Abstract
Purpose of review
Subdural hematomas (SDH) represent common neurosurgical problem associated with significant morbidity, mortality, and high recurrence rates. SDH incidence increases with age; numbers of patients affected by SDH continue to rise with our aging population and increasing number of people taking antiplatelet agents or anticoagulation. Medical and surgical SDH management remains a subject of investigation.
Recent findings
Initial management of patients with concern for altered mental status with or without trauma starts with Emergency Neurological Life Support (ENLS) guidelines, with a focus on maintaining ICP < 22 mmHg, CPP > 60 mmHg, MAP 80–110 mmHg, and PaO2 > 60 mmHg, followed by rapid sequence intubation if necessary, and expedited acquisition of imaging to identify a space-occupying lesion. Patients are administered anti-seizure medications, and their antiplatelet medications or anticoagulation may be reversed if neurosurgical interventions are anticipated, or until hemorrhage is stabilized on imaging.
Summary
Medical SDH care focuses on (a) management of intracranial hypertension; (b) maintenance of adequate cerebral perfusion; (c) seizure prevention and treatment; (d) maintenance of normothermia, eucarbia, euglycemia, and euvolemia; and (e) early initiation of enteral feeding, mobilization, and physical therapy. Post-operatively, SDH patients require ICU level care and are co-managed by neurointensivists with expertise in treating increased intracranial pressure, seizures, and status epilepticus, as well as medical complications of critical illness. Here, we review various aspects of medical management with a brief overview of pertinent literature and clinical trials for patients diagnosed with SDH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subdural hematomas (SDH) are a frequently encountered neurosurgical problem with an increasing prevalence and incidence, especially among the elderly. Balser et al. recently reported the overall incidence of 79.4/100,000 person-years with age-standardized incidence of 39.4/100,000 person-years [1, 2•, 3,4,5]. Chronic SDH alone affect 8.2/100,000 people over 70 annually [6]. SDH are more common in men; one fifth occur bilaterally at presentation [5]. Incidence of SDH in infants and toddlers is higher than in children or adults, amounting to 20–40/100,000; over half of these are attributed to nonaccidental trauma, while others result from birth trauma, external hydrocephalus, hematologic disorders, vascular malformations, genetic disorders or infection [7,8,9]. SDH are associated with significant morbidity and as high as 32% mortality, with recurrence rates ranging anywhere from 1 to 33%; mortality rates as high as 60–90% have been reported in patients with acute traumatic SDH and GCS ≤ 8 [7, 10,11,12,13].
SDHs arise within inner dural cellular layer between dura mater and the arachnoid, with subsequent development of thin membranes along their inner border [14], which may result in formation of multiple septations containing varying ages of hemorrhage. This can negatively affect treatment by precluding complete evacuation and promote recurrence. SDH enlarge via leaky endothelial gap junctions that allow blood extravasation due to increased permeability along thick outer SDH membrane, and repeated membrane micro-hemorrhages from their fragile neovasculature [15,16,17]. Pathogenesis of SDH expansion has multiple proposed mechanisms, which include: (a) recurrent hemorrhage due to hyperfibrinolytic state associated with increased fibrinogen levels triggered by tissue plasminogen activator and increased plasmin levels, which induce pro-inflammatory kallikrein system, (b) SDH liquefaction resulting in an osmotic gradient, which when coupled with increased vascular permeability, may increase SDH compartments; and (c) inflammation and neovascularization, which promote new septations that stretch under pressure exerted by increasing SDH content, resulting in further micro-hemorrhages [15,16,17].
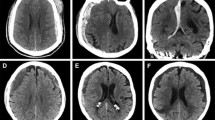
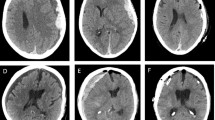
SDHs can be acute (≤ 3 days old), subacute (4 to 20 days) or chronic (≥ 20 days) based on age, and traumatic or spontaneous based on etiology (Fig. 1). They may be unilateral or bilateral, and are diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI). Acute SDH arise from relatively low-pressure venous bleeding due to damaged bridging veins, which may be traumatic or spontaneous, and may cease due to clotting or intracranial hypertension; 20–30% percent of SDH are attributed to arterial rupture [18,19,20]. Prevalence of acute SDH in patients with traumatic brain injury (TBI) is 11–20%, though to result from linear brain acceleration within the skull causing stretch/torque injury to veins or arteries [21,22,23]. Half of these patients present in a coma due to herniation, especially common with posterior fossa hemorrhages; those initially not comatose may undergo further neurologic decline as a result of intracranial hypertension due to compression, decreased perfusion, or ischemia within 6 h after injury [24,25,26].
