Abstract
Recognition of self-antigen and its destruction by the immune system is the hallmark of autoimmune diseases. During the developmental stages, immune cells are introduced to the self-antigen, for which tolerance develops. The inflammatory insults that break the immune tolerance provoke immune system against self-antigen, progressively leading to autoimmune diseases. SH2 domain containing protein tyrosine phosphatase (PTP), SHP-1, was identified as hematopoietic cell-specific PTP that regulates immune function from developing immune tolerance to mediating cell signaling post-immunoreceptor activation. The extensive research on SHP-1-deficient mice elucidated the diversified role of SHP-1 in immune regulation, and inflammatory process and related disorders such as cancer, autoimmunity, and neurodegenerative diseases. The present review focalizes upon the implication of SHP-1 in the pathogenesis of autoimmune disorders, such as allergic asthma, neutrophilic dermatosis, atopic dermatitis, rheumatoid arthritis, and multiple sclerosis, so as to lay the background in pursuance of developing therapeutic strategies targeting SHP-1. Also, new SHP-1 molecular targets have been suggested like SIRP-α, PIPKIγ, and RIP-1 that may prove to be the focal point for the development of therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In concurrence with Newton’s third law of motion, protein tyrosine phosphatases (PTPs) antagonize the action of protein tyrosine kinases (PTKs), thereby maintaining steady state of tyrosine phosphorylation of proteins required for cellular homeostasis. Due to this, research on PTPs function has tremendously accelerated because they are potential therapeutic targets in diseases involving uncontrolled PTKs function [1]. Human genome mapping has revealed more than 100 phosphatases belonging to the PTP family which is further divided into four subfamilies based on substrate specificity (Fig. 1). These are (a) class I, largest group of PTPs further subdivided into classical or dual-specific PTPs, (b) class II or low molecular weight PTPs, (c) class III or cysteine-based PTPs, and (d) class IV or pTyr-based PTPs [2] (Fig. 1). The entire members of PTP superfamily contain a highly conserved active site sequence C(X)5R known as PTP signature motif and similar core structures made of a central parallel β-sheet with flanking α-helices containing a β-loop–α-loop that encompasses the PTP signature motif [3]. Although PTPs encompass structural similarity, functional diversity is achieved through regulatory domains and subunits.
Based on cellular localization, the class I classical PTPs are further subdivided into receptor tyrosine phosphatases (RPTPs) or nonreceptor tyrosine phosphatases (NRPTPs). The receptor tyrosine phosphatases possess transmembrane topology comprising an extracellular domain, a single membrane spanning region, and one or two cytoplasmic PTPase domains [4]. CD45, a well-known regulator of T cell development and TCR signaling, is the best characterized receptor tyrosine phosphatase. On the other hand, the nonreceptor tyrosine phosphatases are intracellular enzymes localized in different cell organelles and comprises a catalytic PTPase domain flanked by various N and C terminal residues engaged in secondary and tertiary structure formation like in SH2 domain [4]. One such cytosolic PTP is SHP-1. SH2 domain containing tyrosine phosphatase (SHP-1) is a 595-amino acid residue long cytosolic protein tyrosine phosphatase expressed primarily in hematopoietic cell [5]. It contains two N-terminal-located SH2 domains, a single catalytic domain and a C terminal tail bearing two tyrosine residues for phosphorylation [5]. Figure 2 diagrammatically represents domain organization of SHP-1 in open (activated) and closed (inactivated) states. Another member of SH2 domain containing PTPs family is SHP-2 that has similar structural topology to SHP-1, but unlike SHP-1 it is ubiquitously expressed. Beside structural homology, the cellular function performed by these enzymes differs markedly [6]. This is due to the difference in the amino acid residues forming SH2 domains, which are involved in the binding of these proteins with the phosphotyrosine residues on the substrate molecule. SHP-1 is a well-known negative regulator of cell signaling pathways where as SHP-2 promotes cell proliferation, differentiation, and growth [7]. Nevertheless, the growing evidences on the cellular functions of these two phosphatases demonstrate that it is not possible to clearly demarcate the role of these two as positive or negative regulator, since their function varies among tissue types depending upon the disease involved.
Motheaten phenotype
In 1965, Jacksons laboratory produced two unusual strains of C57BL/6J mice with patchy absence or thinness of hair [hence the name given “motheaten” (me/me) or “viable motheaten” (mev/mev)] [8]. These mice display patchy dermatitis, extramedullary haematopoiesis, splenomegaly, hemorrhagic pneumonitis, immunological dysfunctions and die within 2–3 (me/me) or 9–12 (mev/mev) weeks of birth [9] (Fig. 3). The phenotype arose from a recessive mutation on chromosome 6 in hematopoietic cell protein tyrosine phosphatase (Hcph) gene [10]. Later, the hematopoietic cell protein tyrosine phosphatase was identified as PTP1C (SHP-1) whose truncated expression or defective catalytic activity results in “motheaten” (me/me) or “viable motheaten” (mev/mev) phenotype, respectively [11]. The anomalies lie in the immunological status of these mice. “Motheaten” or “viable motheaten” mice are immunodeficient but exhibit symptoms of systemic autoimmunity such as hyper-gammaglobulinemia (elevated serum IgM level), presence of antinuclear antibodies, and granular deposition of IgM and IgG in kidney glomeruli [12]. The severe developmental aberrations and systemic autoimmunity exhibited by these animal results from a defect in B and T cell development [13] (Fig. 4). In mev/mev mice, early thymic involution occurs as the loss of intrathymic precursor activity causing thymocytopenia which in part is due to abnormal interleukin-7 (IL-7) production or secretion by hematopoietic-origin accessory cells [14, 15]. Beside B and T cells, development and differentiation of other immune cells such as monocyte/macrophages and dendritic cells are also hampered by SHP-1 deficiency, leading to marked infiltration of these cells in various tissues including skin, peritoneal cavity, spleen, lungs, liver, and kidneys [16]. The increased tumor necrosis factor-α (TNF-α) secretion from macrophages accumulated in the lungs accelerate collagen synthesis by lung fibroblast causing pulmonary fibrosis, being the main reason for the early high mortality rate in mev/mev mice [17]. The polymorphonuclear cells isolated from me/me or mev/mev mice show increased oxidant production, increased adherent property, and decreased chemotactic activity [18]. The pro-inflammatory cytokines level increased due to impaired negative regulation of SHP-1 on cytokine signaling interferes with osteoclast function, e.g., the inflated TNF-α and IL-6 production promotes osteoclastogenesis and hence osteopenic phenotype in mev/mev mice [19]. Also, mev/mev mice are anemic because decreased glutathione level creates highly oxidative environment that ruptures red blood cells (RBCs) by oxidizing erythrocyte membrane phospholipids [20]. The foregoing data reveal the regulation of SHP-1 on development and activation of hematopoietic cell lineage and basis of immunological abnormalities in me/me or mev/mev mice (Table 1).
