Abstract
Purpose
To evaluate the potential role of immunohistochemical changes in stanniocalcin 1 (STC1) and stanniocalcin 2 (STC2) expressions in papillary thyroid cancer (PTC) tissues in the disease’s diagnosis and to investigate their relationship with classical clinicopathological prognostic factors.
Methods
The study included 100 patients with PTC. Normal thyroid tissue adjacent to the tumor was taken as the control group. Clinicopathological prognostic features at the time of diagnosis of patients were recorded. STC1 and STC2 expressions of tumor tissue and adjacent normal tissue were determined immunohistochemically.
Results
The sensitivity of STC1 in the diagnosis of PTC was 93%, the specificity was 94%, positive predictive value (PPV) 93.9%, and negative predictive value (NPV) 93.1%. It was determined that the STC1 staining score in tumor tissue was positively correlated with the disease TNM stage score (r = 0.259, p = 0.009) and the increase in STC1 staining score were independent risk factors that increased the risk of lymph node metastasis (R2 = 0.398, p < 0.001). While 21% of the tumor tissues were stained with STC2, none of the normal thyroid tissues adjacent to the tumor tissue showed any staining with STC2. No correlation was found between STC2 immunohistochemical staining of tumor tissue and clinicopathological risk factors for the disease.
Conclusion
Increased STC1 expression in thyroid lesions may be helpful in diagnosing PTC. In addition, since increased STC1 expression in PTC tissues is associated with the risk of lymph node metastasis, it may be an efficient marker for predicting the prognosis of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine cancer and papillary thyroid cancer (PTC) constitutes 80–85% of these. The incidence of thyroid cancer has been increasing recently, due to this rapid increase in the incidence of PTC, there is a need for accurate pathological diagnosis of benign and malignant thyroid lesions [1, 2]. Histopathological features of primarily diagnosed PTC include typical papillary structures and nuclear changes such as a prominent nuclear membrane, nuclear grooves, intranuclear inclusions, and nuclear overlap. Psammoma bodies and hyaline bodies are other notable histological features of PTC. However, some benign thyroid pathologies can also reveal these morphological changes [3,4,5]. In spite of that, the aforementioned typical nuclear changes may not be observed in some PTC cases. For instance, the cystic variant of PTC shows large edematous papillary structures covered with nucleated cells with scattered chromatin and flattened surfaces [6,7,8]. The role of various immunohistochemical evaluations in the diagnosis of malignant thyroid lesions has been evaluated [9, 10]. However, a sensitive biomarker to provide the definitive diagnosis has not been revealed yet.
It is important to determine the prognosis correctly for the treatment plan in PTC. However, due to the difficulties experienced in estimating PTC cases with aggressive features, the determination of new prognostic biomarkers has gained importance [11,12,13]. The role of potential protein biomarkers, namely galectin-3, cathepsin-B, cytokeratin-19, and E-cadherin in determining the disease prognosis has been investigated so far. Nevertheless, none of these have been proven to be beneficial in determining the risk of PTC [14,15,16].
BRAF mutation is one of the most common molecular events in PTC. This mutation activates the mitogen-activated protein kinase (MAPK) pathway, leading to loss of control in the cellular cycle, which initiates the development of malignancy. The effect of BRAF mutations on the clinical outcomes of PTC is controversial [17]. Various studies have shown that the presence of BRAF V600E mutation is associated with an aggressive clinical course in PTC, such as extrathyroidal invasion and lymph node metastasis [11, 18, 19]. On the other hand, there are studies that do not support these results [20, 21]. Therefore, more reliable biomarkers are needed to predict prognosis of the disease.
Stanniocalcin (STCs) is a glycoprotein hormone involved in various biological processes, including regulation of calcium-phosphate homeostasis, cell proliferation, apoptosis, inflammation, oxidative stress, and cancer development [22]. Two paralogs of STCs (STC1 and STC2) have been identified in mammals and are widely expressed in a variety of tissues. Since they are not detected in serum, they are thought to exert their biological functions via the autocrine and/or paracrine pathway [23, 24] The role of STC1 in diagnosing and determining the prognosis of various cancers including esophageal and gastric cancer have been shown to date [24,25,26]. Another glycoprotein, STC2, a homolog of STC1, is suggested to be a marker in the diagnosis and prognosis of several cancers like renal cell carcinoma, and colorectal cancer [27,28,29].
