Abstract
Subdural hematomas (SDHs), though frequently grouped together, can result from a variety of different etiologies, and therefore many different subtypes exist. Moreover, the high incidence of these lesions in the neurocritical care settings behooves practitioners to have a firm grasp on their diagnosis and management. We present here a review of SDHs, with an emphasis on how different subtypes of SDHs differ from one another and with discussion of their medical and surgical management in the neurocritical care setting. In this paper, we discuss considerations for acute, subacute, and chronic SDHs and how presentation and management may change in both the elderly and pediatric populations. We discuss SDHs that arise in the setting of anticoagulation, those that arise in the setting of active cerebrospinal fluid diversion, and those that are recurrent and recalcitrant to initial surgical evacuation. Management steps reviewed include detailed discussion of initial assessment, anticoagulation reversal, seizure prophylaxis, blood pressure management, and indications for intracranial pressure monitoring. Direct surgical management options are reviewed, including open craniotomy, twist-drill, and burr-hole drainage and the usage of subdural drainage systems. SDHs are a common finding in the neurocritical care setting and have a diverse set of presentations. With a better understanding of the fundamental differences between subtypes of SDHs, critical care practitioners can better tailor their management of both the patient’s intracranial and multi-systemic pathologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subdural hematomas (SDHs) result from a variety of etiologies. Their clinical impact ranges from relatively minor to potentially life threatening, and significant nuances exist in their management. Given the high incidence of SDH in patients with neurocritical care needs, it is important for clinicians with routine exposure to these patients to have a firm grasp on their diagnosis and management. We present a review of SDHs, with particular focus on the various subtypes and how they differ in etiology, epidemiology, presentation, and management.
Classification
All SDHs are defined by the presence of blood between dura and arachnoid layers covering the brain. Aside from the anatomic location of blood collection, relatively few characteristics are consistent across all subtypes of SDHs. SDHs can be further classified by the time course of development (acute, subacute, chronic, acute on chronic), etiology (traumatic, spontaneous, secondary to other pathologies), age of the patient, and other special considerations (e.g., anticoagulation-induced, bilateral, recurrent). Often, multiple variables co-exist, and a given hematoma may harbor attributes of several subtypes.
The origin of subdural bleeding has been classically attributed to disruption of bridging veins running from the cortical surface to the overlying dura, although this is not always the case, as discussed below.
Acute Subdural Hematomas
General Considerations in Adults
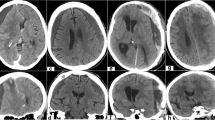
In the young, healthy adult brain, acute SDHs typically follow significant head trauma and are observed in 12–29 % of severe traumatic brain injury (TBI) cases [1–4]. The presence of an acute SDH should be suspected in patients who present with decreased or progressively worsening levels of consciousness, and those with unilateral neurologic deficits (e.g., dilated pupil, motor weakness or posturing, or focal seizure activity). Diagnosis is confirmed on noncontrast computed tomography (CT), where an acute subdural hematoma (SDH) appears as a hyper-attenuating mass overlying the cerebral convexity, falx cerebri, or tentorium cerebelli, classically with a crescent-like or “half-moon” appearance that crosses cranial suture lines (Fig. 1). When present, acute SDHs are associated with high morbidity and mortality, with mortality rates as high as 50–90 % in patients who present with Glasgow Coma Scale (GCS) scores 8 or less [2, 4–8]. The prognosis for patients presenting with higher GCS scores is noticeably better, with studies reporting a 0 % mortality rate in those with a GCS score of 13 or above and a mortality rate of 21–46 % in patients with intermediate GCS scores [6, 7, 9]. On the whole, the presence of SDH in an acute traumatic injury portends poor prognosis [7, 9–11]. Among all patients with acute SDHs, fewer than 25 % ultimately achieve a full recovery without any major neurologic deficits [2, 4, 8, 9, 12, 13].
Classic examples of SDH on CT scan, including a right-sided acute SDH in a 19-year-old male, note the associated diffuse cerebral edema, b right-sided acute SDH in a 93-year-old female, c acute parafalcine SDH in a 78-year-old male on aspirin and clopidogrel, d bilateral subacute SDHs in a 56-year-old male, e chronic SDHs found in a 88-year-old male following a fall (no reported history of previous head trauma), and f acute on chronic SDH in a 79-year-old male who had fallen 2 weeks earlier, but presented with rapid-onset left-sided facial droop and hemiparesis
Several reasons exist for the significant morbidity and mortality associated with acute SDHs. As opposed to in elderly individuals or others with preexisting brain atrophy, blood vessels in the subdural space of young adults are protected by both the overlying skull and ample underlying brain parenchyma. As such, acute SDHs require antecedent high-energy collision or acceleratory forces. Not surprisingly, such mechanisms frequently generate other concurrent brain injuries [14–16]. Up to 83 % of patients presenting with acute SDHs have associated brain contusions, and a substantial number have evidence of diffuse axonal injury [5, 14, 17]. Furthermore, a significant proportion of acute SDHs arise from hemorrhagic conversion of brain contusions and disruption of cortical arteries [2, 18, 19]. Thus, associated damage to brain parenchyma as well as potentially severe systemic injuries often compound the direct impairment caused by subdural bleeding in determining eventual outcome.
Considerations in Elderly Patients
Acute SDHs in older adults can present similarly to those in younger adults, with a few unique considerations. Due to age-related cerebral atrophy, the subdural space increases, placing cortical veins under increased tension and thus, more prone to injury. Therefore, SDHs occur not only more frequently in older adults but may also present with greater clot thickness and more midline shift [20, 21] (Fig. 2). Moreover, older adults harbor more medical comorbidities, elevating the risk of significant medical complications (myocardial infarction, pneumonia, etc.) during a patient’s hospital course.
Formation of a subdural hematoma. Left coronal section taken at the level of the SSS demonstrating tearing (solid arrow) of a bridging vein (BV) after forceful impact to the skull. Right detail of bridging vein tear (curved arrow) and accumulation of blood within the dural border cell layer (straight arrows). ABC arachnoid barrier cell layer, AT arachnoid trabeculae, CC cerebral cortex, D dura, DBC dural border cell layer, EV emissary vein, F falx, P pia, VL venous lacunae. From [125]. Copyright Remi Nader. Published with permission
As such, it is not surprising that mortality from acute SDH is consistently elevated with increasing age. Adults over 60 years of age have significantly higher rates of mortality following acute traumatic SDH, with some studies reporting a four times higher mortality in elderly than in younger patients [5, 7, 8, 20–22].
Considerations in Pediatric Patients
Infants and toddlers also harbor a higher incidence for SDHs compared to older children and adults [23–26]. This is attributable, in part, to nonaccidental trauma in infants, the so-called “shaken baby syndrome.”[23, 26] Abuse accounts for greater than 50 % of SDHs in toddlers and infants, and the finding of an SDH in a child should raise the concern for potential child abuse [27, 28].
