and wPRx-thr) after pediatric cerebral AVM rupture. However, not all variant ICP-derived CA indices were significantly related to outcomes (PAx or RAC). In our cohort, PRx and wPRx had the highest correlation with outcomes. The reasons why PRx, wPRx, and wPRx-thr were associated with outcomes, but not PAx or RAC are unclear. Both RAC and PAx harness ICP pulse amplitude changes instead of ICP itself and may provide better insight in situations of high cerebrovascular compliance, which may not be readily present in the setting of significant intracranial vascular malformations. The pulse transmission of ABP to the ICP waveform may be modified by intracranial vascular malformation presence, and thus the relationship of these specific indices with CA may differ for these patients.
ABI management guided by the LLA has garnered increased interest given that optimal CA can be determined from assessing the U-shaped relationship of CPP and CA indices over time. Deviations from CPPopt have been associated with worse outcomes in a variety of retrospective studies [9,10,11, 18, 19, 23,24,25,26,27,28,29,30,31,32]. Prospectively, a randomized control trial demonstrated that postoperative delirium was decreased during cardiopulmonary bypass when CPPopt was maintained by managing MAP using the transcranial Doppler ultrasound-based mean velocity index [33]. We found that after pediatric cerebral AVM rupture, increased time of CPP below LLA was associated with worsened outcomes (GOSE-PEDs and acquired epilepsy). This relationship was true when using any CA index to determine LLA, except wPRx. Time below LLA based on PRx had the strongest association with outcomes, similar to the assessment of PRx directly. Interestingly, time below LLA based on wPRx was not significantly associated with outcome, though wPRx directly was. Also interesting was that time below LLA based on PAx and RAC was significantly associated with outcomes, though not their direct values. Specific CA indices themselves may offer risk stratification after cerebral AVM rupture, but do not provide immediate clinically actionable information. Time below LLA offers a potentially modifiable biomarker, raising the possibility that maintaining CPP above LLA derived from certain CA indices may improve outcomes. A prospective study evaluating the feasibility, safety, and physiologic implications of CPPopt-guided management is underway in adult severe TBI patients (CPPopt Guided Therapy: Assessment of Target Effectiveness [COGiTATE]]; clinicaltrials.gov identifier NCT02982122) [34]. Similar studies are needed after cerebral AVM rupture. The reason why time below LLA based on wPRx was not associated with outcomes is unclear. A higher threshold for wavelet coherence between ABP and ICP may be needed, as demonstrated by the significance of wPRx-thr to GOSE-PEDs scores and epilepsy. Furthermore, this study may have been underpowered to fully investigate the association of LLA based on wPRx with these outcome measures and may be better elucidated in a larger study.
Our findings demonstrated associations between clinical outcomes and AF. We found lower BRs to be associated with poor outcome. Baroreflex systems represent the first line of defense in preserving blood flow to the brain and aim toward maintaining and stabilizing blood pressure on a timescale of seconds by dynamically controlling ventricular contractility, vascular tone, and total peripheral resistance through autonomic efferent pathways [37]. Lower BRs has been shown to be independently associated with infections after ischemic stroke [22]. Changes in HRV and BRs have been also associated with poor outcome in adults after TBI [12] and after aneurysmal subarachnoid hemorrhage [35, 36]. Our study adds to this body of literature to illustrate the potential utility of HRV and BRs in neurologic monitoring of patients experiencing cerebral AVM rupture. In our cohort, the three measures of HRV had stronger relationships with different outcomes. HRrmssd most correlated with GOSE-PEDs (R = − 0.46), LHF most correlated with acquired epilepsy (R = − 0.49), and HRsd most correlated with LOS (R = − 0.32). HRsd was the HRV index with the strongest association with outcomes overall having a significant association with GOSE-PEDs and LOS, and a near-significant association with the development of epilepsy (p = 0.068). Interestingly, the strength of association between HRrmssd and LHF had strong association with certain outcome measures, but not others. LHF was strongly associated with the development of epilepsy (R = − 0.49), but not to GOSE-PEDs or LOS (R = − 0.1, − 0.06). Likewise, HRrmssd was strongly associated with the GOSE-PEDs (R = − 0.46), but not with acquired epilepsy (R = 0.1). This suggests that various indices of AF may describe different aspects of brain health and combinations of these indices may be valuable predictors of outcomes. HRsd and HRrmssd represent HRV time-domain measures, whereas LHF represents an HRV frequency-domain measure that assesses the degree of parasympathetic function in relation to sympathetic function [38]. Our findings suggest that the interplay between sympathetic and parasympathetic function may relate to acquired epilepsy, as evidenced by the strong relationship of low LHF with epilepsy. The lack of significance of LHF to GOSE-PEDs may suggest that the interplay between sympathetic and parasympathetic function may not relate to functional outcome or may be better elucidated in a larger, sufficiently powered study.