Developmental defects in SHP-1-deficient lymphocytes: The SHP-1-deficient B and T lymphocytes show hyper-responsiveness to self-antigen, and the enhanced signaling through their respective receptors induces apoptosis in these cells. The negative selection or clonal deletion of auto-reactive lymphocytes depletes the mature naïve cell pool engendering immunodeficient phenotype. Alternatively, auto-reactive cells that escape the apoptotic signal induce autoimmunity
Role of SHP-1 in T cell biology
T cell antigen receptor (TCR) is a transmembrane multimeric complex comprising an antigen binding moiety (α and β chains) associated with signal transduction moiety (CD3γ, δ, ε, and ζ) [21]. Antigen receptor assembly recruits activated src-family protein tyrosine kinases (PTKs) with subsequent tyrosine phosphorylation of immunoreceptor tyrosine-based activation motifs or ITAMs [YxxLx/YxxL] present in the cytosolic region of the antigen receptor signaling subunits [22]. The src-family protein tyrosine kinases involved include Lck and Fyn. The phosphorylated ITAMs act as a docking site for the SH2 domain containing PTK, ZAP-70 [22]. Thus, reversible tyrosine phosphorylation of receptor-engaged ITAMs aids in activation of signal transduction via T cell receptor and is therefore crucial for lymphocyte function. Apart from the above-mentioned receptor and downstream molecules, a variety of adaptor proteins (LAT, SLP-76) and co-receptors (CD5, CD28, PEACAM-1) also plays an important role in propagating T cell signaling [23].
In order to dissolve the T cell activation signals, PTPs acts upon tyrosine-phosphorylated residues on signaling molecules, which is essential to maintain cellular homeostasis. Data generated from studies involving me/me mice invincibly suggest the role of SHP-1 as key player in T cell signaling [23]. In me/me mice, TCR-CD3 engagement results in hyper-responsive T cells due to enhanced IL-2 production and sustained activation of Lck and Fyn [24]. In addition, similar findings are obtained from thymocytes expressing dominant negative form of SHP-1 [24]. SHP-1 modulates thymocyte selection and TCR-mediated apoptosis in cell autonomous fashion as confirmed by the findings that the mev mutation or lymphoid-restricted expression of catalytically inert SHP-1 augments TCR-evoked proliferation and T cell selection in H-Y TCR transgenic mice [25]. The association of SHP-1 with TCR raises the minimum threshold required for the transmission of signals downstream TCR. This is consistent with the finding that SHP-1-deficient peripheral T cells are hyperresponsive to TCR-evoked apoptosis due to increased expression of fas ligand and associated Lck and ZAP-70 activity [26, 27]. Contrastingly, some studies showed the normal fas-mediated signaling in SHP-1-deficient cells [28]. Moreover, me/me mice have increased number of regulatory T cells (Treg cells) which are considered as key players in maintaining peripheral tolerance [29]. These cells are known as CD4+ CD25+ Foxp3+ cells. Cumulative data from me/me TCR transgenic mice suggest that developing thymocytes receives signals that precommit them toward Treg lineage in the absence of SHP-1 [29]. This means that SHP-1 deficiency leads to disproportionate deletion of conventional T cells and survival of Tregs cells.
Apart from T cell development and selection, SHP-1 is known to exert inhibitory effect on T cell differentiation into Th1 and Th2 subpopulation, characterized by cytokine profile and lineage-specific transcription factors [30]. This inhibitory effect is thought to be exerted directly through TCR signaling or indirectly via modulating IL-4R or TGF-β signaling [28, 31].
Unlike TCR, the association of SHP-1 with the T cell inhibitory co-receptor is not so well characterized. This is because the inhibitory co-receptors are expressed by subsets of T cell under specific conditions and results are poorly reproducible. The signaling through inhibitory co-receptors such as CD5, CTLA-4, CD28, LAIR-1, and CEACAM-1 has been found to be affected in response to SHP-1 deficiency, but their exact linkage is yet to be explored [23]. Nevertheless, the association of SHP-1 with the downstream molecules in T cells is studied to a large extent. SHP-1 interacts with Lck, Fyn, ZAP-70, and PI3K which play central role in propagating signal for the variety of pathways including cytoskeletal rearrangement, proliferation, and apoptosis [32]. Additionally, it has been reported that 20–30 % of SHP-1 constitutively localized to the lipid rafts, which is considered to be important for imposing its negative regulation over TCR signaling [33].
The above-mentioned findings define the role of SHP-1 in T cell signaling, development, and differentiation (Fig. 5). However, concrete evidences are required to fully assign specific function of SHP-1 in different T cell subsets and its association with signaling molecules, which determines the final biological outcome.