The exact pathophysiology for the increased susceptibility of infants to develop SDHs is still not fully understood [29]. The unfused calvarial bones in infants allow for large fluctuations in dural intravenous pressure during even normal head movement, posing a possible role for venous hypertension in SDH formation [30]. Moreover, in cases of nonaccidental trauma, it has been hypothesized that periods of ischemia may also occur, leading to endothelial injury and subsequent bleeding risk [31].
SDH management in children similarly prioritizes monitoring of clinical decline and evacuating hemorrhages with symptomatic mass effect. Patients with small lesions, no neurologic deficits, and no evidence of midline shift may be observed. Larger symptomatic lesions should prompt surgical evacuation or potentially needle aspiration if an open fontanel still persists [32–34]. It should be noted, however, that in infants, open craniotomy with surgical evacuation bears a higher risk of hypovolemic shock due to low total blood volume. While this consideration alone is not a contraindication to surgical evacuation, it nevertheless should be kept in mind during the intra-operative and post-operative care of young patients.
Subacute Subdural Hematoma
Hematomas begin to liquefy at 2 weeks after formation. Subacute SDHs represent evolving hematomas weeks after an initial insult as liquefaction of the initial clot causes symptomatic expansion [35]. Much like acute SDHs, subacute SDHs should be suspected in patients with a history of trauma, who then present with delayed onset of decreased mental status, unilateral focal deficits, or other signs of increased intracranial pressure (ICP). On noncontrast CT scan, a subacute SDH appears as an isodense mass, which may have been present on previous imaging studies; their density may be equal to that of normal brain tissue, and they may be missed on CT review. Care should be taken to distinguish them from other fluid collections of the subdural space that can arise in a subacute fashion, such as subdural empyemas or hygromas [36, 37]. The former can be distinguished by the presence of fever, leukocytosis, meningismus, and other signs of intracranial bacterial infection and the latter by its noted similarity to cerebrospinal fluid (CSF) on all imaging modalities.
Much like acute SDHs, subacute SDHs are typically associated with trauma. However, they carry a lower associated burden of mortality and morbidity, with some studies reporting a mortality rate one third of that encountered in acute SDHs [38]. Much remains unknown about these entities, however, and the vast majority of the literature is currently devoted toward the study of either acute or chronic SDHs. As such, there is currently little evidence to guide how management of subacute SDHs should differ from the management of acute or chronic SDHs [39–41].
Chronic Subdural Hematoma
SDHs that arise over the course of weeks to months are defined as chronic and manifest with a markedly different tempo of symptoms and management strategy [42]. The incidence of chronic SDHs is estimated at approximately 0.001–0.002 % per year, with a bias toward males and the elderly [43–45]. They commonly present in patients with increased subdural spaces (such as from aging-related brain atrophy), and often there is a history of only minor head trauma or no recallable antecedent trauma [42, 46–48]. Presenting symptoms range from behavioral disturbances and headaches to focal seizures and unilateral focal deficits [45, 48]. On CT scan, chronic SDHs can be identified as hypo-attenuating masses that maintain the crescentic appearance of their acute counterparts.
The pathophysiology of chronic SDHs differs from that of acute hematomas, and it is important to keep in mind that normal SDH absorption relies on counter pressure from the brain parenchyma and the normal anatomic absence of a potential subdural space [47]. Thus, contributive factors in chronic SDHs include chronically lowered ICP from aggressive ventricular shunting (leading to engorgement and stretching of bridging veins), widened potential space between dura and underlying brain parenchyma (as can also be seen from aggressive ventricular shunting and can also occur with brain atrophy from aging, chronic alcoholism, or other causes), and a lack of underlying parenchymal swelling (as is seen in cases of relatively minor trauma) [46]. In the initial formation of the hematoma, minor trauma leads to disruption of venous structures, resulting in small amounts of subdural blood accumulation that cannot be absorbed in normal fashion.
Over time, chronic SDHs may enlarge [46]. The exact mechanisms for enlargement are not fully elucidated, but one hypothesis attributes continued bleeding to the formation of neomembranes from the inner layer of the dura to invest around the hematoma [49]. Neomembranes are triggered by cleavage of the dura-arachnoid space and consist of an inner and outer layer, with the latter being highly vascularized tissue that can contribute to repeated low-volume bleeding [50]. There is also reason to believe that this outer membrane secretes anti-thrombotic and fibrinolytic substances into the inner hematoma space, allowing the hematoma to remain liquefied and expand over time [51, 52].
Mortality associated with chronic SDHs is significantly lower than that of acute SDHs, with estimates ranging from less than 1–5 % in most studies [45, 53]. However, these numbers may underestimate the true morbidity burden of chronic SDHs, particularly in the elderly. One study of elderly chronic SDH patients (mean age 80.6 years) measured an initial 16.7 % in-hospital mortality rate but found that when followed after discharge, mortality rose to 26 % at 6 months and 32 % at 1 year post-hospitalization [54].
Acute on Chronic Subdural Hematomas
Acute on chronic SDHs occurs in the setting of known SDH that expands due to subsequent trauma. Clinically, these patients often have a known or suspected chronic SDH (e.g., elderly patients with complaints of progressively worsening headaches and mental status) and then develop an acute worsening of their headache or decline in neurologic status. CT scan may reveal a lesion that features both acute (hyperdense) and chronic (hypodense) material in the subdural space. Limited literature on this population suggests that an acute component compounds as many as 8 % of chronic SDHs [55]. Similar to acute SDHs, acute on chronic SDHs require rapid treatment to avoid the potentially dangerous sequelae of an enlarging clot burden.
Bilateral Subdural Hematomas
Bilateral chronic SDHs are found in 10–25 % of patients with chronic SDHs and merit special mention for several reasons [47, 48, 56, 57]. Unlike unilateral lesions, bilateral chronic SDHs rarely present with hemiparesis or focal neurologic deficits and are less likely to produce midline shift [57, 58]. As such, the clinical diagnosis of these lesions is more difficult as symptoms may be nonspecific for intracranial masses (e.g., headache, progressive global mental decline). Moreover, as the brain parenchyma is compressed bilaterally, the direction of mass effect trends away from lateral shifts and subfalcine herniation and toward downward shifts and central herniation, a potentially fatal complication if not diagnosed and corrected in a timely fashion. Additionally, when encountered outside of a traumatic setting, bilateral SDHs should prompt suspicion of intracranial hypotension and precipitate investigations into a source of spontaneous CSF leak or overly aggressive CSF shunting. Symptoms and signs that augment suspicion for intracranial hypotension include positional headaches and apparent dural thickening on imaging. In such instances, repair of the CSF leak or reduction in the degree of CSF shunting may lead to spontaneous resolution of the subdural collection without need for more aggressive interventions.