Conversely, spontaneous SDH are associated with antiplatelet or anticoagulation use, hemorrhagic dural lesions (e.g., metastases, meningiomas) [27, 28], intracranial hypotension [29], ruptured aneurysms, dural arteriovenous fistulas or malformations adherent to the arachnoid with associated arterial bleeding [30, 31], and age- or alcohol-related brain atrophy which places bridging veins under stretch. Pathophysiology of infantile SDH is not entirely understood; the role of venous hypertension associated with fluctuations in venous pressure during head movement due to unfused bony calvarium with resulting ischemia have been proposed as a mechanism leading to endothelial injury [32]. Unfortunately, many pediatric SDH result from nonaccidental trauma, requiring comprehensive investigation of a multi-disciplinary team; poor prognosis associated with nonaccidental TBI reflects the extent of parenchymal injury and significant forces required to sustain SDH [33]. Patients undergoing intracranial surgery, e.g., decompressive hemicraniectomy for malignant stroke or trauma, are at risk of developing symptomatic subdural hygromas due to perturbations in CSF flow dynamics, which often require treatment.
Initial management of SDH patients
Management of patients with acute or symptomatic chronic SDH starts with Advanced Trauma Life Support (ATLS) protocols instituted in the pre-hospital and emergency department setting to maintain circulation, airway, and breathing. The Neuro-Critical Care Society developed Emergency Neurological Life Support (ENLS) guidelines [34] to streamline resuscitation and early physiologic management with recommendations that should be individualized (Fig. 2). Recommendations include MAP goals 80–110 mmHg with SBP goals of 100–180 mmHg to prevent excessive and continued intracranial hemorrhage, while maintaining cerebral perfusion pressure (CPP) of > 60 mmHg in adults and 40–65 mmHg in children, although there is no class I evidence to support these specific parameters [35,36,37,38, 39••, 40••] (Fig. 2). Brain oxygenation to PaO2 > 60 mmHg is of paramount importance, as hypoxia with PaO2 < 30 mmHg results in irreversible damage within 3 min, with concomitant vasodilation and ICP elevation as a result of hypercarbia due to inadequate ventilation; however, hyperoxia (PaO2 > 300 mmHg) can be equally damaging with resulting cerebral ischemia due to vasoconstriction [39••, 41]. Adequate oxygenation may require intubation when severe extracranial injuries are present, or if the patient is agitated, intoxicated or has a declining mental status. Intubation is best achieved with pre-oxygenation to SpO2 ≥ 90%, followed by rapid sequence intubation with concomitant sedation and neuromuscular blockade to minimize the risk of aspiration [39••, 42,43,44,45]. Induction with 0.3–0.4 mg/kg etomidate (sedative, hypnotic), 1–2.5 mg/kg propofol (anesthetic, hypnotic, sedative), or 1.5–2 mg/kg ketamine (dissociative, anesthetic) in combination with 2–3 μg/kg fentanyl (analgesic) is frequently used in the trauma setting, with 1.5 mg/kg IV lidocaine administered to prevent airway manipulation-associated increases in ICP [39••]. Neuromuscular blockade is achieved with 1.5–2 mg/kg short acting IV succinylcholine, or longer-acting non-depolarizing agents, e.g., 1.2–1.4 mg/kg rocuronium or 0.15 mg/kg cisatracurium [39••]. Upon intubation, sedation should be titrated to a RASS of 0 to 2 depending on the clinical scenario (e.g., paroxysmal sympathetic hyperactivity, ventilator dyssynchrony, intracranial hypertension). Commonly used sedatives include propofol, dexmedetomidine, or midazolam, while fentanyl or remifentanil are used as analgesia adjuncts.
Imaging
Following resuscitation, patients with AMS require intracranial imaging. Widely available rapid CT imaging has over 96% sensitivity and 98% specificity, while MRI is nearly 100% sensitive in patients with thin SDH along falx or tentorium, and can identify underlying etiologies (e.g., vascular or neoplastic). Intracranial imaging provides information regarding chronicity and size of hemorrhage, presence of midline shift, ventricular trapping, and basal cistern effacement. Patients suspected to have a vascular lesion may require CTA, MRA, or conventional digital subtraction angiography. Clinical deterioration (e.g., decreasing level of arousal, sluggish or dilated pupils, or extensor posturing) reflects intracranial hypertension, for which emergent imaging and surgical intervention could be life-saving.
Management of intracranial hypertension
Intracranial hypertension with increased intracranial pressure (ICP ≥ 22) is associated with worse outcomes and higher mortality rates in TBI patients; ICP values as high as 40–85 mmHg have been reported [46, 47]. Thus, following a treatment algorithm to ensure timely initiation of pressure lowering strategies, including surgical decompression, is critical (Fig. 2). According to the Monro-Kellie doctrine, fixed intracranial vault containing brain parenchyma, blood, and cerebrospinal fluid must accommodate any increase in volume through displacement of one or more of the other components. SDH, often with associated edema, functions mechanically as a space-occupying lesion that can exhaust the brain’s compliance through autoregulatory failure, resulting in intracranial hypertension. Simple initial measures for ICP management include head-of-bed elevation to ≥ 90° and loosening the cervical collar to prevent venous outflow obstruction [25]; these can be followed by short-term hyperventilation to a PaCO2 of 28–30 mmHg to decrease ICPs via vasoconstriction [48, 49].