SHP-1 in T cell biology: SHP-1 negatively regulates T cell proliferation and differentiation via inhibiting the signaling through TCR, IL-2R, CD28, PI3K, Lck, ZAP-70, SLP-76 [23]. Also, the SHP-1 deficiency skews T cell differentiation toward Tregs cells suggesting the role of SHP-1 in propagating the signal that commits developing thymocytes more toward the conventional T cell lineage than the regulatory one. TCR T cell receptor, PI3K phosphoinositide 3-kinase, JAK Janus kinase, STAT signal transducer and activator of transcription, Tregs regulatory T cells
SHP-1 and autoimmune diseases
Once identified as hematopoietic cell-specific protein tyrosine phosphatase, the extensive research on SHP-1-deficient mice elucidated the involvement of SHP-1 in large number of cell functions and related pathologies (especially inflammatory process). For that reason here we attempted to summarize the implication of SHP-1 in the pathogenesis of autoimmune diseases. Also, we try to suggest SHP-1 molecular targets that may prove fruitful for the development of therapeutic strategies.
SHP-1 in allergic asthma
The systemic autoimmunity and severe inflammation in SHP-1-deficient motheaten mice is well characterized. The SHP-1-deficient hyperactive immune cells and their secretions conspire to initiate a cascade of inflammatory response against foreign or self-antigen instigating inflammatory or autoimmune diseases, respectively. The motheaten and viable motheaten mice spontaneously develop Th2-like pulmonary inflammation (a hallmark of allergic asthma) marked by enhanced IL-13 and signal transducer and activator of transcription 6 (STAT6) activation without exposure to known allergen [34]. SHP-1 is a known negative regulator of cytokine signaling including IL-4/IL-13, deficiency of which leads to activation of IL-4/IL-13 pathway engendering Th2 inflammatory response including eosinophilia, mucus metaplasia, airway epithelial hypertrophy, pulmonary fibrosis, increased airway resistance, and hyper-responsiveness in motheaten mice via STAT6 activation and upregulation of STAT6-targeted genes [28, 35]. The Th2 response suggests involvement of innate immune cells in the pathogenesis. The SHP-1 deficiency causes increased mast cell number in the lungs of motheaten mice together with increased Th2 cytokine and IgE production [35]. Thus, it is possible that the pulmonary phenotype exhibited by motheaten viable mice, maybe to some extent, is due to dysregulation of mast cell as supported by the finding that mast cell-deficient motheaten viable mice show reduced pulmonary inflammation [35]. In addition to this, oxidative stress is another known mechanism that contributes to allergic pulmonary inflammation and allergic asthma [36]. The increased oxidative stress is been associated with the inhibition of PTPs and break of immune tolerance [37]. The human alveolar epithelial cell line transfected with catalytically inactive SHP-1 shows increased chemokine ligand 20 (CCL20) and CCL5 (RANTES) levels in response to oxidative stress [36]. CCL20 and CCL5 recruit monocyte/dendritic cell into the lung epithelium, thereby developing and maintaining allergic asthma [37]. Consistent with this, broncho alveolar lavage (BAL) cell from viable motheaten mice exposed to oxidative stress show elevated reactive oxygen species (ROS) levels [37]. Also, the intranasal challenge with ovalbumin significantly increases ROS production, STAT6 phosphorylation, and decreases nuclear factor-kappa B (NF-κB) activation and eosinophilia in these mice. Since SHP-1 is known to suppress oxidative stress by inhibiting nicotinamide adenine dinucleotide (NADH)-oxidase and inducible nitric oxide synthase (iNOS) activity, SHP-1 deficiency causes increased oxidative stress and break in immune tolerance to aeroallergens in viable motheaten mice that promotes allergic airway inflammation. In addition, SHP-1 deficiency and oxidative stress abet mucus hyper-secretion in chronic inflammatory airway diseases that can be blocked by antioxidant treatment [38]. Further, defective SHP-1 in asthmatic epithelial cells contributes to exaggerated response to mycoplasma pneumonia due to increased IL-8 production and phosphatidyl inositol 3 kinase (PI3K)/Akt and NF-κB activation that aggravates asthma [39]. Similarly, in allergic rhinitis also SHP-1 deficiency is linked to the Th2 nasal inflammation involving interferon-gamma (IFN-γ) [40]. Evidently, SHP-1 maintains immunologic homeostasis of upper and lower airways, failure of which gives rise to pulmonary inflammation (Fig. 6).
SHP-1 in atopic dermatitis
The involvement of SHP-1 in skin inflammation is also studied in the PLC-β3-deficient mouse model that develops atopic dermatitis like skin lesions including eczematous skin lesions, hair loss and infiltration of immune cells, and increased IgE levels [41]. Phospholipase C-β3 (PLC-β3), an enzyme that catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate and generates diacylglycerol and inositol 1,4,5-trisphosphate, interacts with SHP-1 which in turn inactivates STAT5, inhibiting the proliferation of hematopoietic stem cells (HSCs) and myeloid cells [42, 43]. In PLC-β3-deficient mice, spontaneous skin lesions development is governed by mast cells as confirmed by mast cell deficiency but not αβ T cell or B cell deficiency can prevent skin lesions [41]. Mast cells are also indispensable for allergen-induced dermatitis in these mice, and the process involves FcεRI [41]. Moreover, mast cells are hyper-responsive to IL-3 due to reduced SHP-1 activity and increased STAT5 activation although IL-3 is not the only factor of STAT5 activity. PLC-β3 regulates expression of thymic stromal lymphopoietin (TSLP) and periostin in keratinocytes and fibroblasts, respectively [41]. These two proteins are critically involved in atopic dermatitis pathogenesis. These results signify the role of SHP-1 in dermatitis development by regulating IL-3 responsiveness of mast cells and STAT5 activation. Figure 7 briefly describes the pathogenesis of atopic dermatitis.