Our study uniquely evaluated multiple indices of CA and AF that could predict acquired epilepsy. Higher PRx and wPRx and more time below LLA based upon PRx, PAx, wPRx-thr, and RAC were most associated with acquired epilepsy (R values ranging from 0.46 to 0.59). This suggests that more time with poor CA may lead to epilepsy development. Lower LHF was also significantly related to acquired epilepsy, suggesting that imbalances in sympathetic/parasympathetic activity may relate to long-term neural hyperexcitability and epileptogenesis. These findings raise the possibility that a precision-based approach to management of children with AVM rupture using real-time measures of CA and AF may aid in improving outcomes and alleviating surviving patients of associated comorbidities such as epilepsy.
Limitations
The small sample size from a single institution and the retrospective nature of this study limit its interpretation. Though limited in number, our cohort was a good representation of the population. Thirteen out of 14 of our patients (93%) had GCS scores < 8, with prior studies showing that up to 50% of children with cerebral AVM rupture may have similar presentation [39]. Two (14%) of our patients had in-hospital mortality, consistent with studies showing immediate mortality rates between 5 and 25% [2, 40,41,42,43]. The analysis was observational and did not prospectively look at the response to therapeutic interventions. This needs to be further studied to understand what may be potential opportunities for clinical management in the future to individualize care and improve outcomes. A number of alternative noninvasive model-based indices of CA, including transcranial Doppler ultrasound-based mean velocity index (Mx) and near-infrared spectroscopy-based cerebral oximetry index (COx) [44, 45], were not investigated in this study and warrant future assessment. Similarly, combinations of indices were not evaluated. High-resolution multimodal monitoring data often carry significant artifact, and while substantial effort was made toward its reduction, some artifact may still exist.
Conclusion
After pediatric cerebral AVM rupture, poor global functional outcome and acquired epilepsy were associated with posterior fossa hemorrhage, decreased AF, and perturbations in CA as measured in a number of model-based indices of cerebral physiology. These findings raise the possibility that a precision-based approach to neurocritical care management of children with AVM rupture using real-time measures of CA and AF may aid in improving outcomes and alleviating surviving patients of associated comorbidities such as epilepsy. Larger prospective work is needed to better understand the value of these indices in clinical decision support for children after pediatric cerebral AVM rupture.
References
Anderson RCE, McDowell MM MM, Kellner CP, Appelboom G, Bruce SS, Kotchekov IS, et al. Arteriovenous malformation-associated aneurysms in the pediatric population. J Neurosurg Pedaitr. 2012;9(1):11–6.
Hoh BL, Olgivy CS, Butler WE, Loeffler JS, Putman CM, Chapman PH. Multimodality treatment of nongalenic arteriovenous malformations in pediatric patients. Neurosurgery. 2000;47(2):346–58.
Shtaya A, Millar J, Sparrow O. Multimodality management and outcomes of brain arterio-venous malformations (AVMs) in children: personal experience and review of the literature, with specific emphasis on age at first AVM bleed. Childs Nerv Syst. 2017;33(4):573–81.
Olgivy CS, Stieg PE, Awad I, Brown RD Jr, Kondziolka D, Rosenwasser R, et al. AHA Scientific Statement: recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32(6):1458–71.
Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2(2):161–92.
Chillon JM. Cerebral vessels during chronic hypertension: from arteries to therapeutics. J Hypertens. 2002;20(5):817–8.
Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;S(2):S282–96.
Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. Neurosurgery. 2001;95:756–63.
Aries MJ, Czosnykia M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63.
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–8.
Petkus V, Preiksaitis A, Krakauskaite S, Zubaviciute E, Rocka S, Rastenyte D, et al. Benefit on optimal cerebral perfusion pressure-targeted treatment for traumatic brain injury patients. J Crit Care. 2017;41:49–55.
Sykora M, Czosnyka M, Liu X, Donnelly J, Nasr N, Diedler J, et al. Autonomic impairment in severe traumatic brain injury: a multimodality neuromonitoring study. Crit Care Med. 2016;44(6):1173–81.