Recurrent Subdural Hematomas
An estimated 10–25 % of patients with chronic SDHs will experience recurrence following evacuation [53, 59–63]. Many factors have been linked to recurrence, including age, anticoagulation usage, persistent post-operative midline shift, large original hematoma size, bilateral lesions, septated or mixed-density lesions, and presence of post-operative seizures [56, 59, 61, 63]. Although the reliability of any single factors to predict recurrence varies between studies, taken as a whole, they suggest that residual hematoma following treatment is an important causative factor in chronic SDH recurrence. They further indicate that initial surgical evacuation may prove difficult in large lesions, septated lesions, and mixed-density lesions [64].
When present, recurrent SDHs are treated similarly to primary lesions, with repeat surgical evacuation a preferred option. Repeat surgical evacuation, even with the exact same methodology as the initial evacuation, is often effective and results in successful treatment in greater than 70 % of patients [53]. Continued recurrence may prompt placement of a subdural-peritoneal shunt for longer term lesion control.
Anticoagulation-Associated Subdural Hematomas
Systemic anticoagulation is a known risk for the development of both acute and chronic SDHs [65, 66]. In population studies, patients on oral anticoagulation therapy are estimated to have a 4 to 15-fold increased risk for SDH [67, 68]. Moreover, for patients on warfarin, an increase in prothrombin ratio from 2.0 to 2.5 has been measured to increase the risk of subdural hemorrhage by greater than sevenfold [69]. Limited evidence suggests that direct thrombin inhibitors may pose an equivalent risk of SDH formation compared with warfarin, in a dose-dependent manner [70]. Although direct thrombin inhibitors pose certain advantages over warfarin, caution must be exerted in the setting of intracranial hemorrhage and the neurocritical care population as no current methods exist to reverse the effects of direct thrombin inhibitors [71].
Although patients on anticoagulation represent a heterogeneous group, SDHs in the setting of anticoagulants are not only more frequent but also present in a more sudden and severe fashion. These lesions can expand rapidly, and unless anticoagulation is quickly reversed, can result in severe symptoms and death.
Secondary Subdural Hematomas
Rarely, SDHs are encountered secondary to a primary vascular, malignant, or other structural abnormalities. From a vascular perspective, SDHs have been reported secondary to dural arteriovenous fistulas, arteriovenous malformations, and aneurysmal rupture (particularly aneurysms arising from the posterior communicating artery) [72–76]. Instances of these are exceedingly rare and data are limited to case reports and small case series. In patients with no other risk factors, spontaneous SDH formation can be investigated for potential underlying vascular etiologies using CT angiogram and potentially invasive angiography. Acute, symptomatic lesions warrant immediate surgical attention and appropriate correction of the underlying lesion.
Malignant lesions have also been known to lead to SDH formation, with primary intrinsic tumors, meningeal tumors, multicentric lymphomatous tumors, and dural metastases all having been implicated in SDH formation [77, 78]. The incidence of SDH in systemic cancer is low, with one study reporting only two incidents in a series of 2508 consecutive autopsy specimens [79]. The pathophysiology of these hematomas has been hypothesized to involve either direct hemorrhage from the tumor itself, or metastatic obstruction of intradural veins, leading to dilation and rupture of collateral capillaries on the inner layer of the dura [80, 81]. A diagnosis of malignancy-associated SDH should be suspected in patients with a known history of metastatic cancer and rapidly progressive focal neurologic deficits or decrease in mental status. Management should be based on the presence of compressive symptoms as well as the individual goals of care in the setting of metastatic disease.
Other structural brain abnormalities can also predispose patients to SDH formation. The most commonly encountered of these are arachnoid cysts, which are known to occur in approximately 2.4 % of chronic SDHs [82]. The exact mechanism for the association between arachnoid cysts and SDHs remains currently unclear and has been hypothesized to involve potential bleeding from disruption of the outer wall of the cyst [83, 84]. Management of arachnoid cyst-associated SDH also remains unclear, with authors reporting success with evacuation of either the hematoma alone or in conjunction with the associated arachnoid cyst [82, 84].
Cerebral Spinal Fluid Collections in the Subdural Space
Not all subdural fluid collections are filled with blood, however. There are a number of clinical circumstances in which there is accumulation of cerebral spinal fluid (CSF) in the subdural space. These circumstances include spontaneous accumulation in some patients, certain pediatric patients, and finally some patients who have undergone complicated intracranial operations. These extra-axial collections have been referred to as subdural hygromas, subdural fluid collections, external brain tamponade, or external hydrocephalus [85–87]. All of these descriptions have an implied etiological connection to hydrocephalus [88, 89]. In our evaluation, the more accurate association is with derangements in intracranial CSF dynamics, which at times does not reach the level of hydrocephalus. Given the focus of this manuscript, we will direct our attention to the presentation and management of those collections that present in the post-operative setting, typical of the neurocritical care setting. The vast majority of these collections follow decompressive craniectomy, although some do present outside of this setting.
The etiology of these extra-axial collections in the setting of decompressive craniectomy is difficult to ascertain. One theory proposes that surgery violates the dural and bony tissue planes and creates the contiguity and communication among the normally distinct subgaleal, epidural, subdural spaces with the subarachnoid space needed for the formation of these collections. This derangement in cerebral hydro dynamics is only accentuated when the normal “closed vault” pressure provided by the intact skull is removed, making the accumulation of these collections more prominent in those patients who have received a craniectomy. Therefore “extra-axial” is the most appropriate anatomical description of the space where post-craniectomy fluid collections occur [89].
Malignant middle cerebral artery (MCA) infarction has been shown to respond favorably to decompressive craniectomy with significant supporting evidence from well-conducted prospective trials, including DECIMAL, DESTINY, and HAMLET [86, 90, 91]. Given this evidence, an increased number of patients are being treated with this modality. These and other studies have also demonstrated that extra-axial fluid collections are known consequences of decompressive hemicraniectomy, often requiring further management [92].
Decompressive craniectomy is also prominently utilized in other settings where uncontrolled ICP is not responsive to medical therapy, such as trauma, subarachnoid hemorrhage, and occasionally tumors associated with significant edema. Most prominent amongst these clinical setting is the application of decompressive craniectomy in trauma. The recent DECRA trial has renewed interest in this controversial surgery [93]. The trial demonstrated that after decompressive craniectomy, patients had decreased ICP and the length of stay in the Intensive Care Unit but increased unfavorable outcomes. Work published by the senior author demonstrates how these collections can, at times, behave in a very aggressive fashion causing mass effect, herniation, and significant worsening in patient clinical status [88, 89]. We described this entity as Craniectomy-Associated Progressive Extra-Axial Collections with Treated Hydrocephalus (CAPECTH; Fig. 3). It is clear that the benefits of the decompressive craniectomy in these settings will not be realized until we deal with the complications associated with the procedure, including the management of these extra-axial collections.