Cancer cells provide most of their energy by glycolytic activity rather than oxidative phosphorylation, which is also known as the Warburg effect. STC1 has an important role in the development of this adaptive response. STC1 upregulates uncoupling protein 2 expression level and decreases mitochondrial membrane potential. Thus, it increases lactate production for anaerobic glycolysis. It also reduces formation of the reactive oxygen species (ROS) resulting decrement in oxidative and endoplasmic reticulum (ER) stresses, and makes tumors resistant to ROS. Thereby facilitating the survival of cancer cells by inhibiting apoptosis [24, 30]. STC1 is also thought to promote proliferation of tumor cells [31]. Ma et al. showed that STC1 stimulates cell cycle progression, increases cancer cell proliferation by increasing the expression of cyclin D1, Cdk4, and Cdk6, and by suppressing p21 expression, accelerating the G1/S transition [32].
STC1 has been evaluated in a limited number of patients with thyroid cancer. In the study conducted by Hayase et al., STC1 was found to be highly expressed in cell cultures obtained from the primary cancer tissue and metastases of Follicular Thyroid Cancer and from the primary tumor of anaplastic thyroid carcinoma [33]. Dai et al. showed that administration of recombinant STC1 in thyroid cancer cell lines increased cell proliferation [34]. On the other hand, the roles of the STC1 and STC2 in the diagnosis and predicting prognosis of PTC disease have not been evaluated until now.
In this study, we aimed to evaluate the role of immunohistochemical changes in STC1 and STC2 expressions in PTC tissues in diagnosis and their relationship with classical clinicopathological prognostic factors (lymph node metastasis, vascular invasion, multifocality, tumor size, aggressive histology, TNM staging, microscopic extra-thyroidal spread, capsular invasion, and macroscopic invasion).
Materials and methods
Selection of the subjects to be included in the study
The G-Power 3.1.9.2 program was used to determine the sample size. When the effect size was 0.35, the type 1 error rate was 0.05, and test power was accepted as 95%, it was determined that it would be appropriate to work with 90 patient tissue samples. In order to stay on the safe side, it was deemed appropriate to design a study with 100 cases. The data of 350 patients who were followed up with the diagnosis of PTC in our clinic between 2016 and 2020 were examined retrospectively. Patients with a previous history of malignancy other than PTC, lost to follow-up and whose pathology specimens were not be able to be obtained were excluded from the study. A hundred patients with histologically confirmed PTC who underwent bilateral or unilateral thyroidectomy were enrolled in this study (between 19 and 69 years, 74 females, 26 males). Normal thyroid tissue adjacent to the tumor tissue was taken as the control group. Based on the data of the patients at the time of diagnosis, risk scores were determined according to the PTC risk score verified by the American Thyroid Association (ATA) in 2015, and patients were classified into two groups as medium or high risk and low risk [35]. Of the patients, 52 (52%) had medium or high risk, while 48 (48%) had low risk.
Determination of clinicopathological features
Clinicopathological prognostic features of the patients at the time of the first diagnosis were documented. Gender, age (under 45 years, 45 years and above), tumor size, tumor number (single, multiple), lymph node metastasis number, side of the tumor (right, left, right, and left), T stage (T1, T2, T3, T4), N stage (N0, N1), TNM stage (1, 2, 3, 4), histopathological variant (classical, follicular, Whartin-like and oncocytic), capsular invasion, vascular invasion, intrathyroidal extension, extrathyroidal extension, multifocality, radioactive iodine (RAI) treatment and disease follow-up times were recorded.
Immunohistochemical evaluation
Trinocular Nikon Eclipse Ni-U microscope (origin; Japan). STC1 immunohistochemical staining using rabbit polyclonal antibody ab229477 (Abcam, origin; USA) at 1/600 dilution, 1/100 dilution using rabbit polyclonal antibody STC2 ab63057 (Abcam, origin; USA) Dako Omnis fully automated immunohistochemistry instrument (origin; Denmark) used to evaluate preparations. Human invasive breast tumor tissue and mouse spleen, and pancreas tissue were used as positive controls. 5-micron sections were taken from the paraffin tissue that best represents the tumor with a Leica rotary microtome (origin; Germany) and stained with Dako Omnis fully automatic immunohistochemistry device. Standard brown DAB (3,3′ diaminobenzidine tetrahydrochloride) was used for positive coloring. Double-blind evaluation of STC1 and STC2 was performed by two pathologists with a trinocular Nikon Eclipse Ni-U microscope. Optical microscope image taken at ×20 and ×100 magnification of preparations with Nikon DS-RI2.
