Abstract
PROX1 is a homeobox transcription factor involved in the development of the lens, liver and heart and found upregulated in colorectal cancers. We studied PROX1 expression by immunohistochemistry in rectal neuroendocrine tumors (NETs). Approximately 10 to 15 % of gastroenteropancreatic NETs occur in the rectum, and some may metastasize. Yet little is known about the molecular pathogenesis of rectal NETs or their metastasis propensity. The objectives were to find out whether PROX1 plays a role in progression of rectal NETs and whether it has value as prognostic marker. In grading of rectal NETs, we applied the WHO 2010 classification. We carried out immunohistochemical staining of PROX1 on 72 primary tumors and six metastases and evaluated nuclear positivity in each tumor. Correlation between PROX1 expression, metastasis and patient survival was then assessed. Annexin A1, a downstream target of PROX1, was immunohistochemically assessed in 18 tumors. PROX1 protein was detected in about half of the tumors, with stronger expression in metastasized cases. PROX1 expression correlated with tumor metastasis and patient prognosis. Annexin A1 was negative in most of the high-grade tumors correlating strongly with grade and metastatic potential. Our results indicate that immunohistochemical detection of PROX1 correlates with a more malignant phenotype in rectal NETs. High PROX1 expression was associated with increased metastatic potential and poor patient survival but not as strongly as grade by the WHO 2010 classification. PROX1 may be involved in progression of rectal NETs as a part of the Wnt pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The PROX1 gene encodes a homeobox transcription factor controlling the embryonic development of various organs, such as retina, lens, lymphatic endothelium and liver [1–3]. PROX1 has been implicated in the progression of intestinal cancer in the APC mouse model [4]. In normal cells, APC, the protein produced by the adenomatous polyposis gene, associates with a protein complex containing cytoplasmic beta-catenin inducing its degradation. When this is inhibited by Wnt signalling, beta-catenin accumulates in the nucleus as a beta-catenin/TCF (T cell factor) complex, which controls the expression of several genes. PROX1 is one target of the beta-catenin/TCF pathway, which is upregulated in colorectal cancer development. When non-neoplastic intestinal mucosa transforms into an adenoma, and further into invasive adenocarcinoma, PROX1 contributes to tumor progression, likely via modulating cell adhesion and extracellular matrix interactions [4, 5]. Annexin A1 is a target of the Wnt pathway; overexpression of PROX1 leads to diminished expression of Annexin A1 [6].
In normal colon, PROX1 is expressed mostly in neuroendocrine epithelial cells and lymphatic vessel endothelial cells [7]. At least 15 different subpopulations of neuroendocrine cells have been detected in the gastrointestinal (GI) tract. PROX1 is expressed in cells expressing the hormone peptide YY (PYY), cholecystokinin (CKK) and glucagon-like peptide-1 (GLP-1) and in part of the serotonin-expressing cells [4].
The development of gastrointestinal neuroendocrine tumors (GI-NETs) and their progression from indolent to a more aggressive form is incompletely known, although precursor lesions such as hyperplasia of neuroendocrine cells have been identified [8]. Approximately 10 to 15 % of GI-NETs are in the rectum [9]. In recent years, their incidence has been about 1/100,000 [10], with a rising tendency [11]. This may in part be due to the improved detection of GI-NETs through increased availability of endoscopy and radiology, but a true increase in incidence cannot be excluded. Most rectal NETs grow slowly, causing symptoms late during their course. The prognosis of GI-NET patients is generally favourable, with 5-year survival of 88 to 91 %, but patients with metastatic disease have a poor prognosis [12–15].
In the WHO 2010 classification of neuroendocrine tumors of the pancreas and GI tract, the tumors are graded according to proliferation index and mitotic rate. In a previous report (Table 1), we re-classified 73 rectal NETs according to the WHO 2010 classification. During follow-up, 10 of these tumors had metastasized. None of the grade (G) 1 tumors were associated with metastasis, whereas of 11 G2 tumors, nine metastasized during the follow-up. We concluded that the WHO 2010 classification was accurate in predicting the metastatic potential of rectal NETs [16].
Here, we have studied the immunohistochemical expression of PROX1 and correlated its expression to patient prognosis in a series of rectal NETs in 72 primary tumors; we also analysed six metastases. The goal was to find out, whether progression of rectal NETs and colorectal adenocarcinoma might have common pathways involving PROX1, and to test prognostic significance of PROX1 expression. Expression of Annexin A1 was evaluated as a downstream target of PROX1.
Materials and methods
Tumor series
The tumor series comprised 73 consecutive rectal NETs from 1980 to 2008 identified from our pathology laboratory database. According to the WHO 2010 classification, the series consisted of 61 G1 NETs, 11 G2 NETs and one G3 NEC of large cell type (Table 1). The TNM stage could not be reliably determined in the tumor series, because many tumors were removed in pieces making the muscle wall invasion often impossible to exclude, and only very few patients were examined radiologically to detect metastasis at the time of the primary diagnosis, because earlier, the malignant nature of these tumors was not appreciated [16]. The mean age of the 28 male and 45 female patients at diagnosis was 58.3 years. Mean follow-up after surgery was 148 months, and median 137 months (23 days to 346 months), with the latest follow-up in October 2013. The study was approved by the Ethics Committee of Helsinki University Central Hospital and by the National Authority for Medicolegal Affairs.
