Abstract
Medullary thyroid carcinoma (MTC) has been shown to express Prospero homeobox protein 1 (Prox1), a transcription factor whose expression is altered in a variety of human cancers. We conducted a retrospective study on a series of 32 patients with MTC to test the correlation of Prox1 expression in MTC with clinicopathological features and to evaluate its prognostic significance. Correlation of Prox1 immunohistochemical expression with tumor size, proliferative index (Ki67), and calcitonin and CEA serum levels prior to surgery was tested for significant correlations. The difference in Prox1 and Ki67 immunohistochemical expression according to the immunohistochemical staining intensity of CEA, chromogranin A, and calcitonin was tested using the Kruskal-Wallis H test and linear regression analysis. The prognostic value of Prox1 and Ki67 for our patient cohort was assessed by Kaplan-Meier log rank survival analysis. We demonstrated a positive correlation between Prox1 expression and Ki67 index. Prox1 also showed significant difference in expression according to chromogranin A and calcitonin immunohistochemical expression, with higher Prox1 expression in tumors with stronger chromogranin A or calcitonin staining. Prox1 expression did not correlate with PFS or OS based on Kaplan-Meier log rank survival analysis. In conclusion, Prox1 expression in MTC is positively correlated with Ki67 and with the immunohistochemical expression of chromogranin A and calcitonin. However, the present study does not support a role for Prox1 in MTC prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor arising from calcitonin-producing C cells of the thyroid; MTC accounts for 1–2% of all thyroid cancers and is the third most common thyroid malignancy after papillary and follicular carcinomas [1, 2]. Despite being relatively uncommon, MTC accounts for more than 14% of thyroid cancer-related deaths [3]. Follicular cell-derived well-differentiated thyroid cancers (namely papillary and follicular thyroid carcinomas) show an excellent prognosis with a 10-year survival rate of 93% for papillary thyroid carcinoma (PTC) and almost 100% for minimally invasive follicular thyroid carcinoma (FTC) [4, 5]. Conversely, patients with MTC have a worse prognosis, with 10-year survival rates of 100, 93, 71, and 21% for patients with disease stages I, II, III, and IV, respectively [1, 6]. Patient age over 50 years and TNM stage are the only independent prognostic factors identified in a series of sporadic and hereditary MTCs [7, 8]; extrathyroidal tumor extension and elevated postoperative calcitonin levels are also considered poor prognostic features [7, 9]. Unfortunately, so far, no biomarker has been proven to be associated with prognosis in multivariate analyses, with the possible exception of proliferative index evaluated with Ki67 staining, although further studies are needed to confirm this observation [10, 11].
Among thyroid cancer types, those of follicular cell origin show a consistent downregulation of Prospero homeobox protein 1 (Prox1), whereas hyperplastic C cells and MTCs have been demonstrated to express Prox1, together with secretory peptides (i.e., calcitonin and chromogranin A) [12, 13]. Prox1 is a homeobox transcription factor that plays a critical role in the development of various organs during embryogenesis; during postnatal life, alterations in its expression have been found in a variety of tumors [14,15,16,17,18,19,20,21]. Among these, in rectal neuroendocrine carcinomas, high Prox1 expression has been associated with increased metastatic potential and poor survival [22]. However, the prognostic utility of Prox1 in MTC has not been addressed yet.
Herein, we analyzed the immunohistochemical expression of Prox1 in a series of 32 MTCs and correlated it with patient outcome and clinicopathological features, including proliferative index (Ki67).
Materials and Methods
Patient Selection
Thirty-two cases of MTC with available clinical and pathological data and material available for review were included in the study. We excluded from our cohort patients affected by hereditary MTC in order to avoid possible bias concerning Prox1 levels in tumors of patients with different genetic mechanism of MTC onset. The study was approved by the Ethics Committee of the Canton of Vaud (no. 2016-01058).
All tissues were formalin-fixed and paraffin-embedded (FFPE). For each case, one representative slide with the corresponding tissue block (including both tumor and non-tumoral thyroid tissue) was selected.