Use of hypertonics (30–120 cm3 of 23.4% saline) or hyperosmolar therapy (0.5–1.5 g/kg mannitol) which result in osmotic gradients and promote fluid shifts from intracellular and intercellular brain compartments into circulation may be counterproductive, and are used only as a temporizing measure until emergent surgical interventions can be implemented. EVD placement is not recommended, as CSF drainage may increase stretch on the draining veins. Long-term use of hyperventilation may not be beneficial due to resulting respiratory alkalosis and secondary ischemia, yet it remains an appropriate adjunct in an acutely deteriorating patient on the way to the operating room. In a recent RCT evaluating the utility of maintaining PaCO2 goals of 25 mmHg for 5 days in patients with TBI, hyperventilation correlated with worse clinical outcomes [50]; however, it may be successfully used to treat reactive post-operative hyperemia resulting from ICP elevation [51].
Likewise, while Na levels of > 165 mEq/L have been associated with poor outcomes, there is no data supporting specific sodium levels or osmolality to correlate with effectiveness of medical ICP management. Choices between hypertonic vs hyperosmolar therapy are made depending on volume status, blood pressure, or medical history (e.g., congestive heart failure, renal failure), making hypertonic therapy the preferred option in hypotensive patients with low volume status, while mannitol is preferred in patients with congestive heart failure. Several institutions across the country are participating in HOPES trial, an RCT investigating the effects of hypothermia to 33 °C on TBI patients with acute surgical SDH, that may have a positive effect by decreasing ICPs through reduction in cerebral blood flow and improve cerebral edema via decreasing metabolic demands [52••]. Recently completed RCT EuroTherm reported a reduction in the number of emergent decompressive hemicraniectomies and necessity for barbiturate coma for ICP control, but suggested that cooling TBI patients to 32–34 °C may result in more complications, including infections and arrhythmias [52••, 53].
Anti-seizure drugs
Administration of prophylactic anti-seizure drugs (ASDs) remains controversial. Some literature reports that 25% of traumatic SDH patients and 11–19% of chronic SDH patients develop seizures either at presentation or immediately post-operatively, especially with SDH involving the tentorium, temporal lobe, or other highly epileptogenic areas [54, 55] (Fig. 2). The Guidelines for the Management of Severe TBI provide level IIA recommendations for phenytoin use to decrease the incidence of early post-traumatic seizures [56•]; seizures can result in increased ICPs and high tissue metabolic demands [57, 58]. Other studies report much lower rates of seizures of 2% [59, 60]. In a retrospective review of 129 patients, 73 of which received prophylactic phenytoin, 2 patients in the no-ASD group developed post-operative seizures, both thought to be related to surgical technique [59]. Phenytoin administration reduced seizure incidence from 14 to 4% according to an RCT comparing phenytoin vs levetiracetam, suggesting similar efficacy [57]. Several observational studies show a more dramatic reduction in seizure incidence from 32 to 2.4% in 98 patients treated with phenytoin [55]. While phenytoin/fosphenytoin were the agents of choice due to convenient route of administration and benign side effect profile, levetiracetam is becoming the ASD of choice with similar effectiveness. Valproate may benefit patients with behavioral issues or psychiatric history [61, 62]. A prospective study of surface and depth EEG in conjunction with microdialysis in 34 patients with severe TBI, all requiring emergent surgical interventions, reported 61% seizures or pseudoperiodic discharges, 42.9% of which were detected on depth EEG, with resulting metabolic crisis as evident from the elevated microdialysis lactate to pyruvate ratios when prolonged [63]. A new international multicenter translational study termed EpiBioS4Rx (The Epilepsy Bioinformatics Study for Antiepileptogenic Therapy) is underway, aimed to understand epileptogenesis in TBI and identify biomarkers of epileptogenesis in animal models and patients [64].
There is little data to support prophylactic ASD use in the elderly or chronic SDH patients. Retrospective analysis of 218 chronic SDH patients undergoing burr hole SDH evacuation reported 13.7% seizure incidence in mixed density chronic SDH group, as compared to 2.4% isodense chronic SDH group, suggesting the correlation between imaging characteristics and seizure risk [65]. Won et al. proposed GATE-24 scoring system based on 24-h post-operative GCS, anticoagulation use, time to OR, and need for prior ASDs, to help assess seizure risk [66]. Patients with acute SDH with GCS 14-15 not on anticoagulation and taken to the OR within 24 h do not require ASDs [66]. A retrospective cohort study of 88 chronic SDH patients undergoing burr hole evacuation, 71% (60/88) of which received ASDs, reported 12.5% incidence of seizures in 11/88 patients, with pre-operative ASD administration being a single predictor of post-operative seizures, suggestive that timing of ASD administration may play a role [67]. However, the exact duration of ASD prophylaxis remains unclear. In patients with no documented post-TBI seizures, ASDs are stopped prior to hospital discharge or weaned as an outpatient. While ASDs are indicated in patients with seizures and low seizure threshold, e.g., alcohol or TBI affecting highly epileptogenic brain areas, ASD use has been associated with increased fall risk in patients > 65 years of age and should be used judiciously and dosed appropriately for patient comorbidities, e.g., renal disease [68]. Continuous or spot EEG may be beneficial in these settings to assess need for ASD use.