The staining score for STC1 and STC2 was calculated semi-quantitatively according to the percentage of positive staining and staining intensity (Figs. 1 and 2). A definite positive reaction was described as a brown signal in the cytoplasm or cell membrane. Staining intensity was scored as 0 (no staining), 1 (weak staining in light yellow), 2 (medium staining in yellow-brown), 3 (strong staining in brown). The percentage of positive staining was scored as ≤10%, 11–50%, 51–80%, and ≥81% as 1, 2, 3, and 4, respectively. The total score obtained from these scorings was accepted as the final staining score. The calculated score ranges from a minimum of 1 to a maximum of 7.
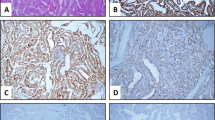
Evaluation staining with STC1 of papillary thyroid carcinoma tissues. A Papillary thyroid carcinoma with prominent papillary structures (B) 1+ staining with STC1 in papillary thyroid carcinoma (C) 2+ staining with STC1 in papillary thyroid carcinoma (D) 3+ staining with STC1 in papillary structures in papillary thyroid carcinoma (A: H&E 40×, B, C, D: DAB 100×)
Evaluation staining with STC2 of papillary thyroid carcinoma tissues. A Papillary thyroid carcinoma with prominent papillary structures and nuclear inclusions (B) 3+ staining with SCT2 in invasive breast carcinoma as positive control (C) 1+ staining with STC2 in papillary thyroid carcinoma (D) 2+ staining with STC2 in papillary thyroid carcinoma (A: H&E 100×, B:DAB 20×, C, D:100×)
Statistical analysis
Descriptive statistics were used to summarize study data. The relationship between categorical variables was assessed using Chi-square and Fisher’s exact tests. The diagnostic power of STC1 and STC2 for PTC was evaluated by receiver operating characteristic (ROC) curve analysis. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated in diagnosing PTC. The relationship between STC1 staining score and clinicopathological findings in PTC patients was evaluated by Spearman correlation analysis. The independent effect of increased STC1 staining score on the risk of lymph node metastasis was evaluated by multivariate logistic regression analysis. SPSS Windows version 22.0 package program was used for statistical analysis and p < 0.05 was considered statistically significant.
Results
Demographic, clinical, and biochemical characteristics of the patients are given in Table 1. Among all of the patients, 99% (99/100 patients) underwent total thyroidectomy (TTx), while only 1% (1/100 patients) underwent lobectomy. Lymph node dissection was performed for 21% of the patients (21/100). Three patients who underwent TTx were re-operated due to recurrence.
According to the ATA risk scoring system, patients were classified into three groups, 48% (48/100 patients) of the patients defined in the low-risk group, 30% (30/100 patients) were in the medium-risk group, and the remaining 22% (22/100 patients) were in the high-risk group. 65% of the cases received RAI treatment. During a mean follow-up period of 42.99 ± 19.68 months, ninety-four patients followed without any progression whereas, three patients developed recurrence after treatment. A total of 3 metastasis and among them 2 deaths were reported.
The status of clinicopathological findings according to the immunohistochemically STC1 staining intensity is shown in Table 2. 93% of PTC tissues and only 6% of normal thyroid tissues adjacent to tumor tissue were stained with STC1. Of the tumor tissues stained with STC1, 34% were stained light, 33% moderate, and 31% high intensity.
STC1 staining intensity in tumor tissue was not found to be associated with age, gender, tumor size, multifocal, intra-, and extra-thyroid extension, lymph node metastasis, distant organ metastasis, and disease stage (Table 2, p < 0.05 for each).
When the relationship between the STC1 staining score and clinicopathological findings was evaluated, the final staining score showed a positive correlation with the disease TNM stage score (r = 0.259, p = 0.009). In the multivariate logistic regression analysis, the increase in the STC1 staining score of the tumor tissue was demonstrated to be an independent risk factor that increases the risk of lymph node metastasis (Table 3, R2 = 0.398, p < 0.001).