A 50-year-old male with severe traumatic brain injury and hemorrhage who developed an extra-axial collection ipsilateral to the craniectomy. Axial noncontrast head CT scans showing a brainstem compression from mass effect; b a large collection slightly denser than cerebrospinal fluid with significant mass effect; and c the collection resolved after cranioplasty and ventriculoperitoneal shunt placement
Management
Immediate Assessment and Nonoperative Interventions
Common to all acute presentations, the ABCs of critical care must be secured. This refers to securing the airway, controlling blood pressure, and circulation or coagulation. Although many of the concepts for this acute critical care medicine are similar for all patients, there are important differences that the practitioner must pay special attention to. In an attempt to increase awareness and education in this important topic, numerous collaborative efforts have been institured, including the formation of the Emergency Neurological Life Support (ENLS) by the Neurocritical Care Society [94, 95].
Mechanical airway ventilation is critical to ensure that critical organs receive proper oxygenation. The central nervous system (CNS) is the most sensitive tissue to the effects of hypoxia, with irreversible damage beginning at 3 min when the PaO2 drops below 30 mm Hg [96]. Another important role of adequate ventilation is to ensure proper removal of CO2, as it is known that hypercapnia may have deleterious results in the neurologically devastated patient, including vasodilation and resultant increases in ICP. The specifics of airway management are beyond the scope of this publication, but readers are guided to important literature on this topic [97]. It has been suggested that any patient with a Glascow Coma Scale (GCS) of 8 or below should be intubated and that approximately 30 % of patients with intracerebral hemorrhage will require to be mechanically intubated [98]. Another advantage of mechanical ventilation is that allows for safer use of sedating medicines, which are helpful in the initial management of increased ICP by lowering patient agitation and decreasing brain tissue metabolic demands.
After a patient’s airway has been secured, attention should be directed to controlling blood pressure to a limited extent. This is because although uncontrolled hypertension has been noted to have deleterious effects on the CNS, aggressive treatment may result in ischemic damage [99]. Similar to recent guidelines for patients with intracerebral hemorrhage, it has been suggested that lowering the systolic blood pressures to around 140 mm Hg may be beneficial [100, 101].
As previously mentioned, reversal of anticoagulation is also important in the setting of anticoagulation-associated hematomas, and administration of fresh frozen plasma (FFP), platelets, protamine sulfate, and/or vitamin K can help stem the aggressive progression of these SDHs. Two classes of drugs that have garnered some attention in the reversal of anticoagulation are pro-thrombin complex concentrates (PCCs) and recombinant factor VIIa (rFVIIa). PCCs have been used to counteract warfarin and although results seem to be equivocal between using them and FFP, they have the added advantage of being low-volume, limiting the adverse effects of volume overload in these patients, which could include unwanted cerebral affects such as worsening cerebral edema, or more systemic effects such as heart failure [102]. Although there was much initial excitement about the use of rFVIIa in the reversal of anticoagulation-related intracerebral hemorrhages, further studies showed that rFVIIa did not generate thrombin as effectively as PCCs and furthermore caused more thromboembolic events [103]. Thus, for patients known to be on Coumadin that present with SDHs and the decision has been made for operative management, it is our practice to administer a PCC with a goal of an international normalized ratio (INR) of 1.4 or below, and this has been shown in small series to decrease the amount of time needed to correct the coagulopathy and time to surgical intervention [104]. It should be noted that the time of effect of PCCs is only a few hours and redosing may be necessary or supplementation with vitamin K. The use of newer oral anticoagulants such as thrombin inhibitors are becoming increasingly popular due to the fact that levels do not have to be continually monitored, but no specific antidotes are widely available in the US [107]. Clinicians should be aware of these newer drugs and the timely consultation of hematologists is a common occurrence in the management of these patients.
Patients on antiplatelet therapy such as aspirin have an increased risk of bleeding and subsequent enlargement of their SDHs, and this risk is increased when they are on other antiplatelet agents such as Plavix. Platelet infusions for patients on antiplatelet therapy did not show any improvements in mortality or functional outcomes in patients with intracranial hemorrhage but is still widely practiced [105, 106]. Similarly, patients with low platelet counts such as those with myelodysplasia are particularly difficult patients to treat and current recommendations suggest that a platelet level of 100,000 mL−1 is required for invasive neurosurgery, and the level should be maintained to 50,000 mL−1 for 2 days after such invasive procedures [108]
It is only after securing the ABCs of critical care that it is safe to think about acquiring imaging to make a diagnosis. For its ability to quickly diagnose most intracerebral pathologies, a noncontrast CT is the initial imaging modality of choice in these instances. Although MRI may be helpful, especially in helping with subdurals that are related to other entities such as neoplasms (see section on secondary SDHs), the time needed for their acquisition makes it less favorable in the acute setting. Following the diagnosis of a SDH, a primary concern is ICP management. As originally formulated over two centuries ago, the Monro–Kellie hypothesis states that the intracranial space has a fixed volume comprised of brain parenchyma, CSF, and intracranial blood [109]. In the setting of a SDH, clot burden and potentially post-traumatic edema further reduce the available amount of intracranial space. After the buffering ability of CSF and cerebral blood flow auto-regulation are exhausted, cerebral ischemia and herniation become worrisome complications. As such, ICP management is an important pillar in the management of all SDH patients. As will be seen in the discussion below, there are important differences in the management of increased ICPs in patients with SDHs compared to those with traumatic brain injuries (TBIs) or other space-occupying lesions.
Unlike the trauma guidelines which suggest that the insertion of a fiberoptic ICP wire or external ventricular drain (EVD) may be helpful in the setting of a patient with GCS of 8 or below and an abnormal CT scan, the placement of such devices may be more detrimental for patients with SDHs, as the drainage of CSF may cause further retraction of draining veins and worsening of the SDH, similar to the subdural collections seen in patients with aggressive ventriculoperitoneal shunts. It is for these reasons that the use of hypertonic saline and mannitol—two therapies that are well documented to help with ICPs are not useful in the context of SDHs (see below). By acting as another constituent in the cranial vault, the SDH has a direct role in increasing ICPs, and it is for this reason that operative management plays such a crucial role (see below).
Post-traumatic seizures are a potentially serious complication, especially within 7 days following the initial insult. SDHs are a known risk factor for early traumatic seizures in TBI patients, with as many as 24 % of patients with traumatic SDHs presenting with an early post-traumatic seizure [110]. Similarly, for acute or acute-on-chronic subdural patients, the rate of post-operative seizures has been reported to be as high as 25 % [111].
Patients with SDHs, particularly those with involvement of the temporal lobe, tentorium, and other epileptogenic regions, frequently require anti-seizure prophylaxis, given a previously reported association between initiation of pharmacologic prophylaxis and reduced incidence of early post-traumatic seizures [112, 113].