Concerning pathological data, tumor (T) and lymph node (N) stage could be reliably determined for all tumors according to the TNM classification for thyroid cancer as outlined in the 8th edition of the AJCC Cancer Staging Manual; M stage was derived from the imaging reports contained in the electronic health records of the patients [23]. Clinical data, carcinoembryonic antigen (CEA), and calcitonin serum levels were retrieved from patient medical records (Table 1). Calcitonin serum levels were measured by electrochemiluminescence immunoassay (ECLIA) (Roche cobas); CEA serum levels by chemiluminescent microparticle immunoassay (CMIA) (Abbott).
Immunohistochemistry
Four-micrometer sections were obtained from selected FFPE tissue blocks and immunohistochemical staining was performed with the antibodies listed in Table 2 on a fully automated immunostainer (Ventana Benchmark XT; Ventana Medical Systems, Tucson, AZ) using a multimer detection kit (Ultra-View; Universal DAB; Ventana Medical Systems). Staining was performed according to the manufacturer’s instructions. Antigen retrieval was carried out in EDTA (pH 8.0) during 30 min and counter-staining was performed with Mayer Hematoxylin.
Immunohistochemical staining for Prox1, Ki67, and chromogranin A was available for all 32 cases of MTC, whereas calcitonin and CEA immunohistochemical staining was performed on 25 out of 32 cases due to tumor exhaustion in the available FFPE tissue block in the remaining 7 cases.
Immunohistochemical Evaluation of Ki67 and Prox1 Expression
Immunohistochemistry slides were scanned using the Hamamatsu C9600 NanoZoomer Digital Pathology slide scanner (Hamamatsu Photonics, Hamamatsu, Japan). Visualization was carried out using Slidepath Digital Image Hub, version 4.0.7 (Slidepath, Leica Biosystems, Milton Keynes, UK).
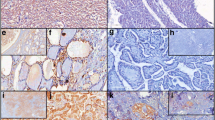
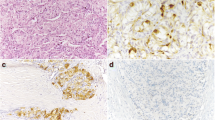
Tumor samples were investigated as follows: Prox1 and Ki67 immunohistochemistry slides (one of each for every study case) were scanned at low magnification (×4) to identify positive hotspots; two tumor hotspots were defined by using a 500-μm side square grid; the selected areas were printed in full-color scale at high magnification (× 20); Prox1- and Ki67-positive and Prox1- and Ki67-negative tumor cells were manually counted in those areas. Ki67-positive cells were counted in the same Prox1 tumor hotspots. Only Prox1 nuclear immunoreactivity was considered as positive, cytoplasmic staining being considered nonspecific (Fig. 1). The percentage of Prox1-positive and Ki67-positive cells was calculated.
Immunohistochemical Evaluation of CEA, Chromogranin A, and Calcitonin Expression
Chromogranin A, CEA, and calcitonin expression was manually scored based on intensity of immunohistochemistry cytoplasmic expression as weak (score = 1), moderate (score = 2), strong (score = 3), and intense (score = 4).
Statistical Analysis
Correlations between Prox1 expression, Ki67 expression, tumor size, and calcitonin and CEA serum levels prior to thyroidectomy were tested using linear regression analysis. The level of expression of Ki67 and Prox1 in different stages was evaluated using linear regression analysis. In addition, the Kruskal-Wallis H test and linear regression analysis were used to evaluate the difference between Prox1 and Ki67 expression in different staining intensity score subgroups of CEA, chromogranin A, and calcitonin.
The prognostic value of low versus high Prox1 expression was assessed using progression-free survival (PFS) and overall survival (OS) as the endpoints. PFS was defined as time from the date of initial diagnosis and treatment to the first occurrence of new metastases based on medical imaging reports, death, or last known consultation (censored). OS was defined as the time from diagnosis to death from any cause or last known consultation (censored). Survival curves were obtained from Kaplan-Meier estimates and compared by the log-rank test using cutoffs as the median value of Prox1 expression and 5% Ki67 expression, as reported by Pan et al. [24].
All statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY) and Stata/SE version 13.0 (StataCorp., College Station, TX). P values < 0.05 were considered as statistically significant.