While 21% of the tumor tissues were stained with STC2, none of the normal thyroid tissues adjacent to the tumor tissue revealed STC2 staining. Macrophages of tumor tissues were stained with STC2. No correlation was found between STC2 expression of tumor tissue and clinicopathological risk factors (p > 0.05).
The diagnostic power of STC1 and STC2 to predict PTC was evaluated by ROC analysis. The area under the curve was determined as 0.979 for STC1 and 0.605 for STC2 (Figs. 3 and 4). The sensitivity of STC1 in the diagnosis of PTC was 93%, the specificity was 94%, PPV 93.9%, and NPV 93.1%. While the specificity of STC2 in the diagnosis of PTC was 100%, its sensitivity was only 21%. When STC1 and STC2 were investigated together, the sensitivity was 57%; the specificity was 97% (Table 4).
Discussion
In the study, it was shown that, the expression of STC1 was significantly higher in PTC tissues compared to normal thyroid tissue. In light of this information, STC1 can be consider as a good marker for the diagnosis of PTC with 93% sensitivity and 94% specificity. In addition, the intense pattern of STC1 staining in pathology specimen shown to be associated with adverse clinicopathological features such as high TNM stage and lymphatic metastasis. This suggests that STC1 can also be used as a biomarker to predict PTC prognosis.
STC1 is located in mitochondria, STC2 is located in the ER and golgi apparatus and regulates the function of these organelles via the autocrine/paracrine pathway [22]. In cancer cells, expression of hypoxia-inducible factor (HIF) increases in response to the hypoxic microenvironment and then it induces STC1 expression [36]. With the Warburg effect, STC1 shifts mitochondrial respiration to a more glycolytic metabolic profile, increasing the tolerance of tumor cells to hypoxia and inhibiting apoptosis [24]. The role of mitochondrial abnormalities in the development of thyroid cancer is also well known [37]. However, the role of STC1, which regulates mitochondrial functions, in the diagnosis of PTC has never been evaluated. In this study, STC1 immunostaining was found to be an efficient marker in the diagnosis of PTC with 93% sensitivity, 94% specificity, 93.9% PPV, and 93.1% NPV. Although most of the PTC tissues were stained with STC1, intensities showed differences. In addition, the staining intensity of normal thyroid tissues was mostly strong in PTCs and weak to moderate in non-PTC lesions. Failure to stain normal thyroid tissue other than PTC may be diagnostically important. Although the specificity of STC2 immunostaining for PTC was 100%, its sensitivity was very low (21%). In the literature, no immunohistochemical marker with this sensitivity and specificity is demonstrated. In a meta-analysis by Xin et al., which asses the markers that may have diagnostic value in PTC; the sensitivity for Gal-3 was 84.2% and specificity 83.2%, for HBME-1 92.8% and specificity 86.4%, and for CK-19 81.6% and specificity 87.2% [32]. For the diagnosis of PTC, MMP9 sensitivity was 83.5%, specificity was 79.1%, PPV was 83.1%, and NPV was 79.6% [33]. In this current study, the increase in STC1 expression in PTC may be related to the regulation of mitochondrial respiration, which has a role in the pathogenesis of the disease. On the other hand, STC2 plays an important role in cytoprotection against ER/oxidative stress-induced apoptosis [23]. The lower expression of STC2 in PTC tissues may be related to the fact that ER stress is not a major problem in the disease.
PTC is a curable disease with a good prognosis, dismally 8–23% of patients present local recurrences and/or distant metastases [38]. In recent years, the primary goal of PTC management has been to identify cases with potentially aggressive diseases that require specific treatment and specific follow-up programs. Disease prognosis is very significant in deciding whether RAI treatment is needed and the level of TSH suppression. Classical risk factors (age at diagnosis, large tumor size, aggressive histological variants, extra-thyroidal extension, lymph node metastasis, and distant metastasis) are occasionally insufficient in determining the prognosis [35, 39]. Therefore, the roles of various biomarkers have been investigated [40].