Although previously popular, phenytoin for post-traumatic seizure prophylaxis has been gradually supplanted by levetiracetam given its more favorable adverse effect profile [114]. Data to support this switch are somewhat lacking, but at least one retrospective study found that levetiracetam has a similar efficacy profile as phenytoin in preventing seizures in patients with acute or subacute SDHs, while being associated with a lower risk of adverse effects [115]. Equivalent efficacies may not persist for more severe lesions, however. For patients with midline shift noted on initial head CT, levetiracetam has been associated with a higher risk of electrographic seizures compared to phenytoin [115].
In chronic SDH patients, about 11 % develop seizures following surgery for hematoma evacuation, although this rate varies and has been reported to be as high as 19 % [42, 116]. The use of antiepileptic drugs for seizure prophylaxis in chronic SDH patients remains controversial.
To date, no controlled prospective study has studied the effects of timing, dosage, and type of antiepileptic medication on post-operative seizures in SDH patients. One study found that 2.4 % of patients who received prophylactic anticonvulsive medications developed seizures, as compared to 32 % of patients who did not receive therapeutic levels of prophylactic medication [116]. Another study found that, although preoperative antiepileptic drug prophylaxis was associated with a lower risk of post-operative seizures in chronic SDH patients treated with burr-hole drainage, the use of such medication did not affect discharge outcomes [117]. Given the strong correlation between post-operative seizures and mortality, as well as the significant increase in morbidity in patients with new seizures, there is a need for more controlled studies to establish standard guidelines in managing post-operative seizures in the setting of SDH [116].
It is because of these complex issues and the association of acute SDHs and severe trauma that it is our practice to admit most symptomatic SDHs to our neurocritical intensive care unit (NICU) until operative management of the SDHs is arranged.
Operative Intervention in Acute Subdural Hematomas
The management of acute SDHs is influenced by the clinical course, radiologic findings, and patient prognosis. The latter consideration warrants special emphasis as, due to the association between acute SDHs and very severe trauma, an unfortunately high number of patients with acute SDHs are unsalvageable and thus are not surgical candidates. Current guidelines recommend surgical evacuation with open craniotomy in cases of acute SDH with maximal thickness of greater than or equal to 1 cm or greater than or equal to 0.5 cm of midline shift [1]. Lesions that do not meet the aforementioned criteria may still prompt operative evacuation if they have a GCS score of 8 or less and meet one of the following conditions: (1) a decrease in GCS score by 2 or more points from the time of injury to hospital presentation, (2) asymmetric or fixed and dilated pupils, or (3) patients with ICP greater than 20 mm Hg [1].
It is important to note that these guidelines are based on observational data, and no randomized controlled trials exist to guide surgical management of acute SDHs. Clot thickness and midline shift have both been linked to poor outcomes, though the exact numerical values at which morbidity and mortality begin to rise vary across studies [5, 11, 17, 20]. Likewise, clot thicknesses of greater than 1 cm and greater than 0.5 cm of midline shift have been associated with the failure of nonoperative therapy and the need for delayed surgery in multiple studies [3, 118, 119]. Thus, consistent evidence suggests that larger hematomas benefit from surgical evacuation. The evidence for surgical evacuation for smaller hematomas is more limited. In one prospective series of 65 patients, the 15 patients with ICP less than 20 mm Hg and with hematomas less than 5 mm of thickness were initially treated nonoperatively. Three of the 15 died from their injuries (two from progression of intracranial pathology, one unlisted) and two subsequently required surgical treatment. Decrease in GCS from time of injury was linked to failure of nonoperative management [3].
Despite advancements in diagnostic and therapeutic options over the years, acute traumatic subdural in adults remains a serious, life-threatening condition that is typically associated with underlying structural brain injury. In addition to prompt surgical evaluation and hematoma evacuation when indicated, medical management of cerebral edema and ICP are equally important in maximizing patient outcomes after this serious injury. It is for this reason that many, if not all patients post-operatively are readmitted to the NICU after operative management. With the successful obliteration of the potential space of the SDH and the interval placement of subdural drains, more traditional methods of ICP management such as hypertonic therapy and placements of EVD are safer. It is also our practice to leave drains in the subdural space as well as in the subgaleal space for 1 or 2 days to help reduce the chance of recurrence, borrowing from the evidence of efficacy of drains in chronic subdural evacuations (see below).
Operative Intervention in Chronic Subdural Hematomas
Management of chronic SDHs is somewhat controversial, with debate on the optimal method of surgical evacuation. While reports of successful nonoperative management of chronic SDHs exist, most authors agree that the majority of chronic SDHs do require surgical drainage as their very existence suggests compromise in the body’s ability to naturally reabsorb the hematoma [120]. Moreover, the natural history of these lesions is to frequently expand over time, leading to further cerebral compression [46]. In one randomized controlled trial, mannitol therapy was compared to surgical evacuation of chronic SDHs, the trial was terminated due to failure of mannitol alone in all nonoperative patients [121]. In an interesting study, a single institution hypothesized that hyperfibrinolysis plays a major role in liquefaction of chronic SDHs. Thus, they gave 21 patients an oral regimen of the antifibrinolytic drug, tranexamic acid. They showed over an 80 % reduction in the volume of chronic subdurals and no patients’ subdurals increased in size [122]. Larger studies are needed to corroborate these results. No specific guidelines currently exist on the indications for surgery in chronic SDHs, all symptomatic lesions causing focal symptoms should be considered potential operative candidates.
Methods of surgical evacuation include open craniotomy (involving removal of a bone window in the operating room and direct hematoma drainage, irrigation, and/or neomembrane resection), burr-hole craniotomy (involving a large hole drilled into the cranium in the operating room, and subsequent dural puncture and irrigation), and twist-drill craniotomy (involving a smaller hole placed in the cranium, which can be performed at bedside, with subsequent placement of a drainage catheter). Any of these procedures can also be augmented with the placement of a subdural drain, to allow for continued post-operative drainage of hematoma contents. To date, no class I evidence exists to compare the various ways of gaining access through the cranium [53, 123]. There is little evidence to suggest that excision of the hematoma membrane improves outcomes, making the use of large opened craniotomies for this purpose less frequent [95]. Moreover, it should be noted that evidence exists to support the use of subdural drains. In one randomized controlled trial of 269 patients undergoing burr-hole drainage, drain placement was associated with lower rates of hematoma recurrence and reduced mortality [124].
As the optimal method of chronic SDH evacuation remains an area of continued research, other patient-specific factors may heavily influence a clinician’s approach to SDH drainage. In patients who are unstable for transport to the operating room or are poor candidates to undergo general anesthesia, twist-drill craniotomy may have significant advantages over more invasive methods.