Anticoagulation/antiplatelet use
The incidence of spontaneous acute SDH has been steadily growing in our aging population, who are at a higher risk of developing SDH due to medical comorbidities that require oral anticoagulant (AC) or antiplatelet agents, many of which cannot be easily reversed [69, 70, 71•] (Fig. 2). Timely AC reversal is the cornerstone of acute medical management in patients with acute SDH to prevent hematoma expansion and poor functional outcomes [70, 71•]. Patients taking coumadin or other vitamin K antagonists (VKA) should receive 10 mg vitamin K, followed by four-factor prothrombin complex concentrate (PCC) at 25–50 u/kg depending on patient’s INR, with recombinant factor VIIa available for emergency reversal of warfarin in the setting of acute SDH [72,73,74,75]. Reversal of direct thrombin inhibitors (DTIs, e.g., dabigatran) or factor Xa inhibitors (e.g., apixaban, rivaroxaban, edoxaban) in patients with acute SDH remain problematic; FDA approved monoclonal antibody agent idarucizumab (Praxbind) for DTIs (phase III trial RE-VERSE AD), andexanet alfa for fXa inhibitors rivaroxaban/apixaban, and PER977 for edoxaban (phase II trial, awaiting FDA approval) are under investigation; dialysis or plasmapheresis remain other available options [71•,76,77,78,79].
PATCH trial compared standard of care with platelet transfusion in acute SDH patients on antiplatelet agents and showed worse outcomes and increased risk of death in patients who received platelet transfusions. Current recommendations suggest avoiding platelet transfusion, unless the patient requires neurosurgical interventions or is thrombocytopenic (defined as < 75,000 platelet count) [71•, 80]. Additional 0.4 μg/kg ddAVP may be administered to patients with acute SDH [81]. Nearly one third of severe TBI patients (GCS = < 8) manifest profound coagulopathy in the setting of a presumed massive systemic release of tissue factor and phospholipids. This causes consumptive coagulopathy referred to as disseminated intravascular coagulation (DIC), which can be abated by transfusion of coagulation factors and cryoprecipitate (including fibrinogen) with plasma or PCC [82, 83].
A recent retrospective study of 116 acute SDH patients by Won et al. reported 65% of patients taking AC (54.1% VKA, 37.8% thrombocyte inhibitors, 8.1% direct oral anticoagulants), associated with older age, > 4 comorbidities (atrial fibrillation, hypertension, diabetes), higher likelihood of remaining comatose 24 h post-operatively, and poorer functional outcomes [66]. In this study, patients taking thrombocyte inhibitors were given 0.4 μg/kg ddAVP, 1–2 g tranexamic acid, 2u platelets pre-operatively, while other patients received 50 IU/kg PCC and 10 mg vitamin K. Patients on DTIs had the highest degree of midline shift (mean 11.4 mm), while patients on thrombocyte inhibitors had highest SDH recurrence (39.2%). While perioperative mortality was highest in patients on DTIs (OR 3.3), patients on VKAs had the highest 6-month mortality rates (OR 2.7) [66].
AC resumption remains an unaddressed issue. Retrospective studies suggest that rates of thrombotic complications are similar for patients who are not restarted on AC post-operatively as compared to those who do not take AC at baseline [84,85,86,87,88,89]. Kawamata et al. reported that in 27 patients with intracranial hemorrhage (11/27 chronic, 3/27 acute SDH) associated with coumadin use, no SDH recurrence was reported after starting AC 3 days post-operatively [85]. Other studies reported no change in morbidity and recurrence in patients that underwent pre-operative AC reversal [86]. Based on recommendations from American College of Chest Physicians for high- and low-risk patients, sequential compression devices and low-dose unfractionated heparin (HSQ) should be initiated within 12–24 h post-operatively; however, resumption of full dose AC for high-risk patients needs to be addressed on an individual basis, accounting for the degree of hemostasis achieved intra-operatively and risk of SDH recurrence [87]. Similarly, patients on antiplatelet agents did not differ in outcomes if reversed with platelets/ddAVP pre-operatively and antiplatelet agent restarted 1 week post-operatively [88].
General ICU care
Patients are started on subcutaneous heparin (HSQ) prophylaxis after SDH stability has been established on serial imaging, preferably within 72 h (Fig. 2). Normothermia is achieved with Tylenol for fever control (NSAIDs avoided due to their inhibitory effects on platelet cyclooxygenase and impaired platelet aggregation) and a surface-counterwarming blanket, as needed. Glucose levels are maintained within 120–180 range. Patients are kept euvolemic with isotonic fluids to avoid fluid shifts, which could exacerbate cerebral edema and raise ICP. Patients are started on enteral feeds via nasogastric tube, or if able taking PO, within 24-48 h. Proton pump inhibitors or H2 receptor blockers are used to avoid stress ulcer formation and GI bleeding in high-risk ICU patients (e.g., intubated, unfed, taking AC/steroids).