Tumor microenvironment is important in tumor progression and metastasis. As a result of rapid proliferation of tumor cells, a hypoxic environment occurs and HIF expression increases. Increased STC1 in response to HIF stimulates cell proliferation, reduces apoptosis, thus promotes metastasis and invasion [24, 41, 42]. A recent meta-analysis showed that high STC1 adversely affects the prognosis of solid tumors [43]. The role of STC1 in predicting PTC prognosis has not been evaluated to date. In this study, when the relationship between the percentages of staining of tumor tissue with STC1 and clinicopathological findings was evaluated by Spearman correlation analysis, it was shown that the increase in the percentage of STC1 immunostaining was associated with more unfavorable clinicopathological features such as lymph node metastasis, ATA risk score and TNM stage (respectively, r = 0.236, p = 0.018 and r = 0.201, p = 0.045). In the multivariate logistic regression analysis, being over 45 years old and the increase in the percentage of STC1 shown to be responsible for the increased risk of lymph node metastasis (R2 = 0.398, p < 0.001). The higher expression of STC1 in PTC cases with poor prognostic features may be related to their more hypoxic microenvironment. Wang et al. found higher expression of HIF-1α in PTC tissues with lymph node metastases [44]. Burrows et al. also revealed that reduction of HIF-1 α activity with phosphoinositide 3-kinase inhibitor in follicular thyroid cancer cells reduced tumor growth and metastatic lung colonization [45]. In hypoxic conditions, thyroid cancer cell mitochondria produce high levels of ROS. Mitochondrial ROS contribute to tumor growth, epithelial-mesenchymal transition, cancer invasion, and metastasis [37]. In addition, STC1 stimulates tumor neo-angiogenesis, thereby providing sufficient oxygen and nutrients for tumor cell survival and proliferation and promoting distant metastasis [24].
There are no approved drug treatments other than tyrosine kinase inhibitors for PTC patients resistant to radioiodine therapy [46]. STC1 may be a potential new target for the development of targeted therapies, especially in this patient group. Further studies are needed on this subject.
In our study, STC2 did not show immunostaining in normal thyroid tissue adjacent to the tumor tissue. Only 21% of the tumor tissues were stained. No correlation was found between PTC prognosis and STC2 immunostaining of tumor tissue. These results suggest that immunohistochemical evaluation of STC2 will not be useful in predicting PTC prognosis.
The strength of this study is that the rate of staining with STC1 is low (6%) in normal thyroid tissue and high (93%) in PTC tissues, suggesting its potential to be used as a diagnostic marker. However, the study also has some limitations. In this study, the expression of STC1 and STC2 was evaluated only immunohistochemically. Expression levels of these genes and protein levels in tissue could not be measured by PCR. In addition, the level of HIF-1, which indicates tissue hypoxia, was not evaluated.
In conclusion, because of its high sensitivity and specificity, STC1 may be an efficient marker to confirm the diagnosis of PTC cases that do not show typical histopathological features. We also think that it will be useful in determining the prognosis of PTC. Further studies are needed.
References
E.L. Mazzaferri, R.T. Kloos, Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 86, 1447–163 (2001)
L. Leenhardt, P. Grosclaude, L. Chérié-Challine, Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 14, 1056–1060 (2004)
Z.W. Baloch, V.A. LiVolsi, S.L. Asa, J. Rosai, M.J. Merino, G. Randolph et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine‐Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 36, 425–437 (2008)
V.A. LiVolsi, Papillary thyroid carcinoma: an update. Mod. Pathol. 24, 1–9 (2011)
R.A. DeLellis, Pathology and genetics of tumours of endocrine organs, (IARC, 2004), pp. 50–66.