Atopic dermatitis: SHP-1 inhibits the proliferation of myeloid cells via interacting with PLC-β3. In PLC-β3-deficient mice, mast cells are hyper-responsive toward the IL-3 and show enhanced STAT5 expression due to reduced SHP-1 activity. The increased expression of STAT5, TSLP, and periostin from mast cell, keratinocytes, and fibroblast, respectively, contributes toward the pathogenesis of atopic dermatitis. PLC-β3 phospholipase C-β3, TSLP thymic stromal lymphopoietin
SHP-1 in neutrophilic dermatosis
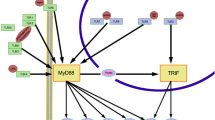
Neutrophilic dermatosis is a group of skin disorder characterized by intense infiltration of neutrophils into the epidermis and dermis causing skin lesions [44]. The screening of cDNA and genomic DNA sequences of PTPN6 from patients with neutrophilic dermatosis identified novel splice variants which were not detected in healthy controls. The missense mutation causing insertion of B2 element into the exon6 of mouse Ptpn6 gene (mice are called as meB2 mice) was identified that expresses functionally altered SHP-1 protein [45]. The mice homozygous for this mutation develops autoinflammatory symptoms resembling neutrophilic dermatosis in humans [45]. For example, increased neutrophil number in peripheral blood, bone marrow and other organs due to decreased NF-κB pro-apoptotic activity and extended half life is observed [45]. The increased neutrophil number leads to the infiltration of these cells in various organs including epidermis causing skin ulceration and pustules formation. Other symptoms include hyper-gammaglobulinemia with IgG and IgM deposition in the kidneys and presence of anti-DNA, antinuclear, and anti-histone antibodies, high levels of pro-inflammatory cytokines and acute phase proteins (serum amyloid-A and C-reactive protein) [45]. Since the etiology of human neutrophilic dermatosis has been hampered by the lack of experimental system, the observations from the above-mentioned study bring about the importance of meB2 mice as an experimental model for the study of this heterogenous group of cutaneous diseases. The N-ethyl-N-nitrosourea (ENU)-induced recessive mutation in C57BL/6J mice gave rise to a new phenotype called as spin (spontaneous inflammation) that harbors Y208N amino acid substitution in the carboxy-terminal SH2 domain of SHP-1 [46]. The chronic inflammation in homozygotes for spin mutation resembles that of neutrophilic dermatosis which includes footpad swelling, salivary glands and lungs inflammation and presence of antichromatin antibodies [45]. The persistent inflammation and tissue damage are triggered by dysregulated SHP-1 activity and receptor-interacting protein-1 (RIP-1)-mediated IL-1α production [47, 48]. IL-1α has been associated with the process of wound healing, and its uncontrolled secretion orchestrates inflammation and tissue destruction in SHP-1-deficient mice [47–49]. Consequently, SHP-1/RIP-1/IL-1α signaling axis proves to be a recent target for the treatment of neutrophilic dermatosis (Fig. 8). Recent evidences suggest the interaction of SHP-1 with phosphatidylinositol phosphate kinase type Iγ (PIPKIγ) inhibits neutrophil migration to inflamed tissue [50]. PIPKIγ is an enzyme involved in generation of secondary messenger phosphatidylinositol 4,5-bisphosphate (PI4,5P2) required for cytoskeletal arrangement [51]. Throwing some light on this pathway can reveal new molecular targets to cure this disease.
Neutrophilic dermatosis: The SHP-1-deficient mice (meB2 mice and spin mutants) represent promising experimental models to study the molecular mechanism of human neutrophilic dermatosis. These mice develop skin lesions similar to human neutrophilic dermatosis and aid in exploring therapeutic targets such as PIPKIγ, RIP-1, and IL-1α for the same. PIPKIγ phosphatidylinositol phosphate kinase type Iγ, RIP-1 receptor-interacting protein
SHP-1 in rheumatoid arthritis
Based upon type of receptor engagement immune receptors generate signals which may be either pro- or anti-inflammatory. For example, human Fc gamma receptor IIA (hFcγRIIA or CD32A) functions as dual activating/inhibitory receptor [52]. In rheumatoid arthritis, cross-linking of hFcγRIIA by IgG immune complex and subsequent phosphorylation of its two tyrosine residues within immunoreceptor tyrosine-based activation motif (ITAM) recruits and activates spleen tyrosine kinase (Syk) initiates an inflammatory response [53]. Contrastingly, in normal controls or engagement of hFcγRIIA ITAM with either anti-hFcγRIIA F(ab′)2 or intravenous human IgG results in phosphorylation of single tyrosine (Y304) residue and further recruitment of Syk and SHP-1 [53]. The association of SHP-1 with Syk dissociates and inactivates Syk, thereby inhibiting ROS generation via upregulating ITAMi signaling [53]. The stable recruitment and activation of Syk are involved in the RA pathogenesis indicating dysregulated SHP-1 activity which needs further investigation. These findings suggest the role of SHP-1 in deciding the final outcome of receptor engagement via modulating RTKs function. Another mechanism in the development of rheumatoid arthritis is the bidirectional interaction between inflammatory macrophages and T cells. The IL-23-induced Th17 cells promote polarization of M1 macrophages via increased granulocyte-monocyte colony-stimulating factor (GM-CSF) and interferon regulatory factor 5 (IRF5) activities in RA [54]. The increased GM-CSF activity may result from decreased SHP-1 expression as supported by the finding that viable motheaten mice have enhanced GM-CSF activity and increased M1 phenotype macrophage production [54] (Fig. 9). SHP-1 associates with the death receptor 5 (DR5) and negatively regulates GM-CSF-mediated survival signaling in neutrophils and SHP-1-deficient cells circumvent apoptosis signal engendering chronic inflammation [54]. One of the therapeutic approaches for autoimmune diseases is to eliminate these cells from the system via sensitizing them to apoptosis signal. For example, TRA8, an anti-DR5 agonistic antibody, inhibits survival signals and induces apoptosis signal in inflammatory IRF-5+ IL-23+ inflammatory macrophages and GM-CSF+ Th17 cell population in SHP-1-depleted cells [54].