Recent clinical trials address contributions of various non-surgical factors on functional outcomes in SDH patients, including SDH recurrence, or even preventing the need for surgical interventions altogether. Dexamethasone was proposed as an adjunct to help reduce SDH recurrence or avoid surgical interventions [90,91,92]. Pilot RCT investigated 12 mg/day dexamethasone administration for 3 weeks (followed by a taper) and showed no benefit [93, 94], with patients developing side effects of chronic steroid use. Double blind RCT DRESH of 820 patients undergoing surgical interventions for chronic SDH is underway to help determine the utility of 4–16 mg/day dexamethasone use 48 h post-operatively to help reduce chronic SDH recurrence [93, 94]. SDH recurrence has been associated with pneumocephalus and increased serum levels of IL-6, IL-8, fibrinogen, and D-dimer [95]. Park et al. analyzed fibrinogen (key in fibrin clot formation) and D-dimer (cross-linked fibrin degradation product with anti-thrombin effects inhibiting fibrin polymerization) levels in 31 chronic SDH patients undergoing burr hole evacuation with drain placement, and implicated their elevated levels with evolution of mixed density SDH, suggesting that targeting this pathway may be beneficial [96].
Other studies investigated the association between SDH biology and inflammatory state. COXIBRAIN is a prospective randomized phase II/III study of chronic SDH patients designed to address the utility of Cox-2 inhibitors to reduce SDH recurrence by inhibiting VEGF-mediated neovascularization of SDH membranes, in which elevated VEGF levels are thought to depend on Cox-2-mediated eicosanoid, prostaglandin and thromboxane synthesis. This study was terminated prematurely due to (a) prior Cox-2 inhibitor use for rheumatic disease in 55% of patients, who developed chronic SDH, and (b) strict contraindications for Cox-2 inhibitor use in 66.6% patients (only 9.3% of chronic SDH patients eligible for the study) [97]. An ongoing TRACS trial, a multicenter double blind RCT, is investigating the use of 750 mg tranexamic acid (TXA) for a maximum of 20 weeks in chronic SDH patients to assess chronic SDH resolution without surgical procedures [98]. TXA may inhibit fibrinolysis and SDH-associated inflammatory response possibly by binding plasminogen, with resulting decrease in plasmin levels and reduced activation of the pro-inflammatory kallikrein system [99]. Multicenter double blind RCT titled ATOCH is investigating the utility of 20 mg nightly atorvastatin for 8 weeks in chronic SDH patients due to its proposed anti-inflammatory effects [100].
Conclusions
Subdural hematomas remain a common neurosurgical problem requiring specialized aspects of neuro-critical care tailored to the patient population affected by this disease. While medical management of patients with subdural hematomas has been outlined in broad strokes over the years, multiple variables in neuro-critical care remain under investigation, with newer research studies focusing on implementation of targeted medical therapies based on the understanding of underlying disease processes. Patients with subdural hematomas are served best by specialized multi-disciplinary management with a focus on individualized decision-making, with appropriate pre-operative and post-operative medical management.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural haematoma in the elderly—a North Wales experience. J R Soc Med. 2002 Jun;95(6):290–2.
• Balser D, Farooq S, Mehmood T, et al. Actual and projected incidence rates for chronic subdural hematomas in United States veterans administration and civilian populations. J Neurosurg. 2015;123(5):1209–15. A recent study of SDH incidence.
Bourgeois P, Sleiman M, Louis E, Haddad E, Touzet G, Fichten A, et al. Chronic subdural hematoma in patients over 80 years of age. Neurochirurgie. 1999;45(2):124–8.
Cousseau DH, Echevarría Martín G, Gaspari M, Gonorazky SE. Chronic and subacute subdural haematoma. An epidemiological study in a captive population. Rev Neurol. 2001;32(9):821–4.
Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992;32(4):207–9.
Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002;78(916):71–5.
Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet. 2000;356(9241):1571–2.
Talvik I, Metsvaht T, Leito K, Põder H, Kool P, Väli M, et al. Inflicted traumatic brain injury (ITBI) or shaken baby syndrome (SBS) in Estonia. Acta Paediatr. 2006;95(7):799–804.
Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharples P, et al. Subdural haemorrhages in infants: population based study. BMJ. 1998;317(7172):1558–61.
Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418–22.
Tanikawa M, Mase M, Yamada K, Yamashita N, Matsumoto T, Banno T, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir. 2001;143(6):613–8.
Seelig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981;304(25):1511–8.
Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Correlation between CT scan findings and outcome. Acta Neurochir. 1996;138(2):185–91.
Haines DE, Harkey HL, Al-Mefty O. The “subdural” space: a new look at an outdated concept. Neurosurgery. 1993;32(1):111–20.
Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975;43(5):569–78.
Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 1983;59(2):298–303.
Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61(6):523–7.