M. Miettinen, A.J. Kovatich, P. Kärkkäinen, Keratin subsets in papillary and follicular thyroid lesions. Virchows Arch. 431, 407–413 (1997)
Y. Yamamoto, K. Izumi, H. Otsuka, An immunohistochemical study of epithelial membrane antigen, cytokeratin, and vimentin in papillary thyroid carcinoma. Recognition of lethal and favorable prognostic types. Cancer 70, 2326–2333 (1992)
K. McLaren, D. Cossar, The immunohistochemical localization of S100 in the diagnosis of papillary carcinoma of the thyroid. Hum. Pathol. 27, 633–636 (1996)
H. Liu, F. Lin, Application of immunohistochemistry in thyroid pathology. Arch. Pathol. Lab Med. 139, 67–82 (2015)
L.L. de Matos, A.B. Del Giglio, C.O. Matsubayashi, M. de Lima Farah, A. Del Giglio, M.A. da Silva Pinhal, Expression of CK-19, galectin-3 and HBME-1 in the differentiation of thyroid lesions: systematic review and diagnostic meta-analysis. Diagn. Pathol. 7, 1–10 (2012)
T.H. Kim, Y.J. Park, J.A. Lim, H.Y. Ahn, E.K. Lee, Y.J. Lee et al. The association of the BRAFV600E mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta‐analysis. Cancer 118, 1764–1773 (2012)
X. Liu, C. Su, J. Xu, D. Zhou, H. Yan, W. Li et al. Immunohistochemical analysis of matrix metalloproteinase 9 predicts papillary thyroid carcinoma prognosis. Oncol. Lett. 17, 2308–2316 (2019)
H. Toy, O. Etli, Z.E. Celik, A.S. Alikanoglu, Associations between nucleus size, and immunohistochemical Galectin-3, Cytokeratine-19 and Hbme-1 markers in thyroid papillary carcinoma: a morphometric analyze. Pathol. Oncol. Res. 25, 401–408 (2019)
O.L. Griffith, C.G. Chiu, A.M. Gown, S.J. Jones, S.M. Wiseman, Biomarker panel diagnosis of thyroid cancer: a critical review. Expert Rev. Anticancer Ther. 8, 1399–1413 (2008)
Q. Song, D. Wang, Y. Lou, C. Li, C. Fang, X. He et al. Diagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn. Pathol. 6, 1–6 (2011)
L.M. Brown, S.M. Helmke, S.W. Hunsucker, R.T. Netea‐Maier, S.A. Chiang, D.E. Heinz et al. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol. Carcinog. 45, 613–626 (2006)
R.S. Scheffel, J.M. Dora, A.L. Maia, BRAF mutations in thyroid cancer. Curr. Opin. Oncol. 34, 9–18 (2022)
Y. Tao, F. Wang, X. Shen, G. Zhu, R. Liu, D. Viola et al. BRAF V600E Status Sharply Differentiates Lymph Node Metastasis-associated Mortality Risk in Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 106, 3228–3238 (2021)
A. Czarniecka, M. Oczko-Wojciechowska, M. Barczyński, BRAF V600E mutation in prognostication of papillary thyroid cancer (PTC) recurrence. Gland Surg. 5, 495–505 (2016)
D. Ahn, J.S. Park, J.H. Sohn, J.H. Kim, S.K. Park, A.N. Seo et al. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx 39, 198–203 (2012)
A. Nasirden, T. Saito, Y. Fukumura, K. Hara, K. Akaike, A. Kurisaki-Arakawa et al. In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF V600E mutation. Virchows Arch. 469, 687–696 (2016)
B. Yeung, A. Law, C.K. Wong, Evolution and roles of stanniocalcin. Mol. Cell Endocrinol. 349, 272–280 (2012)
A.D. Joshi, New Insights Into Physiological and Pathophysiological Functions of Stanniocalcin 2. Front. Endocrinol. (Lausanne) 11, 172 (2020)
F. Zhao, G. Yang, M. Feng, Z. Cao, Y. Liu, J. Qiu et al. Expression, function and clinical application of stanniocalcin-1 in cancer. J. Cell Mol. Med. 24, 7686–7696 (2020)
M. Shirakawa, Y. Fujiwara, Y. Sugita, J.H. Moon, S. Takiguchi, K. Nakajima et al. Assessment of stanniocalcin-1 as a prognostic marker in human esophageal squamous cell carcinoma. Oncol. Rep. 27, 940–946 (2012)
T. Arigami, Y. Uenosono, S. Ishigami, T. Hagihara, N. Haraguchi, D. Matsushita et al. Expression of stanniocalcin 1 as a potential biomarker of gastric cancer. Oncology 83, 158–164 (2012)
H.A. Meyer, A. Tölle, M. Jung, F.R. Fritzsche, B. Haendler, I. Kristiansen et al. Identification of stanniocalcin 2 as prognostic marker in renal cell carcinoma. Eur. Urol. 55, 669–678 (2009)
K. Ieta, F. Tanaka, T. Yokobori, Y. Kita, N. Haraguchi, K. Mimori et al. Clinicopathological significance of stanniocalcin 2 gene expression in colorectal cancer. Int J. Cancer 125, 926–931 (2009)
S. Li, Q. Huang, D. Li, L. Lv, Y. Li, Z. Wu, The significance of Stanniocalcin 2 in malignancies and mechanisms. Bioengineered 12, 7276–7285 (2021)