Rheumatoid arthritis: The engagement of hFcγRIIA with a either anti-hFcγRIIA F(ab′)2 or intravenous human IgG phosphorylates single tyrosine residue that recruits Syk and SHP-1, thereby inhibiting inflammatory responses, whereas b cross-linking with IgG immune complex results in phosphorylation of two tyrosine residue disturbs the interaction between Syk and SHP-1 and initiates inflammation leading to rheumatoid arthritis. Also, the diminished SHP-1 expression skews macrophages toward M1 phenotype via upregulating IL-23 signaling-mediated GM-CSF level
SHP-1 in psoriasis
Psoriasis is another autoimmune skin disease known to involve infiltration of immune cells specifically activated T cells and increased pro-inflammatory cytokine production due to dysregulated SHP-1 activity [55]. The T cells from psoriasis subjects show hyper-responsiveness to IFN-α and enhanced JAK/STAT signaling [55]. Since SHP-1 negatively regulates cytokine signaling via SOCS3 (suppressor of cytokine signaling 3), decreased SHP-1 and SOCS3 expressions contribute toward T cell hyper-responsiveness to IFN-α [55]. Furthermore, SHP-1 promoter demethylation and enhanced SHP-1 isoform II transcription have also been reported in psoriasis [56].
SHP-1 in multiple sclerosis
The myelin deficiency displayed by SHP-1-deficient motheaten mice arises from reduced myelin basic protein (MBP) production in oligodendrocytes or increased innate inflammatory response in the CNS [57, 58]. Multiple sclerosis (MS), a chronic inflammatory demyelinating disease of the central nervous system (CNS), involves multiple leukocyte cell types including lymphocytes, macrophages, and dendritic cells that form sclerotic lesions [59]. Among these macrophages are the major cells involved in destruction of myelin sheath in MS and animal model of MS [60]. The macrophages and peripheral blood mononuclear cells (PBMCs) from multiple sclerosis patients are SHP-1 deficient and show heightened activation of STAT6, STAT1, and NF-κB that promotes demyelination [61–64]. The current treatment for MS is IFN-β1a that downregulates NF-κB and STAT6 activation by means of upregulating SHP-1 activity [62]. The bisulfite sequencing of PBMCs from MS patients revealed increased SHP-1 transcripts II promoter methylation that causes aberrant silencing of SHP-1 expression [61]. Similarly, SHP-1 promoter hyper-methylation-associated defective SHP-1 expression has been reported in schizophrenic subjects [65]. While macrophages are the ultimate culprit in the pathogenesis of MS, T cell actually initiates and perpetuates the brain inflammation in MS. The link between SHP-1 and TCR signaling is very well characterized [24]. In animal model of MS, i.e., experimental autoimmune encephalomyelitis (EAE), following TCR engagement with autoantigens CD4+ Th1 cell mediates inflammatory demyelination in the CNS [66]. The immunization of mice heterozygous for deletion of the SHP-1 gene (me(v±)) with immunodominant epitope of myelin basic protein (MBP Ac1-11) enhances T cell proliferation and IFN-γ production due to decrease in activation threshold for autoreactive T cell in the absence of SHP-1 activity [66] (Fig. 10). Additionally, motheaten viable heterozygous mice challenged with myelin oligodendrocyte glycoprotein MOG35–55 or MOG35–55 MHC variant 45D exhibit symptoms of experimental autoimmune encephalomyelitis following immunization with MHC variant peptides demonstrating the control of SHP-1 over T cell anergy induced by unstable interaction between peptide and MHC complex [67]. CD47–SIRP-α signaling pathway is another important pathway that contributes to experimental autoimmune encephalomyelitis which is regulated by SHP-1 [68]. Signal regulatory protein a (SIRP-α) or SH2 domain containing protein tyrosine phosphatase substrate-1 (SHPS-1) is a transmembrane protein which consists of three extracellular immunoglobulin-like domains and cytoplasmic domain containing four tyrosine phosphorylation sites that binds with SHP-1 [68].
Multiple sclerosis: The enhanced SHP-1 promoter methylation in PBMCs/macrophages from multiple sclerosis patient witnesses the role of this enzyme in the pathogenesis of this diseases. Moreover, in experimental autoimmune encephalomyelitis, the decreased SHP-1 activity is associated with decreased level of activation threshold for T cells that causes recognition of autoantigen by these cells and immune destruction of myelin
SHP-1 in virus-induced demyelination
The enhanced susceptibility to virus-induced demyelination in mice genetically lacking SHP-1 (me/me) originates from macrophage neuroinvasion due to increased macrophage chemoattractant protein-1 (MCP-1) activity [69]. The intracranial or intraperitoneal inoculation of BeAn strain of Theiler’s murine encephalomyelitis virus (TMEV) in SHP-1-deficient mice enhanced blood borne macrophage infiltration into the CNS, virus replication, and inflammatory demyelination compared to infected wild-type mice [69]. In concordance, the clodronate liposome-based deletion of monocyte/macrophage significantly reduces this inflammatory response and onset of TMEV-induced paralysis in the spinal cords of me/me mice compared to those in wild-type littermates [70]. The virus-induced interferons and pro-inflammatory cytokines production leads to increased SHP-1 level to counteract viral replication via upregulating iNOS activity [71]. SHP-1 also regulates differentiation, distribution, and cytokine production by microglia within the murine central nervous system (CNS) and SHP-1 deficiency leads to higher viral replication through STAT-6-dependent arginase I gene expression and reduced nitric oxide production in CNS glia [71]. In me/me mice cochlear ablation causes increased neuronal loss within the auditory brainstem due to abnormal microglia activation [72]. Following lipopolysaccharide (LPS) stimulation me/me microglia releases increased levels of neurotoxic mediators, i.e., nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), and interleukin-1beta (IL-1beta) [73]. Also, SHP-1 regulates glial activation and proliferation in the ischemic/injured CNS [74]. Beside microglia, SHP-1 controls pro-inflammatory signaling in astrocytes and oligodendrocytes as supported by the finding that IFN-γ-induced me/me astrocytes have high levels of NF-κB, IRF-1, and major histocompatibility complex I (MHC class I) molecule and in IL-6-induced me/me oligodendrocytes show higher levels of STAT3 phosphorylation and STAT3-responsive c-fos gene expression [26, 58, 66].