Gennarelli TA, Thibault LE. Biomechanics of acute subdural hematoma. J Trauma. 1982;22(8):680–6.
Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir. 1988;90(3–4):111–6.
Maxeiner H, Wolff M. Pure subdural hematomas: a postmortem analysis of their form and bleeding points. Neurosurgery. 2002;50(3):503–8.
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Surgical Management of Traumatic Brain Injury Author Group. Neurosurgery. 2006;58(3 Suppl):S16–24.
Servadei F, Nasi MT, Cremonini AM, Giuliani G, Cenni P, Nanni A. Importance of a reliable admission Glasgow Coma Scale score for determining the need for evacuation of posttraumatic subdural hematomas: a prospective study of 65 patients. J Trauma. 1998;44(5):868–73.
Servadei F, Nasi MT, Giuliani G, Cremonini AM, Cenni P, Zappi D, et al. CT prognostic factors in acute subdural haematomas: the value of the ‘worst’ CT scan. Br J Neurosurg. 2000;14(2):110–6.
Schreiber MA, Aoki N, Scott BG, Beck JR. Determinants of mortality in patients with severe blunt head injury. Arch Surg. 2002;137(3):285–90.
Mayer SA, Chong JY. Critical care management of increased intracranial pressure. J Int Care Med. 2002;17(2):55–67.
Salvant JB Jr, Muizelaar JP. Changes in cerebral blood flow and metabolism related to the presence of subdural hematoma. Neurosurgery. 1993;33(3):387–93.
Bergmann M, Puskas Z, Kuchelmeister K. Subdural hematoma due to dural metastasis: case report and review of the literature. Clin Neurol Neurosurg. 1992;94(3):235–40.
Okuno S, Touho H, Ohnishi H, Karasawa J. Falx meningioma presenting as acute subdural hematoma: case report. Surg Neurol. 1999;52(2):180–4.
Beck J, Gralla J, Fung C, Ulrich CT, Schucht P, Fichtner J, et al. Spinal cerebrospinal fluid leak as the cause of chronic subdural hematomas in nongeriatric patients. J Neurosurg. 2014;121(6):1380–7.
Rengachary SS, Szymanski DC. Subdural hematomas of arterial origin. Neurosurgery. 1981 Feb;8(2):166–72.
Inamasu J, Saito R, Nakamura Y, Ichikizaki K, Suga S, Kawase T, et al. Acute subdural hematoma caused by ruptured cerebral aneurysms: diagnostic and therapeutic pitfalls. Resuscitation. 2002;52(1):71–6.
Cowan F, Thoresen M. Changes in superior sagittal sinus blood velocities due to postural alterations and pressure on the head of the newborn infant. Pediatrics. 1985;75(6):1038–47.
Geddes JF, Tasker RC, Hackshaw AK, Nickols CD, Adams GG, Whitwell HL, et al. Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol Appl Neurobiol. 2003;29(1):14–22.
Smith MD, Kishikova L, Norris JM. Surgical management of chronic subdural haematoma: one hole or two? Int J Surg. 2012;10(9):450–2.
Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. INTERACT2 investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–65.
Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(Suppl 1):S59–64.
Elf K, Nilsson P, Ronne-Engström E, Howells T, Enblad P. Cerebral perfusion pressure between 50 and 60 mm Hg may be beneficial in head-injured patients: a computerized secondary insult monitoring study. Neurosurgery. 2005;56(5):962–71.
Jaeger M, Dengl M, Meixensberger J, Schuhmann MU. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med. 2010;38(5):1343–7.
•• Rajajee V, Riggs B, Seder DB. Emergency neurological life support: airway, ventilation, and sedation. Neurocrit Care. 2017;27(Suppl 1):4–28. A summary of critical care guidelines for initial resuscitation of neurologically ill patient.
•• Garvin R, Mangat HS. Emergency neurological life support: severe traumatic brain injury. Neurocrit Care. 2017;27(Suppl 1):159–69. A summary of critical care guidelines for patients with severe traumatic brain injury.
Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998;317(7161):798–801.
Kortbeek JB, Al Turki SA, Ali J, Antoine JA, Bouillon B, Brasel K, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64(6):1638–50.
Li J, Murphy-Lavoie H, Bugas C, Martinez J, Preston C. Complications of emergency intubation with and without paralysis. Am J Emerg Med. 1999;17(2):141–3.
Tayal VS, Riggs RW, Marx JA, Tomaszewski CA, Schneider RE. Rapid-sequence intubation at an emergency medicine residency: success rate and adverse events during a two-year period. Acad Emerg Med. 1999;6(1):31–7.
Bernard SA, Nguyen V, Cameron P, Masci K, Fitzgerald M, Cooper DJ, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann Surg. 2010;252(6):959–65.
Badri S, Chen J, Barber J, Temkin NR, Dikmen SS, Chesnut RM, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38(11):1800–9.
Verweij BH, Muizelaar JP, Vinas FC. Hyperacute measurement of intracranial pressure, cerebral perfusion pressure, jugular venous oxygen saturation, and laser Doppler flowmetry, before and during removal of traumatic acute subdural hematoma. J Neurosurg. 2001;95(4):569–72.