Results
Patient Series and Clinicopathological Features
Thirty-two MTCs were analyzed (9 males and 23 females), with an average age of 50.6 years (range 21–72) (Table 1). The average lesion size was 2.4 (range 0.6–6.2) cm. Based on the 8th edition of the AJCC (American Joint Committee on Cancer) TNM system [23], 12/32 patients (37.5%) were classified as stage I, 6/32 (18.8%) as stage II, 1/32 (3%) as stage III, and 13/32 (40.7%) as stage IV.
Mean values of Prox1 and Ki67 expression were 59.1% (range 1.2–98.2%) and 1.6% (range 0.2–15.8%), respectively. Only two cases had foci of C-cell hyperplasia: one case with a single focus, of which no further material was available for immunohistochemical analysis after re-cut of the paraffin block, and a second one with two foci that showed weak reactivity.
Chromogranin A immunohistochemical intensity score was weak in 4/32 cases, moderate in 6/32 cases, strong in 15/32 cases, and intense in 7/32 cases. Calcitonin immunohistochemical intensity score was weak in 6/25 cases, moderate in 6/25 cases, strong in 8/25 cases, and intense in 5/25 cases. CEA immunohistochemical intensity score was low in 6/25 cases, moderate in 4/25 cases, strong in 6/25 cases, and very strong in 9/25 cases.
The median value of preoperative serum CEA was 29.9 ng/l (range 3.3–354.3); the median value of preoperative serum calcitonin was 828 ng/l (range 21.5–11,630).
Prox1 Expression in Medullary Thyroid Carcinoma
Prox1 expression significantly predicted Ki67 expression in regression analysis (beta = 0.373, p = 0.036).
Ki67 expression, but not Prox1 expression, was significantly higher in patients with stage IV disease (p = 0.010 and p = 0.174, respectively) and with lymph node metastasis (p = 0.03 and p = 0.321, respectively) based on linear regression analysis. Ki67 expression (beta = 0.518, p = 0.002), but not Prox1 expression (beta = 0.069, p = 0.708), was a predictor of the presence of distant metastasis using linear regression test.
Immunohistochemical intensity staining score subgroups of chromogranin A and calcitonin, but not CEA, significantly predicted Prox1 expression (p = 0.0004, p = 0.004, and p = 0.336, respectively) using linear regression analysis; accordingly, Prox1 expression showed significant difference using the Kruskal-Wallis H test analysis in different chromogranin A (p = 0.016) and calcitonin (p = 0.013) but not CEA (p = 0.293) immunohistochemical intensity staining score subgroups, with higher mean Prox1 expression in subgroups showing higher staining score, notably for chromogranin A (Fig. 2). No difference in Ki67 expression was observed in the respective immunohistochemical intensity staining score subgroups (p = 0.208, p = 0.239, and p = 0.237, respectively). No correlation was observed between Ki67 expression and preoperative serum levels of CEA or calcitonin (p = 0.516 and p = 0.796, respectively).
Both Prox1 and Ki67 did not show significant association with survival based on Kaplan-Meier log rank survival analyses using the median as a cutoff for Prox1 and using 5% expression as a cutoff for Ki67 (Fig. 3).
In Kaplan-Meier Log Rank survival analysis, a higher Ki67 expression showed a trend for association with a shorter OS (p = 0.066), b but did not affect PFS (p = 0.166) using 5% expression as a cutoff; blue line: Ki67 < 5%, red line: Ki67 > 5%. c Prox1 did not affect OS (p = 0.908) or d PFS (p = 0.841) using the median value of Prox1 expression as a cutoff; blue line: Prox1 expression < 9.1%, (median value), Prox1 expression > 59.1% (median value)
Discussion
Among patients affected by MTC, up to 70% present nodal metastases and up to 10% distant metastases [11]. Patients with progressive or metastatic disease who cannot be treated by surgery or other localized strategies are candidates for systemic therapy. However, conventional chemotherapy with cytotoxic agents provides limited benefit in patients with MTC, and approved tyrosine kinase inhibitors extend PFS but not OS [25,26,27]. Deeper understanding of the molecular pathways involved in MTC biology is critical to improve prognosis and to develop more effective treatments for improving loco-regional control, as well as to treat distant metastatic and recurrent disease. Apart from age (> 50 years) and TNM stage that are independent prognostic factors, no biomarkers have been identified as prognostic or predictive parameters to date. In the search for new biomarkers of aggressiveness that could help to better stratify MTC patients, we studied the expression of Prox1 in a series of 32 MTCs. We compared Prox1 expression with Ki67 proliferative index, with immunohistochemical staining intensity of chromogranin A, calcitonin, and CEA, and with preoperative serum levels of calcitonin and CEA.