Other autoimmune diseases such as myasthenia gravis and systemic lupus erythematosus (SLE) manifest due to decrease in activation threshold for TCR and BCR signaling, respectively, owing to diminished SHP-1 activity [75, 76]. Indeed, SHP-1 plays significant role in the pathogenesis of autoimmune diseases. SHP-1-deficient mouse models represent acceptable experimental system for further investigation required to explore the SHP-1 circuitry involved in the same so as to find new targets for drug development.
Summary
Autoimmune diseases depicts the destroying behavior of immune system, which otherwise is the defense mechanism of the body to combat diseases. Number of genes has been reported that regulates immune cell function, and alteration in their expression causes disturbances in inflammatory settings. The defects in immune cell function and symptoms of autoimmunity exhibited by SHP-1-deficient mice ignited the scientific world to deeply investigate the potential of this tyrosine phosphatase as a drug target for the treatment of autoimmune diseases. The association of SHP-1 with the immunoreceptors (activation and inhibitory), nonreceptor kinases, adaptor molecules, transcription factors, etc., in immune cells is required to switch off the cell activation signal. In addition, SHP-1 interaction with immunoreceptor during developmental stages ascertains the tolerance to self-antigen. Therefore, the SHP-1-deficient immune cells respond to self-antigen and elicit immune response against it. The present review describes how low SHP-1 expression affects immune cell signaling and results in autoimmune diseases such as allergic asthma, neutrophilic dermatosis, atopic dermatitis, rheumatoid arthritis, and multiple sclerosis (Fig. 11). Therapeutic strategies can be thrived by resolving the following issues like:
-
Further scrutinizing the SHP-1 biology in generation of self-tolerance during immune cell development and its correlation with autoimmunity.
-
In case of neutrophilic dermatosis, the SHP-1/RIP-1/IL-α signaling axis is not very well understood. Delineating this pathway might prove valuable to find cure for this disease.
-
Putting glance at new signaling molecules such as SIRP-α and PIPKIγ which have been found to be an SHP-1 target can further boost our knowledge regarding immune cell infiltration, an important mechanism behind progression of autoimmune disorder.
-
The human genome encodes many tyrosine phosphatases that bear structural similarity with SHP-1. Because of this, sole targeting of SHP-1 is rarely achieved. The identification and development of specific molecular antagonists for SHP-1 can aid in developing curative strategies for autoimmune diseases, SHP-1-deficient mice being the experimental system (Table 2).
Table 2 List of the compounds showing inhibitory effect on SHP-1
References
He R, Yu Z, Zhang R, Zhang Z. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin. 2014;35:1227–46.
Alonso A, Sasin J, Bottini N, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711.
Xu K, Li S, Yang W, et al. Structural and biochemical analysis of tyrosine phosphatase related to biofilm formation A (TpbA) from the opportunistic pathogen Pseudomonas aeruginosa PAO1. PLoS One. 2015;10(4):e0124330.
Johnson KG, Van VD. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24.
Marín-Juez R, Jong-Raadsen S, Yang S, Spaink HP. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J Endocrinol. 2014;222:229–41.
Mannell H, Krotz F. SHP-2 regulates growth factor dependent vascular signalling and function. Mini Rev Med Chem. 2014;14:471–83.
Kozicky LK, Sly LM. Phosphatase regulation of macrophage activation. Semin Immunol. 2015;27:276–85.
Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38:489–501.
Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genet Pathol J Hered. 1975;66:250–8.
Bignon JS, Siminovitch KA. Identification of PTP1C mutation as the genetic defect in motheaten and viable motheaten mice: a step toward defining the roles of protein tyrosine phosphatases in the regulation of hemopoietic cell differentiation and function. Clin Immunol Immunopathol. 1994;73:168–79.
Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–9.
Kozlowski M, Mlinaric-Rascan I, Feng GS, Shen R, Pawson T, Siminovitch KA. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178:2157–63.
Sidman CL, Marshall JD, Allen RD. Murine “viable motheaten” mutation reveals a gene critical to the development of both B and T lymphocytes. Proc Natl Acad Sci USA. 1989;86:6279–82.
Hayes SM, Shultz LD, Greiner DL. Thymic involution in viable motheaten (me(v)) mice is associated with a loss of intrathymic precursor activity. Dev Immunol. 1992;2:191–205.
Christianson SW, Greiner DL, Deluca D, et al. T cell developmental defects in ‘viable motheaten’ mice deficient in SHP-1 protein-tyrosine phosphatase. Developmental defects are corrected in vitro in the presence of normal hematopoietic-origin stromal cells and in vivo by exogenous IL-7. J Autoimmun. 2002;18:119–30.
Nakayama K, Takahashi K, Shultz LD, Miyakawa K, Tomita K. Abnormal development and differentiation of macrophages and dendritic cells in viable motheaten mutant mice deficient in haematopoietic cell phosphatase. Int J Exp Pathol. 1997;78:245–57.
Thrall RS, Vogel SN, Evans R, Shultz LD. Role of tumor necrosis factor-alpha in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am J Pathol. 1997;151:1303–10.
Kruger J, Butler JR, Cherapanov V, et al. Deficiency of Src homology 2-containing phosphatase 1 results in abnormalities in murine neutrophil function: studies in motheaten mice. J Immunol. 2000;165:5847–59.
Aoki K, Didomenico E, Sims NA, et al. The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in me(v)/me(v) mutant mice. Bone. 1999;25:261–7.
Lyons BL, Lynes MA, Burzenski L, Joliat MJ, Hadjout N, Shultz LD. Mechanisms of anemia in SHP-1 protein tyrosine phosphatase-deficient “viable motheaten” mice. Exp Hematol. 2003;31:234–43.
Malissen B, Grégoire C, Malissen M, Roncagalli R. Integrative biology of T cell activation. Nat Immunol. 2014;15:790–7.