Coles JP, Minhas PS, Fryer TD, Smielewski P, Aigbirihio F, Donovan T, et al. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30(9):1950–9.
Marion DW, Puccio A, Wisniewski SR, Kochanek P, Dixon CE, Bullian L, et al. Effect of hyperventilation on extracellular concentrations of glutamate, lactate, pyruvate, and local cerebral blood flow in patients with severe traumatic brain injury. Crit Care Med. 2002;30(12):2619–25.
Muizelaar JP, Marmarou A, Ward JD, Kontos HA, Choi SC, Becker DP, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75(5):731–9.
Obrist WD, Langfitt TW, Jaggi JL, Cruz J, Gennarelli TA. Cerebral blood flow and metabolism in comatose patients with acute head injury. Relationship to intracranial hypertension. J Neurosurg. 1984;61(2):241–53.
•• Clinicaltrials.gov. Bethesda (MD): United States National Library of Medicine http://clinicaltrials.gov Accessed April 2018. Comprehensive database of past and ongoing clinical trials.
Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Eurotherm3235 trial collaborators. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373(25):2403–12.
Rabinstein AA, Chung SY, Rudzinski LA, Lanzino G. Seizures after evacuation of subdural hematomas: incidence, risk factors, and functional impact. J Neurosurg. 2010;112(2):455–60.
Sabo RA, Hanigan WC, Aldag JC. Chronic subdural hematomas and seizures: the role of prophylactic anticonvulsive medication. Surg Neurol. 1995;43(6):579–82.
• Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. Additional guidelines for patients with traumatic brain injury.
Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502.
Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. 2001;4:CD000173.
Ohno K, Maehara T, Ichimura K, Suzuki R, Hirakawa K, Monma S. Low incidence of seizures in patients with chronic subdural haematoma. J Neurol Neurosurg Psychiatry. 1993;56(11):1231–3.
Rubin G, Rappaport ZH. Epilepsy in chronic subdural haematoma. Acta Neurochir. 1993;123(1–2):39–42.
Inaba K, Menaker J, Branco BC, Gooch J, Okoye OT, Herrold J, et al. A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. J Trauma Acute Care Surg. 2013;74(3):766–71.
Radic JA, Chou SH, Du R, Lee JW. Levetiracetam versus phenytoin: a comparison of efficacy of seizure prophylaxis and adverse event risk following acute or subacute subdural hematoma diagnosis. Neurocrit Care. 2014;21(2):228–37.
Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79(4):579–90.
EpiBioS4Rx. USC Mark and Mary Stevens Neuroimaging and Informatics Institute. http://epibios.loni.usc.edu Accessed April 2018.
Chen CW, Kuo JR, Lin HJ, Yeh CH, Wong BS, Kao CH, et al. Early post-operative seizures after burr-hole drainage for chronic subdural hematoma: correlation with brain CT findings. J Clin Neurosci. 2004;11(7):706–9.
Won SY, Dubinski D, Bruder M, Cattani A, Seifert V, Konczalla J. Acute subdural hematoma in patients on oral anticoagulant therapy: management and outcome. Neurosurg Focus. 2017;43(5):E12.
Grobelny BT, Ducruet AF, Zacharia BE, Hickman ZL, Andersen KN, Sussman E, et al. Preoperative antiepileptic drug administration and the incidence of postoperative seizures following bur hole-treated chronic subdural hematoma. J Neurosurg. 2009;111(6):1257–62.
Ferreri S, Roth MT, Casteel C, Demby KB, Blalock SJ. Methodology of an ongoing, randomized controlled trial to prevent falls through enhanced pharmaceutical care. Am J Geriatr Pharmacother. 2008;6(2):61–81.
Gaist D, García Rodríguez LA, Hellfritzsch M, Poulsen FR, Halle B, Hallas J, et al. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 2017;317(8):836–46.
Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39(2):69–72.
• Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: executive summary. A statement for healthcare professionals from the Neurocritical Care Society and the Society of Critical Care Medicine. Crit Care Med. 2016;44(12):2251–7. Thorough review of guidelines for reversal of antithrombotics in patients with intracranial hemorrhage.
Chai-Adisaksopha C, Hillis C, Siegal DM, Movilla R, Heddle N, Iorio A, et al. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal. A systematic review and meta-analysis. Thromb Haemost. 2016;116(5):879–90.
Desborough MJ, Oakland KA, Landoni G, Crivellari M, Doree C, Estcourt LJ, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2017;15(2):263–72.
Narayan RK, Maas AI, Marshall LF, Servadei F, Skolnick BE, Tillinger MN. rFVIIa traumatic ICH study group. Recombinant factor VIIA in traumatic intracerebral hemorrhage: results of a dose-escalation clinical trial. Neurosurgery. 2008;62(4):776–86.