Operative Intervention in Cerebral Spinal Fluid Collections in the Subdural Space
The treatment alternatives for management of CSF collections vary to a great extent on which clinical setting they accompany. Collections that accompany replacement of the bone flap tend to follow a much more benign course, as is the case with those that accompany decompressive procedures for MCA infarction. These same collections in the setting of trauma and subarachnoid hemorrhage can have a much more aggressive clinical course and therefore require a different approach.
In the setting of a benign evolution of these collections, it is often sufficient to manage with careful observation and close follow-up. This will avoid unnecessary surgical intervention while monitoring that they resolve spontaneously. In the intermediate setting of persistent collections that cause clinical alterations, the choices for management include temporary drainage of the collection itself, treatment of underlying hydrocephalus with a ventriculoperitoneal shunt, or draining of the subdural collection itself with a subdural to peritoneal shunt. In our own published experience with this intermediate group of patients following MCA infarction and decompression, we found that prompt cranioplasty, when permitted by the clinical situation, resolved these collections without additional interventions [89].
In the setting where these collections follow an aggressive course with mass effect and clinical deterioration, it is often the case where multiple management techniques are required, including local drainage, treatment of hydrocephalus with ventriculoperitoneal shunts, treatment of local collections with subdural-peritoneal shunts and rapid cranioplasty. For those patients in this setting, we have developed a treatment algorithm (Fig. 4) [88].
Follow-Up
Following stabilization of patients with SDH, regular follow-up is an important step not to be missed. As previously mentioned, hematoma recurrence and progression are not uncommon, and follow-up with serial imaging is critical to ensure that recurrent hematoma formation is caught and addressed before it becomes symptomatic. An immediate post-operative CT scan can demonstrate the amount of remaining clot-burden left and serves as a baseline for future comparisons if a patient develops symptoms suspicious for SDH recurrence. Follow-up imaging within 1–2 weeks is also commonly pursued in otherwise asymptomatic patients to monitor for signs of progression or recurrence.
Conclusions
SDHs are a common finding in neurocritical care patients and represent heterogeneous pathologies with different etiologies, epidemiology, and management strategies. It is clear that a multi-faceted approach, including critical care and surgical management is needed for the optimal care of these often very sick patients (Fig. 5). Properly selected patients can benefit substantially from clot evacuation and alleviation of mass effect, but it is important not to overlook the importance of management of other concurrent injuries both intracranially and elsewhere in the body.
References
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16–24 (discussion Si-iv).
Seelig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981;304(25):1511–8.
Servadei F, Nasi MT, Cremonini AM, Giuliani G, Cenni P, Nanni A. Importance of a reliable admission Glasgow Coma Scale score for determining the need for evacuation of posttraumatic subdural hematomas: a prospective study of 65 patients. J Trauma. 1998;44(5):868–73.
Wilberger JE Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74(2):212–8.
Kotwica Z, Brzezinski J. Acute subdural haematoma in adults: an analysis of outcome in comatose patients. Acta Neurochir (Wien). 1993;121(3–4):95–9.
Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Correlation between CT scan findings and outcome. Acta Neurochir (Wien). 1996;138(2):185–91.
Koc RK, Akdemir H, Oktem IS, Meral M, Menku A. Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev. 1997;20(4):239–44.
Servadei F. Prognostic factors in severely head injured adult patients with acute subdural haematoma’s. Acta Neurochir (Wien). 1997;139(4):279–85.
Dent DL, Croce MA, Menke PG, Young BH, Hinson MS, Kudsk KA, et al. Prognostic factors after acute subdural hematoma. J Trauma. 1995;39(1):36–42 (discussion-3).
Cruz J, Minoja G, Okuchi K. Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses of mannitol: a randomized trial. Neurosurgery. 2001;49(4):864–71.
Zumkeller M, Behrmann R, Heissler HE, Dietz H. Computed tomographic criteria and survival rate for patients with acute subdural hematoma. Neurosurgery. 1996;39(4):708–12 (discussion 12-3).
Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol. 1993;40(1):22–5.
Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir (Wien). 1988;90(3–4):111–6.
Sahuquillo-Barris J, Lamarca-Ciuro J, Vilalta-Castan J, Rubio-Garcia E, Rodriguez-Pazos M. Acute subdural hematoma and diffuse axonal injury after severe head trauma. J Neurosurg. 1988;68(6):894–900.
Depreitere B, Van Lierde C, Sloten JV, Van Audekercke R, Van der Perre G, Plets C, et al. Mechanics of acute subdural hematomas resulting from bridging vein rupture. J Neurosurg. 2006;104(6):950–6.
Gennarelli TA, Thibault LE. Biomechanics of acute subdural hematoma. J Trauma. 1982;22(8):680–6.
Servadei F, Nasi MT, Giuliani G, Cremonini AM, Cenni P, Zappi D, et al. CT prognostic factors in acute subdural haematomas: the value of the ‘worst’ CT scan. Br J Neurosurg. 2000;14(2):110–6.
Shenkin HA. Acute subdural hematoma. Review of 39 consecutive cases with high incidence of cortical artery rupture. J Neurosurg. 1982;57(2):254–7.
Maxeiner H, Wolff M. Pure subdural hematomas: a postmortem analysis of their form and bleeding points. Neurosurgery. 2002;50(3):503–8 (discussion 8-9).
Howard MA 3rd, Gross AS, Dacey RG Jr, Winn HR. Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg. 1989;71(6):858–63.
Kotwica Z, Jakubowski JK. Acute head injuries in the elderly. An analysis of 136 consecutive patients. Acta Neurochir (Wien). 1992;118(3–4):98–102.
Cagetti B, Cossu M, Pau A, Rivano C, Viale G. The outcome from acute subdural and epidural intracranial haematomas in very elderly patients. Br J Neurosurg. 1992;6(3):227–31.
Ludwig S, Warman M. Shaken baby syndrome: a review of 20 cases. Ann Emerg Med. 1984;13(2):104–7.
Ingraham FD, Matson DD. Subdural hematoma in infancy. J Pediatr. 1944;24(1):1–37.
Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharples P, et al. Subdural haemorrhages in infants: population based study. BMJ. 1998;317(7172):1558–61.
Kempe CH, Silverman FN, Steele BF, Droegemueller W, Silver HK. The battered-child syndrome. JAMA. 1962;181:17–24.
Feldman KW, Bethel R, Shugerman RP, Grossman DC, Grady MS, Ellenbogen RG. The cause of infant and toddler subdural hemorrhage: a prospective study. Pediatrics. 2001;108(3):636–46.
Hettler J, Greenes DS. Can the initial history predict whether a child with a head injury has been abused? Pediatrics. 2003;111(3):602–7.
Squier W, Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci Int. 2009;187(1–3):6–13.
Cowan F, Thoresen M. Changes in superior sagittal sinus blood velocities due to postural alterations and pressure on the head of the newborn infant. Pediatrics. 1985;75(6):1038–47.