Love PE, Hayes SM. ITAM-mediated signaling by the T-Cell antigen receptor. Cold Spring Harb Perspect Biol. 2010;2:a002485.
Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–59.
Fu G, Rybakin V, Brzostek J, Paster W, Acuto O, Gascoigne NR. Fine-tuning T cell receptor signaling to control T cell development. Trends Immunol. 2014;35:311–8.
Fu G, Casas J, Rigaud S. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013;504:441–5.
Park HS, do Jun Y, Han CR, Woo HJ, Kim YH. Proteasome inhibitor MG132-induced apoptosis via ER stress-mediated apoptotic pathway and its potentiation by protein tyrosine kinase p56lck in human Jurkat T cells. Biochem Pharmacol. 2011;82:1110–25.
Robles-Escajeda E, Lerma D, Nyakeriga AM, Ross JA, Kirken RA, Aguilera RJ, Varela-Ramirez A. Searching in mother nature for anti-cancer activity: anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS One. 2013;8:e73508.
Johnson DJ, Pao LI, Dhanji S, Murakami K, Ohashi PS, Neel BG. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J Exp Med. 2013;210:1419–31.
Iype T, Sankarshanan M, Mauldin IS, Mullins DW, Lorenz U. The protein tyrosine phosphatase SHP-1 modulates the suppressive activity of regulatory T cells. J Immunol. 2010;185:6115–27.
Yu WM, Wang S, Keegan AD, Williams MS, Qu CK. Abnormal Th1 cell differentiation and IFN-gamma production in T lymphocytes from motheaten viable mice mutant for Src homology 2 domain-containing protein tyrosine phosphatase-1. J Immunol. 2005;174:1013–9.
Park IK, Shultz LD, Letterio JJ, Gorham JD. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain containing phosphatase-1. J Immunol. 2005;175:5666–74.
Stanford SM, Rapini N, Bottini N. Regulation of TCR signalling by tyrosine phosphatases: from immune homeostasis to autoimmunity. Immunology. 2012;137:1–19.
Sankarshanan M, Ma Z, Iype T, Lorenz U. Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 1. J Immunol. 2007;179:483–90.
Zhang L, Oh SY, Wu X, et al. SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung. J Immunol. 2010;184:1180–90.
Dwivedi G, Gran MA, Bagchi P, Kemp ML. Dynamic redox regulation of IL-4 signaling. PLoS Comput Biol. 2015;11(11):e1004582.
Li X, Kwon O, Kim DY, Taketomi Y, Murakami M, Chang HW. NecroX-5 suppresses IgE/Ag-stimulated anaphylaxis and mast cell activation by regulating the SHP-1-Syk signaling module. Allergy. 2016;71:198–209.
Frijhoff J, Dagnell M, Godfrey R, Ostman A. Regulation of protein tyrosine phosphatase oxidation in cell adhesion and migration. Antioxid Redox Signal. 2014;20:1994–2010.
Zhou L, Oh SY, Zhou Y, et al. SHP-1 regulation of mast cell function in allergic inflammation and anaphylaxis. PLoS One. 2013;8:e55763.
Wang Y, Zhu Z, Church TD, et al. SHP-1 as a critical regulator of Mycoplasma pneumoniae-induced inflammation in human asthmatic airway epithelial cells. J Immunol. 2012;188:3371–81.
Cho SH, Oh SY, Lane AP, et al. Regulation of nasal airway homeostasis and inflammation in mice by SHP-1 and Th2/Th1 signaling pathways. PLoS One. 2014;4(9):e103685.
Ando T, Xiao W, Gao P, et al. Critical role for mast cell Stat5 activity in skin inflammation. Cell Rep. 2014;6:366–76.
Xu X, Jin T. The novel functions of the PLC/PKC/PKD signaling axis in g protein-coupled receptor-mediated chemotaxis of neutrophils. J Immunol Res. 2015;2015:817604.
Hsiao WY, Lin YC, Liao FH, Chan YC, Huang CY. Dual-specificity phosphatase 4 regulates STAT5 protein stability and helper T cell polarization. PLoS ONE. 2015;10:e0145880.
Alavi A, Sajic D, Cerci FB, Ghazarian D, Rosenbach M, Jorizzo J. Neutrophilic dermatoses: an update. Am J Clin Dermatol. 2014;15:413–23.
Demosthenous C, Han JJ, Hu G, Stenson M, Gupta M. Loss of function mutations in PTPN6 promote STAT3 deregulation via JAK3 kinase in diffuse large B-cell lymphoma. Oncotarget. 2015;6:44703–13.
Caignard G, Eva MM, van Bruggen R, et al. Mouse ENU mutagenesis to understand immunity to infection: methods, selected examples, and perspectives. Genes. 2014;5:887–925.
Lukens JR, Kanneganti TD. SHP-1 and IL-1α conspire to provoke neutrophilic dermatoses. Rare Dis. 2014;31(2):e27742.
Lukens JR, Vogel P, Johnson GR, et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 2013;498:224–7.
Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6.
Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–9.
Stadtmann A, Block H, Volmering S, et al. Cross-talk between Shp1 and PIPKIγ controls leukocyte recruitment. J Immunol. 2015;195:1152–61.
Xu Q, Zhang Y, Xiong X, et al. PIPKIγ targets to the centrosome and restrains centriole duplication. J Cell Sci. 2014;127:1293–305.
Mkaddem SB, Hayem G, Jönsson F, et al. Shifting FcγRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J Clin Invest. 2014;124:3945–59.
Li J, Yang P, Wu Q, et al. Death receptor 5-targeted depletion of interleukin-23-producing macrophages, Th17, and Th1/17 associated with defective tyrosine phosphatase in mice and patients with rheumatoid arthritis. Arthritis Rheum. 2013;65:2594–605.
Eriksen KW, Woetmann A, Skov L, et al. Deficient SOCS3 and SHP-1 expression in psoriatic T cells. J Invest Dermatol. 2010;130:1590–7.