Metastases
During the follow-up, 10 tumors had metastasized, of which nine had metastasized to the liver. In five cases, there were liver metastases exclusively, and in four cases, metastases were found also in another organ in addition to the liver, in regional lymph nodes, pelvis, bone and lymph nodes of the lung hilus. One patient had metastases only in the peritoneum. Concomitant metastases were present in seven patients, and three patients developed metastases later. Tissue material was available for immunohistochemical analysis from six patients with metastasized rectal NET; three patients underwent resection of liver metastases, one had a liver transplantation, one had a biopsy obtained from a liver metastasis and one from a peritoneal metastasis. The core needle biopsies from liver metastasis and peritoneal metastasis represent only a small proportion of metastatic tumor tissue. Material from liver resection and liver transplantation consists of several blocks in each case; immunohistochemistry was carried out on the most representative block.
Immunohistochemistry
The tumor series consisted of 73 primary tumors, and tissue material for immunohistochemistry was available from all but one. The immunostainings were carried out on deparaffinised tissue sections by using a goat anti-PROX1 antibody (R&D Systems, Minneapolis, MN, USA; dilution 1:2000). Annexin A1 staining was carried out on a limited series of 18 tumors including 10 G1 tumors and 8 G2–G3 tumors (BD Biosciences, New Jersey, USA; dilution 1:5000). Pretreatment and staining took place in an Autostainer 480 (LabVision, Fremont, CA, USA) by use of Dako REAL EnVision Detection System, Peroxidase/DAB+ (Dako Glostrup, Denmark).
Scoring
The two pathologists (J. J. and J. H.) who calculated nuclear positivity independently were blinded to the clinicopathological data. In cases with discordancy, a consensus score served for further analysis. Strong nuclear staining for PROX1 was scored 0 to 4: 0 = negative; 1 = weak expression (<30 % of tumor cells); 2 = moderate (30–50 %); 3 = strong (50–80 %); 4 = very strong (> 80 %). Weak nuclear staining was considered unspecific and thus negative. Similar scoring was used for nuclear and cytoplasmic positivity for Annexin. For statistical analyses, the tumors were divided into two groups: tumors with PROX1 expression 0 to 2 classified as low and 3 to 4 as high expression.
Statistics
Statistical analysis was by the chi-square test and Fisher exact test. Kaplan-Meyer life tables had distant metastasis and death as a result of metastasized rectal NET as their end-points. Multivariate analysis by Cox regression had disease-free survival as the end-point. Factors entering the multivariate analysis were expression of PROX1, tumor size, WHO 2010 classification, patient’s age and gender and expression of cyclin A, which in our earlier study correlated with prognosis [17]. Correlations between Annexin A1 and PROX1 expression, metastatic potential as well as grade were computed. p values under 0.05 were considered statistically significant.
Results
PROX1 expression in normal rectal epithelium
Scattered PROX1-positive neuroendocrine cells were found in normal epithelium adjacent to the tumors. The majority of mucosal epithelial cells were PROX1 negative with the exception of cells in the bottom of the mucosal crypts. Endothelial cells of lymphatic vessels were positive, whereas blood vessel endothelial cells were negative.
Expression of PROX1 in rectal NETs
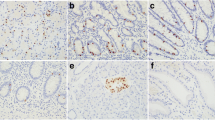
Nuclear PROX1 staining was absent in 36 (50 %), weak in 13 (18 %), moderate in 10 (14 %), strong in 9 (13 %) and very strong in 4 (6 %) tumors (Fig. 1). In the primary tumors, mean PROX1 score was 1.1, with a median of 1. In metastatic tumors, the mean score was 2.4 with a median of 3, and in non-metastatic tumors, 1 with a median of 0.
Expression of Annexin A1
Of 8 G2–G3 cases, all but one were negative for Annexin A1, whereas all G1 tumors showed positivity for Annexin A1. Expression of Annexin A1 correlated strongly with grade and metastatic potential (p < 0.001), and correlation between Annexin A1 and PROX1 was nearly significant (p = 0.07).
Expression of PROX1 in metastases
Five of the six metastases were very strongly positive for PROX1. In these patients, the primary tumor was very strongly positive in one, strongly positive in three and moderately positive in one case, and the proliferation index was higher in the metastasis than in the primary tumor in all but one case. One metastasis was PROX1 negative, as was the corresponding primary tumor, and the metastasis showed higher proliferation index than the primary tumor. The level of expression of PROX1 or Ki-67 did not have an effect on prognosis.