High Prox1 mRNA levels have been documented in several tumors of neuroendocrine origin [22, 28,29,30,31,32,33,34]. In rectal neuroendocrine tumors (NETs), immunohistochemical expression of Prox1 has been associated with metastatic potential and poor survival; moreover, all but one metastases from rectal NETs showed very strong Prox1 immunoreactivity [22]. The exact mechanism through which Prox1 regulates proliferation, migration, and invasion of cancer cells is likely highly tissue-specific. In colorectal cancer, Prox1 is a direct target of overactivated WNT signaling, where it promotes productive autophagy and metabolic fitness of intestinal stem cells [34, 35]. In thyroid cancer cells of follicular origin (i.e., follicular, papillary and anaplastic carcinomas), Prox1 mRNA expression has shown a consistent downregulation as compared with adjacent normal tissues. In addition, restoration of Prox1 function suppresses not only the expression of genes associated with thyroid cancer development but also the malignant phenotype of thyroid carcinoma in vitro and in vivo. [12] Recently, matrix metalloproteinase-14 (MMP14) was shown to be a direct target suppressed by Prox1 in multiple cancer types [36], but it remains to be investigated whether loss of MMP14 contributes to the tumor suppressor role of Prox1 in thyroid cancer. Nevertheless, in thyroid cancers deriving from follicular cells, inactivation of Prox1 contributes to the malignant phenotype and may represent an essential step in thyroid cancer development [12].
On the contrary, hyperplastic C cells and MTCs have been shown to specifically express Prox1, together with secretory peptides, including calcitonin and chromogranin A. In these cells, Prox1 seems to be directly associated with the formation of secretory granules and products, as its knockdown reduces the number of secretory granules detected by electron microscopy and decreases the expression of secretory granule-related genes; Prox1 thus acts as a master transcriptional regulator of secretory granule biogenesis [13]. Our study confirmed the presence of higher Prox1 expression specifically in cases with higher score of immunohistochemical staining intensity of calcitonin and chromogranin A, but not CEA. This interesting finding is consistent with what has been shown in vitro, namely that the role of Prox1 in neuroendocrine maturation and its direct involvement as a transcriptional enhancer of the chromogranin A gene and other secretory granule-related genes [13]. The same study showed that forced expression of Prox1 did not alter the expression of calcitonin or calcitonin-related polypeptide alpha in cultured thyroid cancer cells [13]. Thus, overall, the evidence suggests that Prox1 is associated primarily with the status of neurosecretory granules in MTC cells. However, it is important to note that all of the MTC samples analyzed in that study [13] as well as in the present study did express calcitonin. It will therefore be interesting to also analyze Prox1 expression in calcitonin-negative MTC.
Our results showed a near-significant association between higher Ki67 and lower OS, in line with the recent meta-analysis by Pan et al. that demonstrated an association between higher Ki67 expression and more aggressive behavior of MTC, with lymph node and distant metastatic potential [24].
Even though Prox1 had a significant positive correlation with Ki67 in our patient population, it did not show a negative effect on patient survival, and was not correlated with higher stage of disease nor lymph node and distant metastatic potential of MTC. Therefore Prox1, in spite of its correlation with tumor proliferative index, does not seem to have a clinical impact on patient prognosis. Further studies are needed to elucidate Prox-1 expression in C-cell hyperplasia and in hereditary forms of MTC and to correlate these data with RET mutational status.