Chandra A, Ray A, Senapati S, Chatterjee R. Genetic and epigenetic basis of psoriasis pathogenesis. Mol Immunol. 2015;64:313–23.
Blank T, Prinz M. NF-κB signaling regulates myelination in the CNS. Front Mol Neurosci. 2014;7:47.
Kim JH, Choi DJ, Jeong HK, et al. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: a novel anti-inflammatory function of DJ-1. Neurobiol Dis. 2013;60:1–10.
Hernández-Pedro NY, Espinosa-Ramirez G, de la Cruz VP, Pineda B, Sotelo J. Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol. 2013;2013:413465.
Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog Neurobiol. 2015;127–128:1–22.
Kumagai C, Kalman B, Middleton FA, Vyshkina T, Massa PT. Increased promoter methylation of the immune regulatory gene SHP-1 in leukocytes of multiple sclerosis subjects. J Neuroimmunol. 2012;246:51–7.
Christophi GP, Gruber RC, Panos M, Christophi RL, Jubelt B, Massa PT. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin Immunol. 2012;142:308–19.
Christophi GP, Panos M, Hudson CA, et al. Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab Invest. 2009;89:742–59.
Christophi GP, Hudson CA, Gruber RC, et al. SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients. Lab Invest. 2008;88:243–55.
Pesce M, Ferrone A, Rizzuto A, et al. The SHP-1 expression is associated with cytokines and psychopathological status in unmedicated first episode schizophrenia patients. Brain Behav Immun. 2014;41:251–60.
Gruber RC, LaRocca D, Minchenberg SB, et al. The control of reactive oxygen species production by SHP-1 in oligodendrocytes. Glia. 2015;63:1753–71.
Sauer EL, Cloake NC, Greer JM. Taming the TCR: antigen-specific immunotherapeutic agents for autoimmune diseases. Int Rev Immunol. 2015;34:460–85.
Murata Y, Saito Y, Kaneko T, et al. Autoimmune animal models in the analysis of the CD47–SIRPα signaling pathway. Methods. 2014;65:254–9.
Watson NB, Schneider KM, Massa PT. SHP-1-dependent macrophage differentiation exacerbates virus-induced myositis. J Immunol. 2015;194:2796–809.
Son KN, Lipton HL. Inhibition of Theiler’s virus-induced apoptosis in infected murine macrophages results in necroptosis. Virus Res. 2015;195:177–82.
Heneberg P. Reactive nitrogen species and hydrogen sulfide as regulators of protein tyrosine phosphatase activity. Antioxid Redox Signal. 2014;20:2191–209.
Zhao J, Lurie DI. Cochlear ablation in mice lacking SHP-1 results in an extended period of cell death of anteroventral cochlear nucleus neurons. Hear Res. 2004;189:63–75.
Kaminska B, Mota M, Pizzi M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta. 2016;1862:339–51.
Alig SK, Stampnik Y, Pircher J, et al. The tyrosine phosphatase SHP-1 regulates hypoxia inducible factor-1α (HIF-1α) protein levels in endothelial cells under hypoxia. PLoS One. 2015;10(3):e0121113.
Deng C, Wu B, Yang H, et al. Decreased expression of Src homology 2 domain-containing protein tyrosine phosphatase 1 reduces T cell activation threshold but not the severity of experimental autoimmune myasthenia gravis. J Neuroimmunol. 2003;138:76–82.
Youinou P, Renaudineau Y. CD5 expression in B cells from patients with systemic lupus erythematosus. Crit Rev Immunol. 2011;31:31–42.
Kundu S, Fan K, Cao M, et al. Novel SHP-1 inhibitors tyrosine phosphatase inhibitor-1 and analogs with preclinical anti-tumor activities as tolerated oral agents. J Immunol. 2010;184:6529–36.
Lu L, Wang S, Zhu M, et al. Inhibition protein tyrosine phosphatases by an oxovanadium glutamate complex, Na2[VO(Glu)2(CH3OH)](Glu = glutamate). Biometals. 2010;23:1139–47.
Yi T, Elson P, Mitsuhashi M, et al. Phosphatase inhibitor, sodium stibogluconate, in combination with interferon (IFN) alpha 2b: phase I trials to identify pharmacodynamic and clinical effects. Oncotarget. 2011;2:1155–64.
Yang JW, He XP, Li C, et al. A unique and rapid approach toward the efficient development of novel protein tyrosine phosphatase (PTP) inhibitors based on ‘clicked’ pseudo-glycopeptides. Bioorg Med Chem Lett. 2011;21:1092–6.
Li Y, Lu L, Zhu M, et al. Potent inhibition of protein tyrosine phosphatases by copper complexes with multi-benzimidazole derivatives. Biometals. 2011;24:993–1004.
Wang Q, Zhu M, Lu L, Yuan C, Xing S, Fu X. Potent inhibition of protein tyrosine phosphatases by quinquedentate binuclear copper complexes: synthesis, characterization and biological activities. Dalton Trans. 2011;40:12926–34.
Lu L, Gao X, Zhu M, et al. Exploration of biguanido–oxovanadium complexes as potent and selective inhibitors of protein tyrosine phosphatases. Biometals. 2012;25:599–610.
Akiba H, Sumaoka J, Hamakubo T, Komiyama M. Conjugation-free, visual, and quantitative evaluation of inhibitors on protein tyrosine kinases and phosphatases with a luminescent Tb(III) complex. Anal Bioanal Chem. 2014;406:2957–64.
Acknowledgments
YS acknowledge University Grant Commission (UGC) for awarding senior research fellowship (SRF). FK thank Indian Council of Medical Research (ICMR) for funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, Y., Bashir, S., Bhardwaj, P. et al. Protein tyrosine phosphatase SHP-1: resurgence as new drug target for human autoimmune disorders. Immunol Res 64, 804–819 (2016). https://doi.org/10.1007/s12026-016-8805-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-016-8805-y