S. Ohkouchi, G.J. Block, A.M. Katsha, M. Kanehira, M. Ebina, T. Kikuchi et al. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol. Ther. 20, 417–423 (2012)
J. Hou, J. Cheng, Z. Dai, N. Wei, H. Chen, S. Wang et al. Molecular and Clinical Significance of Stanniocalcin-1 Expression in Breast Cancer Through Promotion of Homologous Recombination-Mediated DNA Damage Repair. Front Cell Dev. Biol. 9, 731086 (2021)
X. Ma, L. Gu, H. Li, Y. Gao, X. Li, D. Shen et al. Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma. J. Transl. Med. 13, 56 (2015)
S. Hayase, Y. Sasaki, T. Matsubara, D. Seo, M. Miyakoshi, T. Murata et al. Expression of stanniocalcin 1 in thyroid side population cells and thyroid cancer cells. Thyroid 25, 425–436 (2015)
D. Dai, Q. Wang, X. Li, J. Liu, X. Ma, W. Xu, Klotho inhibits human follicular thyroid cancer cell growth and promotes apoptosis through regulation of the expression of stanniocalcin-1. Oncol. Rep. 35, 552–558 (2016)
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016)
H.Y. Yeung, K.P. Lai, H.Y. Chan, N.K. Mak, G.F. Wagner, C.K. Wong, Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 146, 4951–4960 (2005)
S.A. Dabravolski, N.G. Nikiforov, A.D. Zhuravlev, N.A. Orekhov, L.M. Mikhaleva, A.N. Orekhov, The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. Int J. Mol. Sci. 23, 460 (2021)
K. Guo, Z. Wang, Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int. J. Clin. Exp. Pathol. 7, 5393–5403 (2014)
R.M. Tuttle, A.S. Alzahrani, Risk stratification in differentiated thyroid cancer: from detection to final follow-up. J. Clin. Endocrinol. Metab. 104, 4087–4100 (2019)
G. Riesco-Eizaguirre, P. Santisteban, Advances in the molecular pathogenesis of thyroid cancer: lessons from the cancer genome. Eur. J. Endocrinol. 175, 203–217 (2016)
L. Barthel, M. Hadamitzky, P. Dammann, M. Schedlowski, U. Sure, B.K. Thakur et al. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 41, 53–75 (2022)
N. Kheshtchin, J. Hadjati, Targeting hypoxia and hypoxia-inducible factor-1 in the tumor microenvironment for optimal cancer immunotherapy. J. Cell Physiol. 237, 1285–1298 (2022)
R. Li, R. Liu, S. Wu, S. Zheng, L. Ye, Y. Shao, Prognostic value of STC1 in solid tumors: a meta-analysis. Biomark. Med. 16, 253–263 (2022)
Y. Wang, Q. Hua, Clinical significance of HIF-1 alpha,VEGF and VEGF-C expression in papillary thyroid carcinoma. Lin. Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 21, 204–206 (2007)
N. Burrows, M. Babur, J. Resch, S. Ridsdale, M. Mejin, E.J. Rowling et al. GDC-0941 inhibits metastatic characteristics of thyroid carcinomas by targeting both the phosphoinositide-3 kinase (PI3K) and hypoxia-inducible factor-1α (HIF-1α) pathways. J. Clin. Endocrinol. Metab. 96, E1934–E1943 (2011)
P. Fallahi, S.M. Ferrari, M.R. Galdiero, G. Varricchi, G. Elia, F. Ragusa et al. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin Cancer Biol. 79, 180–196 (2022)
Author information
Authors and Affiliations
Contributions
H.K. and S.S. was responsible for the design, management, data collection, analysis and writing of the study. M.C. was involved in the design of the study and the immunohistochemical evaluation of tissues. S.M.B. was responsible for data collection. R.O.Y. and M.K. were responsible for the immunohistochemical evaluation of tissues.
Funding
This study has receieved financial support from Suleyman Demirel University Scientific Research Support Fund (TTU-2020-8216).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no competing interests that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
This study was conducted according to the principles of the Helsinki Declaration after it was approved by local ethical committee.
Informed consent
Both written and verbal consents were obtained from voluntary participants before including in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sengun, S., Korkmaz, H., Ciris, M. et al. Diagnostic and prognostic value of Stanniocalcin 1 expression in papillary thyroid cancer. Endocrine 78, 95–103 (2022). https://doi.org/10.1007/s12020-022-03126-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03126-4