Geddes JF, Tasker RC, Hackshaw AK, Nickols CD, Adams GG, Whitwell HL, et al. Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol Appl Neurobiol. 2003;29(1):14–22.
Khoshyomn S, Tranmer BI. Diagnosis and management of pediatric closed head injury. Semin Pediatr Surg. 2004;13(2):80–6.
Aoki N, Masuzawa H. Infantile acute subdural hematoma. Clinical analysis of 26 cases. J Neurosurg. 1984;61(2):273–80.
McLaurin RL, Isaacs E, Lewis HP. Results of nonoperative treatment in 15 cases of infantile subdural hematoma. J Neurosurg. 1971;34(6):753–9.
Scotti G, Terbrugge K, Melancon D, Belanger G. Evaluation of the age of subdural hematomas by computerized tomography. J Neurosurg. 1977;47(3):311–5.
de Beer MH, van Gils AP, Koppen H. Mimics of subacute subdural hematoma in the ED. Am J Emerg Med. 2013;31(3):634.e1-3.
Stone JL, Lang RG, Sugar O, Moody RA. Traumatic subdural hygroma. Neurosurgery. 1981;8(5):542–50.
Rosenorn J, Gjerris F. Long-term follow-up review of patients with acute and subacute subdural hematomas. J Neurosurg. 1978;48(3):345–9.
Takeuchi S, Takasato Y, Otani N, Miyawaki H, Masaoka H, Hayakawa T, et al. Subacute subdural hematoma. Acta Neurochir Suppl. 2013;118:143–6.
Galvez JC, Hecht S. Subacute subdural hematoma in a Karate instructor after noncontact head trauma. Curr Sports Med Rep. 2011;10(1):11–3.
Williams KA Jr, Kouloumberis P, Engelhard HH. Subacute subdural hematoma in a 45-year-old woman with no significant past medical history after a roller coaster ride. Am J Emerg Med. 2009;27(4):517.e5-6.
Cameron MM. Chronic subdural haematoma: a review of 114 cases. J Neurol Neurosurg Psychiatry. 1978;41(9):834–9.
Foelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochir (Wien). 1975;32(3–4):247–50.
Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural haematoma in the elderly–a North Wales experience. J R Soc Med. 2002;95(6):290–2.
Ramachandran R, Hegde T. Chronic subdural hematomas–causes of morbidity and mortality. Surg Neurol. 2007;67(4):367–72 (discussion 72-3).
Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54(5):637–45.
Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418–22.
Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223–9.
Friede RL, Schachenmayr W. The origin ofsubdural neomembranes. II. Fine structural of neomembranes. Am J Pathol. 1978;92(1):69–84.
Yamashima T, Yamamoto S. How do vessels proliferate in the capsule of a chronic subdural hematoma? Neurosurgery. 1984;15(5):672–8.
Ito H, Yamamoto S, Komai T, Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976;45(1):26–31.
Ito H, Komai T, Yamamoto S. Fibrinolytic enzyme in the lining walls of chronic subdural hematoma. J Neurosurg. 1978;48(2):197–200.
Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74(7):937–43.
Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011;114(1):72–6.
Lee KS, Shim JJ, Yoon SM, Doh JW, Yun IG, Bae HG. Acute-on-Chronic Subdural Hematoma: not Uncommon Events. J Korean Neurosurg Soc. 2011;50(6):512–6.
Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95(2):256–62.
Huang YH, Yang KY, Lee TC, Liao CC. Bilateral chronic subdural hematoma: what is the clinical significance? Int J Surg. 2013;11(7):544–8.
Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma. 2010;68(3):571–5.
Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2012;154(9):1541–8.
Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98(6):1217–21.
Stanisic M, Lund-Johansen M, Mahesparan R. Treatment of chronic subdural hematoma by burr-hole craniostomy in adults: influence of some factors on postoperative recurrence. Acta Neurochir (Wien). 2005;147(12):1249–56 (discussion 56-7).
Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3):220–5.
Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63(6):1125–9 (discussion 9).
Tanikawa M, Mase M, Yamada K, Yamashita N, Matsumoto T, Banno T, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien). 2001;143(6):613–8 (discussion 8-9).
Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004;27(4):263–6.
Reymond MA, Marbet G, Radu EW, Gratzl O. Aspirin as a risk factor for hemorrhage in patients with head injuries. Neurosurg Rev. 1992;15(1):21–5.
Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39(2):69–72.
Mattle H, Kohler S, Huber P, Rohner M, Steinsiepe KF. Anticoagulation-related intracranial extracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1989;52(7):829–37.
Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902.
Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6):1511–7.
Garber ST, Sivakumar W, Schmidt RH. Neurosurgical complications of direct thrombin inhibitors–catastrophic hemorrhage after mild traumatic brain injury in a patient receiving dabigatran. J Neurosurg. 2012;116(5):1093–6.
Datta NN, Chan KY, Kwok JC, Poon CY. Posterior fossa subdural hematoma due to ruptured arteriovenous malformation. Case report. Neurosurg Focus. 2000;8(6):1–4.
Krishnaney AA, Rasmussen PA, Masaryk T. Bilateral tentorial subdural hematoma without subarachnoid hemorrhage secondary to anterior communicating artery aneurysm rupture: a case report and review of the literature. AJNR Am J Neuroradiol. 2004;25(6):1006–7.
Lasjaunias P, Chiu M, ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64(5):724–30.
Korosue K, Kondoh T, Ishikawa Y, Nagao T, Tamaki N, Matsumoto S. Acute subdural hematoma associated with nontraumatic middle meningeal artery aneurysm: case report. Neurosurgery. 1988;22(2):411–3.
Duffau H, Lopes M, Janosevic V, Sichez JP, Faillot T, Capelle L, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90(1):78–84.
McKenzie CR, Rengachary SS, McGregor DH, Dixon AY, Suskind DL. Subdural hematoma associated with metastatic neoplasms. Neurosurgery. 1990;27(4):619–24 (discussion 24-5).
Mehta AB, Rahemtulla A, Kumaran TO, Marsh GW. Chronic subdural haematoma: possible association with chronic granulocytic leukaemia in lymphoid transformation. Br Med J (Clin Res Ed). 1985;291(6488):108.
Ambiavagar PC, Sher J. Subdural hematoma secondary to metastatic neoplasm: report of two cases and a review of the literature. Cancer. 1978;42(4):2015–8.
Leech RW, Welch FT, Ojemann GA. Subdural hematoma secondary to metastatic dural carcinomatosis. J Neurosurg. 1974;41(5):610–3.
Turner DM, Graf CJ. Nontraumatic subdural hematoma secondary to dural metastasis: case report and review of the literature. Neurosurgery. 1982;11(5):678–80.