Conclusion
To summarize, we show here that Prox1 expression in MTC is associated with Ki67 expression and is positively correlated with higher levels of chromogranin A and calcitonin on immunohistochemical studies, thus potentially playing a role in MTC development in keeping with a positive regulatory effect on the calcitonin and chromogranin A genes. However, our results did not show statistically significant correlation between Prox1 or Ki67 expression and PFS or OS, even though an association was found between Ki67 and advanced stage. Studies of Prox1 in MTC have potential to help elucidate mechanisms related to C-cell biology and MTC pathobiology. Its potential role in clinical management of MTC patients warrants investigations in larger series.
References
Wells SA, Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF et al (2015) Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25:567–610
Raue F, Frank-Raue K (2015) Epidemiology and Clinical Presentation of Medullary Thyroid Carcinoma. Recent Results Cancer Res 204:61–90
Jaskula-Sztul R, Eide J, Tesfazghi S, Dammalapati A, Harrison AD, Yu XM, Scheinebeck C, Winston-McPherson G, Kupcho KR, Robers MB, Hundal AK, Tang W, Chen H (2015) Tumor-suppressor role of Notch3 in medullary thyroid carcinoma revealed by genetic and pharmacological induction. Mol Cancer Ther 14:499–512
Hundahl SA, Fleming ID, Fremgen AM, Menck HR (1998) A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer 15:2638–2648
Piana S, Frasoldati A, Di Felice E, Gardini G, Tallini G, Rosai J (2010) Encapsulated well-differentiated follicular-patterned thyroid carcinomas do not play a significant role in the fatality rates from thyroid carcinoma. Am J Surg Pathol 34:868–872
Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, Schlumberger M, Bigorgne JC, Dumontier P, Leclerc L, Corcuff B, Guilhem I (1998) Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC study group. Groupe d’étude des tumeurs à calcitonine. Clin Endocrinol (Oxf) 48:265–273
DeLellis RA, Al Ghuzlan A, Albores Saavedra J, Baloch ZW, Basolo F, Eliseri R, et al (2017) Medullary thyroid carcinoma. In: Lloyd R, Osamura RY, Kloppel G, Rosai J (ed) WHO Classification of Tumours of Endocrine Organs, 4th edn. IARC, Lyon, pp 211–214
Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH (2000) Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88:1139–1148.
Scopsi L, Sampietro G, Boracchi P, Del Bo R, Gullo M, Placucci M, Pilotti S (1996) Multivariate analysis of prognostic factors in sporadic medullary carcinoma of the thyroid. A retrospective study of 109 consecutive patients. Cancer 78:2173–2183
Tisell LE, Oden A, Muth A, Altipamark G, Molne J, Ahlman H, Nilsson O (2003) The Ki67 index as a prognostic marker in medullary thyroid carcinoma. Br J Cancer 89:2093–2097
DeLellis RA, Mangray, S (2017) Medullary thyroid carcinoma: a contemporary perspective. AJSP-Reviews and Reports 22:196–208
Choi D, Ramu S, Park E, Jung E, Yang S, Jung W, Choi I, Lee S, Kim KE, Seong YJ, Hong M, Daghlian G, Kim D, Shin E, Seo JI, Khatchadourian V, Zou M, Li W, de Filippo R, Kokorowski P, Chang A, Kim S, Bertoni A, Furlanetto TW, Shin S, Li M, Chen Y, Wong A, Koh C, Geliebter J, Hong YK (2016) Aberrant Activation of Notch Signaling Inhibits PROX1 Activity to Enhance the Malignant Behavior of Thyroid Cancer Cells. Cancer Res 76:582–593
Ishii J, Yazawa T, Chiba T, Shishido-Hara Y, Arimasu Y, Sato H, Kamma H (2016) PROX1 Promotes Secretory Granule Formation in Medullary Thyroid Cancer Cells. Endocrinology 157:1289–1298
Elsir T, Smits A, Lindstrom MS, Nistér M (2012) Transcription factor PROX1: its role in development and cancer. Cancer metastasis reviews 31:793–805
Lavado A, Oliver G (2007) Prox1 expression patterns in the developing and adult murine brain. Dev Dyn 236:518–524
Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G (2002) Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225:351–357
Wigle JT, Chowdhury K, Gruss P, Oliver G (1999) Prox1 function is crucial for mouse lens-fibre elongation. Nature Genet 21:318–322
Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev 118:147–155, 2002
Dyer MA, Livesey FJ, Cepko CL, Oliver G (2003) Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 34:53–58
Risebro CA, Searles RG, Melville AA, Ehler E, Jina N, Shah S, et al (2009) Prox1 maintains muscle structure and growth in the developing heart. Development 136:495–505
Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K (2002) Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21:4593–4599
Jernman J, Kallio P, Hagstrom J, Valimaki MJ, Haapasalo H, Alitalo K, Arola J, Haglund C (2015) PROX1 is involved in progression of rectal neuroendocrine tumors, NETs. Virch Archiv 467:279–284
American Joint Committee on Cancer. Thyroid – Medullary. In: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017: 891.