Veshchev I, Elran H, Salame K. Recombinant coagulation factor VIIa for rapid preoperative correction of warfarin-related coagulopathy in patients with acute subdural hematoma. Med Sci Monit. 2002;8(12):CS98–100.
Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017 Aug 3;377(5):431–41.
Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso M, Brown K, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371(22):2141–2.
Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–24.
Garber ST, Sivakumar W, Schmidt RH. Neurosurgical complications of direct thrombin inhibitors--catastrophic hemorrhage after mild traumatic brain injury in a patient receiving dabigatran. J Neurosurg. 2012;116(5):1093–6.
Nishijima DK, Zehtabchi S, Berrong J, Legome E. Utility of platelet transfusion in adult patients with traumatic intracranial hemorrhage and preinjury antiplatelet use: a systematic review. J Trauma Acute Care Surg. 2012;72(6):1658–63.
Naidech AM, Maas MB, Levasseur-Franklin KE, Liotta EM, Guth JC, Berman M, et al. Desmopressin improves platelet activity in acute intracerebral hemorrhage. Stroke. 2014;45(8):2451–3.
Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir. 2008;150(2):165–75.
Allard CB, Scarpelini S, Rhind SG, Baker AJ, Shek PN, Tien H, et al. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma. 2009;67(5):959–67.
Göksu E, Akyüz M, Uçar T, Kazan S. Spontaneous resolution of a large chronic subdural hematoma: a case report and review of the literature. Ulus Travma Acil Cerrahi Derg. 2009;15(1):95–8.
Kawamata T, Takeshita M, Kubo O, Izawa M, Kagawa M, Takakura K. Management of intracranial hemorrhage associated with anticoagulant therapy. Surg Neurol. 1995;44(5):438–42.
Stanisic M, Lund-Johansen M, Mahesparan R. Treatment of chronic subdural hematoma by burr-hole craniostomy in adults: influence of some factors on postoperative recurrence. Acta Neurochir. 2005;147(12):1249–56.
Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ. Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):71S–109S.
Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63(6):1125–9.
Rust T, Kiemer N, Erasmus A. Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci. 2006;13(8):823–7.
Yao Z, Hu X, Ma L, You C. Dexamethasone for chronic subdural haematoma: a systematic review and meta-analysis. Acta Neurochir. 2017;159(11):2037–44.
Qian Z, Yang D, Sun F, Sun Z. Risk factors for recurrence of chronic subdural hematoma after burr hole surgery: potential protective role of dexamethasone. Br J Neurosurg. 2017;31(1):84–8.
Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, Fernández-Arconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur). 2009;20(4):346–59.
Prud'homme M, Mathieu F, Marcotte N, Cottin S. A pilot placebo controlled randomized trial of dexamethasone for chronic subdural hematoma. Can J Neurol Sci. 2016;43(2):284–90.
Emich S, Richling B, McCoy MR, Al-Schameri RA, Ling F, Sun L, et al. The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural hematoma—the DRESH study: straightforward study protocol for a randomized controlled trial. Trials. 2014;15:6.
Frati A, Salvati M, Mainiero F, Ippoliti F, Rocchi G, Raco A, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100(1):24–32.
Park SH, Kang DH, Park J, Hwang JH, Hwang SK, Sung JK, et al. Fibrinogen and D-dimer analysis of chronic subdural hematomas and computed tomography findings: a prospective study. Clin Neurol Neurosurg. 2011;113(4):272–6.
Schaumann A, Klene W, Rosenstengel C, Ringel F, Tüttenberg J, Vajkoczy P. COXIBRAIN: results of the prospective, randomised, phase II/III study for the selective COX-2 inhibition in chronic subdural haematoma patients. Acta Neurochir. 2016;158(11):2039–44.
Iorio-Morin C, Blanchard J, Richer M, Mathieu D. Tranexamic acid in chronic subdural hematomas (TRACS): study protocol for a randomized controlled trial. Trials 2016;17(1):235.
Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991;5(5):467–73.
Jiang R, Wang D, Poon WS, Lu YC, Li XG, Zhao SG, et al. Effect of atorvastatin on chronic subdural hematoma (ATOCH): a study protocol for a randomized controlled trial. Trials. 2015;16:528.
Acknowledgements
The editors would like to thank Dr. Myrna Rosenfeld for taking the time to review this manuscript. Elena I Fomchenko, Charles C Matouk, and Jason L Gerrard are supported by Yale Neurosurgery. Emily J Gilmore and Kevin N Sheth are supported by Yale Department of Clinical Neurosciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elena I Fomchenko, Emily J Gilmore, Charles C Matouk, and Jason L Gerrard, each declare no conflicts of interest.
Kevin N Sheth is a section editor for Current Treatment Options in Neurology.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
About this article
Cite this article
Fomchenko, E.I., Gilmore, E.J., Matouk, C.C. et al. Management of Subdural Hematomas: Part I. Medical Management of Subdural Hematomas. Curr Treat Options Neurol 20, 28 (2018). https://doi.org/10.1007/s11940-018-0517-2
Published:
DOI: https://doi.org/10.1007/s11940-018-0517-2