Beers SR, Wisniewski SR, Garcia-Filion P, Tian Y, Hahner T, Berger RP, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma. 2012;29(6):1126–39.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65(4):476–83.
Zeiler FA, Donnelly J, Calviello L, Lee JK, Smielewski P, Brady K, et al. Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation, part I: experimental intracranial hypertension. J Neurotrauma. 2018;35(23):2803–11.
Liu X, Donnelly J, Czosnyka M, Aries MJH, Brady K, Cardim D, et al. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: a retrospective study. PLoS Med. 2017;14(7):e1002348.
Zeiler FA, Donnelly J, Menon DK, Smielewski P, Hutchinson PGA, Czosnyka M. A description of a new continuous physiological index in traumatic brain injury using the correlation between pulse amplitude of intracranial pressure and cerebral perfusion pressure. J Neurotrauma. 2018;35:963–74.
Aries MJ, Wesselink R, Elting JW, Donnelly J, Czosnyka M, Ercole A, et al. Enhanced visualization of optimal cerebral perfusion pressure over time to support clinical decision making. Crit Care Med. 2016;44(10):e996–9.
Donnelly J, Czosnyka M, Adams H, Robba C, Steiner LA, Cardim D, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45(9):1464–71.
No authors listed. Heart rate variability. Heart rate variability. Standards of measurement, physiologic interpretation, and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81.
Westerhof BE, Gisolf J, Stok WJ, Wesseling KH, Karemaker JM. Time-domain cross-correlation baroreflex-sensitivity: performance on the EUROBAVAR data set. J Hypertens. 2004;22(7):1371–80.
Sykora M, Siarnik P, Szabo J, Turcani P, Krebs S, Lang W, et al. Baroreflex sensitivity is associated with post-stroke infections. An open, prospective study. J Neurol Sci. 2019;15(406):116450.
Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C, Smielewski P, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16:258–66.
Zeiler FA, Ercole A, Cabeleira M, Zoerle T, Stocchetti N, Menon DK, et al. Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a Center-TBI study. Acta Neurochir. 2019;161:1217–27.
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon DK, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1996;41:11–7.
Zeiler FA, Donnelly J, Calviello L, Smielewski P, Menon DK, Czosnyka M. Pressure autoregulation measurement techniques in adult traumatic brain injury, part II: a scoping review of continuous methods. J Neurotrauma. 2017;34:3227–37.
Zeiler FA, Ercole A, Cabeleira M, Carbonara M, Stocchetti N, Menon DK, et al. Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult TBI: a Center-TBI study. J Neurotrauma. 2018;36:1505–17.
Svedung Wettervik T, Howells T, Enblad P, Lewen A. Temporal neurophysiological dynamics in traumatic brain injury: role of pressure reactivity and optimal cerebral perfusion pressure for predicting outcome. J Neurotrauma. 2019;36:1818–27.
Freeman WD. Management of intracranial pressure. Contin Minneap Minn. 2015;21(5 Neurocritical Care):1299–323.
Dias C, Maia I, Cerejo A, Smielewski P, Paiva JA, Czosnyka M. Plateau waves of intracranial pressure and multimodality brain monitoring. Acta Neurochir Suppl. 2016;122:143–6.
Donnelly J, Czosnyka M, Adams H, Robba C, Steiner LA, Cardim D, et al. Pressure reactivity-based optimal cerebral perfusion pressure in a traumatic brain injury cohort. Acta Neurochir Suppl. 2018;126:209–12.
Kramer AH, Couillard PL, Zygun DA, Aries MJ, Gallagher CN. Continuous assessment of ‘optimal’ cerebral perfusion pressure in traumatic brain injury: a cohort study of feasibility, reliability, and relation to outcome. Neurocrit Care. 2019;30:51–61.
Brown CH, Neufield KJ, Tian J, Probert J, LaFlam A, Max L, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surg. 2019;154(9):819–26.
Zeiler FA, Ercole A, Czosnyka M, Smielewski P, Hawryluk G, Hutchinson PJA, et al. Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: a narrative review of advances in neurocritical care. Brit J Anesth. 2020;124(4):440–53.
Uryga A, Burzynska M, Tabakow P, Kasprowicz M, Budohoski KP, Kazimierska A, et al. Baroreflex sensitivity and heart rate variability are predictors of mortality in patients with aneurysmal subarachnoid hemorrhage. J Neurol Sci. 2018;394:112–9.