Pan DH, Wen DY, Luo YH, Chen G, Yang H, Chen JQ, He Y (2017) The diagnostic and prognostic values of Ki-67/MIB-1 expression in thyroid cancer: a meta-analysis with 6,051 cases. Onco Targets Ther 10:3261–3276
Roy M, Chen H, Sippel RS (2013) Current understanding and management of medullary thyroid cancer. Oncologist 18:1093–1100
Hadoux J, Schlumberger M (2017) Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Pract Res Clin Endocrinol Metab 31:335–347
Liu JW, Chen C, Loh EW, Chu CC, Wang MY, Ouyang HJ, Chang YT, Zhuang WZ, Chou CW, Huang DJ, Lee CH, Yen Y, Tam KW (2018) Tyrosine kinase inhibitors for advanced or metastatic thyroid cancer: a meta-analysis of randomized controlled trials. Curr Med Res Opinion 34:795–803
Elsir T, Eriksson A, Orrego A, Lindstrom MS, Nistér M (2010) Expression of PROX1 Is a common feature of high-grade malignant astrocytic gliomas. J Neuropathol Exp Neurol 69:129–138
Liu Y, Ye X, Zhang JB, Ouyang H, Shen Z, Wu Y, Wang W, Wu J, Tao S, Yang X, Qiao K, Zhang J, Liu J, Fu Q, Xie Y (2015) PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing beta-catenin expression and nuclear translocation. Oncogene 34:5524–5535
Dudas J, Mansuroglu T, Moriconi F, Haller F, Wilting J, Lorf T, Fuzesi L, Ramadori G (2008) Altered regulation of Prox1-gene-expression in liver tumors. BMC Cancer 8:92
Shimoda M, Takahashi M, Yoshimoto T, Kono T, Ikai I, Kubo H (2006) A homeobox protein, prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res 12:6005–6011
Wiener Z, Hogstrom J, Hyvonen V, Band AM, Kallio P, Holopainen T, et al (2014) Prox1 promotes expansion of the colorectal cancer stem cell population to fuel tumor growth and ischemia resistance. Cell Rep 8:1943–1956
Skog M, Bono P, Lundin M, Louhimo J, Linder N, Petrova TV, Andersson LC, Joensuu H, Alitalo K, Haglund CH (2011) Expression and prognostic value of transcription factor PROX1 in colorectal cancer. Br J Cancer 105:1346–1351
Petrova TV, Nykanen A, Norrmen C, Ivanov KI, Andersson LC, Haglund C, et al (2008) Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 13:407–419
Ragusa S, Cheng J, Ivanov KI, Zannger N, Ceteci F, Bernier-Latmani J, et al (2014) PROX1 promotes metabolic adaptation and fuels outgrowth of Wnt (high) metastatic colon cancer cells. Cell Rep 8:1957–1973
Gramolelli S, Cheng J, Martinez-Corral I, Vaha-Koskela M, Elbasani E, Kaivanto E, et al (2018) PROX1 is a transcriptional regulator of MMP14. Sci Rep 8:9531
Acknowledgements
GPS is partly supported by a 2016 Leenaards Foundation Fellowship for Academic Advancement in Clinical Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saglietti, C., La Rosa, S., Sykiotis, G.P. et al. Expression of Prox1 in Medullary Thyroid Carcinoma Is Associated with Chromogranin A and Calcitonin Expression and with Ki67 Proliferative Index, but Not with Prognosis. Endocr Pathol 30, 138–145 (2019). https://doi.org/10.1007/s12022-019-9576-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-019-9576-5