Parsch CS, Krauss J, Hofmann E, Meixensberger J, Roosen K. Arachnoid cysts associated with subdural hematomas and hygromas: analysis of 16 cases, long-term follow-up, and review of the literature. Neurosurgery. 1997;40(3):483–90.
Mori K, Yamamoto T, Horinaka N, Maeda M. Arachnoid cyst is a risk factor for chronic subdural hematoma in juveniles: twelve cases of chronic subdural hematoma associated with arachnoid cyst. J Neurotrauma. 2002;19(9):1017–27.
Domenicucci M, Russo N, Giugni E, Pierallini A. Relationship between supratentorial arachnoid cyst and chronic subdural hematoma: neuroradiological evidence and surgical treatment. J Neurosurg. 2009;110(6):1250–5.
Tanaka Y, Mizuno M, Kobayashi S, Sugita K. Subdural fluid collection following craniotomy. Surg Neurol. 1987;27(4):353–6.
Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007;38(9):2506–17.
Yoshimoto Y, Wakai S, Hamano M. External hydrocephalus after aneurysm surgery: paradoxical response to ventricular shunting. J Neurosurg. 1998;88(3):485–9.
Nalbach SV, Ropper AE, Dunn IF, Gormley WB. Craniectomy-associated Progressive Extra-Axial Collections with Treated Hydrocephalus (CAPECTH): redefining a common complication of decompressive craniectomy. J Clin Neurosci. 2012;19(9):1222–7.
Ropper AE, Nalbach SV, Lin N, Dunn IF, Gormley WB. Resolution of extra-axial collections after decompressive craniectomy for ischemic stroke. J Clin Neurosci. 2012;19(2):231–4.
Juttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke. 2007;38(9):2518–25.
Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8(4):326–33.
Aarabi B, Chesler D, Maulucci C, Blacklock T, Alexander M. Dynamics of subdural hygroma following decompressive craniectomy: a comparative study. Neurosurg Focus. 2009;26(6):E8.
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–502.
Smith WS, Weingart S. Emergency Neurological Life Support (ENLS): what to do in the first hour of a neurological emergency. Neurocrit Care. 2012;17(Suppl 1):S1–3.
Seder DB, Riker RR, Jagoda A, Smith WS, Weingart SD. Emergency neurological life support: airway, ventilation, and sedation. Neurocrit Care. 2012;17(Suppl 1):S4–20.
Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998;317(7161):798–801.
Heidegger T, Gerig HJ, Henderson JJ. Strategies and algorithms for management of the difficult airway. Best Pract Res Clin Anaesthesiol. 2005;19(4):661–74.
BrainTraumaFoundation. Guidelines for the management of severe traumatic brain injury: part I. Blood pressure and oxygenation. J Neurotrauma. 2007;24(suppl 1):S7–13.
Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004;255(2):257–65.
Arima H, Anderson CS, Wang JG, Huang Y, Heeley E, Neal B, et al. Lower treatment blood pressure is associated with greatest reduction in hematoma growth after acute intracerebral hemorrhage. Hypertension. 2010;56(5):852–8.
Delcourt C, Huang Y, Wang J, Heeley E, Lindley R, Stapf C, et al. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2). Int J Stroke. 2010;5(2):110–6.
Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–29.
Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–37.
Yanamadala V, Walcott BP, Fecci PE, Rozman P, Kumar JI, Nahed BV, et al. Reversal of warfarin associated coagulopathy with 4-factor prothrombin complex concentrate in traumatic brain injury and intracranial hemorrhage. J Clin Neurosci. 2014;21(11):1881–4.
Campbell PG, Sen A, Yadla S, Jabbour P, Jallo J. Emergency reversal of antiplatelet agents in patients presenting with an intracranial hemorrhage: a clinical review. World Neurosurg. 2010;74(2–3):279–85.
Creutzfeldt CJ, Weinstein JR, Longstreth WT Jr, Becker KJ, McPharlin TO, Tirschwell DL. Prior antiplatelet therapy, platelet infusion therapy, and outcome after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2009;18(3):221–8.
Akwaa F, Spyropoulos AC. Novel oral anticoagulants: a review of the literature and considerations in special clinical situations. Hosp Pract. 2013;41(1):8–18.
British Committee for Standards in Haematology BTTF. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122(1):10–23.
Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746–8.
Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44(Suppl 10):18–20.
Rabinstein AA, Chung SY, Rudzinski LA, Lanzino G. Seizures after evacuation of subdural hematomas: incidence, risk factors, and functional impact. J Neurosurg. 2010;112(2):455–60.
Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502.
Chang BS, Lowenstein DH. Quality Standards Subcommittee of the American Academy of N. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60(1):10–6.
Kruer RM, Harris LH, Goodwin H, Kornbluth J, Thomas KP, Slater LA, et al. Changing trends in the use of seizure prophylaxis after traumatic brain injury: a shift from phenytoin to levetiracetam. J Crit Care. 2013;28(5):883.e9-13.
Radic JA, Chou SH, Du R, Lee JW. Levetiracetam versus phenytoin: a comparison of efficacy of seizure prophylaxis and adverse event risk following acute or subacute subdural hematoma diagnosis. Neurocrit Care. 2014;21(2):228–37.
Sabo RA, Hanigan WC, Aldag JC. Chronic subdural hematomas and seizures: the role of prophylactic anticonvulsive medication. Surg Neurol. 1995;43(6):579–82.
Grobelny BT, Ducruet AF, Zacharia BE, Hickman ZL, Andersen KN, Sussman E, et al. Preoperative antiepileptic drug administration and the incidence of postoperative seizures following bur hole-treated chronic subdural hematoma. J Neurosurg. 2009;111(6):1257–62.
Mathew P, Oluoch-Olunya DL, Condon BR, Bullock R. Acute subdural haematoma in the conscious patient: outcome with initial non-operative management. Acta Neurochir (Wien). 1993;121(3–4):100–8.
Wong CW. Criteria for conservative treatment of supratentorial acute subdural haematomas. Acta Neurochir (Wien). 1995;135(1–2):38–43.
Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31(2):73–9.
Gjerris F, Schmidt K. Chronic subdural hematoma. Surgery or mannitol treatment. J Neurosurg. 1974;40(5):639–42.
Kageyama H, Toyooka T, Tsuzuki N, Oka K. Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg. 2013;119(2):332–7.
Kolias AG, Sinha R, Park H, Santarius T, Hutchinson PJ. Surgical management of chronic subdural hematomas: in need of better evidence. Acta Neurochir (Wien). 2013;155(1):183–4.
Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374(9695):1067–73.
Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. 2014;120(6):1378–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Huang, K.T., Bi, W.L., Abd-El-Barr, M. et al. The Neurocritical and Neurosurgical Care of Subdural Hematomas. Neurocrit Care 24, 294–307 (2016). https://doi.org/10.1007/s12028-015-0194-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0194-x