Nasr N, Gaio R, Czosnyka M, Budohoski K, Liu X, Donnelly J, et al. Baroreflex impairment after subarachnoid hemorrhage is associated with unfavorable outcome. Stroke. 2018;49:1632–8.
Tasker RC. Intracranial pressure and cerebrovascular autoregulation in pediatric critical illness. Semin Pediatr Neurol. 2014;21(4):255–62.
Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
LoPresti MA, Goethe EA, Lam S. Surgical strategies for management of pediatric arteriovenous malformation rupture: the role of initial decompressive craniectomy. Child Nerv Syst. 2020;36:1445–52.
Meyer PG, Orliaguet GA, Zerah M, Charron B, Jarreau MM, Brunelle F, et al. Emergency management of deeply comatose children with acute rupture of cerebral arteriovenous malformations. Can J Anaesth. 2000;47(8):758–66.
Dorfer C, Czech T, Bavinzski G, Kitz K, Mert A, Knosp E, et al. Multimodality treatment of cerebral AVMs in children: a single-Centre 20 years experience. Childs Nerv Syst. 2010;26(5):681–7.
Ma L, Chen XL, Chen Y, Wu CX, Ma J, Zhao YL. Subsequent haemorrhage in children with untreated brain arteriovenous malformation: higher risk with unbalanced inflow and outflow angioarchitecture. Eur Radiol. 2017;27(7):2868–76.
Beecher JS, Lyon K, Ban VS, Vance A, McDougal C, Whitworth LA, et al. Delayed treatment of ruptured brain AVMs: is it ok to wait? Neurosurgery. 2018;128(4):999–1005.
Budohoski KP, Reinhard M, Aries MJ, Czosnyka Z, Smielewski P, Pickard JD, et al. Monitoring cerebral autoregulation after head injury. Which component of transcranial Doppler flow velocity is optimal? Neurocrit Care. 2012;2:211–8.
Healy RJ, Vorrilla-Vaca A, Ziai W, Mirski MA, Hogue CW, Geocadin R, et al. Glasgow coma scale score fluctuations are associated with a NIRS-based index of cerebral autoregulation in acutely comatose patients. J Neurosurg Anesthesiol. 2019;31:306–10.
Acknowledgements
We thank Hamy Temkit, Ph.D., for discussions of the statistical tests implemented in this study. We also thank Xiuyun Liu, Ph.D., for guidance in the analysis of wPRx and wPRx-thr implemented in this study.
Funding
No external funding sources were utilized for conducting this study.
Author information
Authors and Affiliations
Contributions
BA provided substantial contribution to the design, analysis and interpretation of the data for this work, drafting and revising of this manuscript for important intellectual content, provided final approval of the version to be published, and agrees to be accountable for all aspects of this work. SF, BTB and AJ contributed substantially to the analysis and interpretation of the data for this work, revising of this manuscript for important intellectual content, provided final approval of the version to be published, and agree to be accountable for all aspects of this work. TA, VB, AW, TM, VG, IM and PDA contributed substantially to the interpretation of data for this work, revising of this manuscript for important intellectual content, provided final approval of the version to be published, and agree to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Conflict of interest
The co-authors have no relevant conflicts of interest to disclose. I, Dr. Appavu, report a completed research grant from Moberg ICU Solutions as well as research grants from the American Heart Association (19CDA34760291) and United States Department of Defense Congressionally Directed Medical Research Programs Epilepsy Research Program (W81XWH-19-1-0514), outside of the submitted work. This study was performed under all ethical research guidelines at Phoenix Children’s Hospital, and the Institutional Review Board (IRB) at Phoenix Children’s Hospital approved this study (IRB #17-899). We confirm the use of the reporting checklist, STROBE.
Ethical approval/Informed consent
This study was performed under all ethical research guidelines at Phoenix Children’s Hospital, and the Institutional Review Board at Phoenix Children’s Hospital approved this study (IRB #17-899).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All work was performed at the Barrow Neurological Institute at Phoenix Children’s Hospital.
Rights and permissions
About this article
Cite this article
Appavu, B., Foldes, S., Burrows, B.T. et al. Multimodal Assessment of Cerebral Autoregulation and Autonomic Function After Pediatric Cerebral Arteriovenous Malformation Rupture. Neurocrit Care 34, 537–546 (2021). https://doi.org/10.1007/s12028-020-01058-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01058-3