PROX1 as a prognostic marker
In the chi-square test, PROX1 expression correlated with metastatic potential (p < 0.001), WHO 2010 grade (p < 0.001), cyclin A expression (p < 0.001) and tumor size (p = 0.02) but not with age or sex (Table 2). Correlation of PROX1 with metastatic potential and disease-specific survival is shown in the life table analyses shown in Figs. 2 and 3 (log-rank test, p < 0.001). When G1 and G2 tumors were evaluated as separate groups, PROX1 did not correlate with patient age or gender. In the metastasized tumors, PROX1 did not correlate with progression-free survival or disease-specific survival. In multivariate analysis, only WHO 2010 grade emerged as an independent prognostic factor.
Discussion
We report here that PROX1 is expressed in a significant number of rectal NETs. Strong expression of PROX1 was associated with metastatic potential, poor patient survival and high WHO grade.
In colorectal adenocarcinomas, PROX1 predicts aggressive clinical course and poor survival [7]. In Kaposiform hemangioendothelioma, overexpression of PROX1 is associated with more aggressive tumor behaviour by inducing genes involved in cell migration and adhesion, thus enabling infiltration into surrounding tissues [18, 19]. Gliomas of the central nervous system also express PROX1 [18]. Now, rectal NETs can be added to this list of PROX1-positive tumors, since we detected PROX1 positivity of in about one half of the rectal NETs. Of 12 G2–G3 NETs, only two tumors (17 %) were negative for PROX1, compared to 34 (57 %) of 60 in G1 NETs. High-grade tumors were mostly negative for Annexin A1, and G1 tumors were all positive supporting the hypothesis that Annexin A1 expression is lower in the more advanced PROX1-positive tumors. Six metastases were available for immunohistochemistry, and all but one showed very strong PROX1 expression, which was clearly increased in comparison with the corresponding primary tumors. In one case, both the primary tumor and the metastasis expressed PROX1 very strongly, and in one patient, both were negative. Samples of metastases represent only part of the metastatic tumor tissue, which can be considered a weak point in this study. These results suggest that the APC/beta-catenin/TCF pathway that is central in the pathogenesis of colorectal adenocarcinomas may be involved also in the progression of the rectal NETs. We found that PROX1 correlated with cyclin A expression, but the exact mechanisms, by which PROX1 exerts its function on other genes, cell cycle and proliferation remain uncovered.
In summary, we report that a significant number of rectal NETs express the PROX1 transcription factor and that the expression correlates with the metastatic potential and patient survival. Our results implicate that Wnt pathway activity level is involved in the development of high-grade rectal NETs. Yet, grade according to the WHO 2010 classification still has stronger prognostic significance than PROX1. Future studies should reveal if PROX1 or its downstream genes provide suitable targets for the treatment of these rare tumors.
References
Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P (1993) Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev 44:3–16
Sosa-Pineda B, Wigle JT, Oliver G (2000) Hepatocyte migration during liver development requires Prox1. Nat Genet 25:254–255
Dyer MA, Livesey FJ, Cepko CL, Oliver G (2003) Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 34:53–58
Petrova TV, Nykanen A, Norrmen C, Ivanov KI, Andersson LC, Haglund C, Puolakkainen P, Wempe F, von Melchner H, Gradwohl G, Vanharanta S, Aaltonen LA, Saharinen J, Gentile M, Clarke A, Taipale J, Oliver G, Alitalo K (2008) Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 13:407–419
Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87:159–170
Wiener Z, Hogstrom J, Hyvonen V, Band AM, Kallio P, Holopainen T, Dufva O, Haglund C, Kruuna O, Oliver G, Ben-Neriah Y, Alitalo K (2014) Prox1 promotes expansion of the colorectal cancer stem cell population to fuel tumor growth and ischemia resistance. Cell Rep 8:1943–1956
Skog M, Bono P, Lundin M, Lundin J, Louhimo J, Linder N, Petrova TV, Andersson LC, Joensuu H, Alitalo K, Haglund CH (2011) Expression and prognostic value of transcription factor PROX1 in colorectal cancer. Br J Cancer 105:1346–1351
Calender A (2000) Molecular genetics of neuroendocrine tumors. Digestion 62(Suppl 1):3–18
Niederle MB, Hackl M, Kaserer K, Niederle B (2010) Gastro-entero-pancreatic neuroendocrine tumours—the current incidence and staging based on the WHO and ENETS classification. Endocr Relat Canc 17:909–918
Ellis L, Shale MJ, Coleman MP (2010) Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol 105:2563–2569
Tsikitis VL, Wertheim BC, Guerrero MA (2012) Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer 3:292–302
Modlin IM, Lye KD, Kidd M (2003) A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97:934–959
Kang H, O’Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY (2007) Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis 22:183–189
Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H, Japanese Society for Cancer of the Colon and Rectum (2007) Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 56:863–868
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB (2008) One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26:3063–3072
Jernman J, Valimaki MJ, Louhimo J, Haglund C, Arola J (2012) The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology 95:317–324
Jernman J, Valimaki MJ, Hagstrom J, Louhimo J, Haapasalo H, Arola J, Haglund C (2014) Cyclin A predicts metastatic potential of rectal neuroendocrine tumors. Hum Pathol 45:1605–1609
Elsir T, Qu M, Berntsson SG, Orrego A, Olofsson T, Lindstrom MS, Nister M, von Deimling A, Hartmann C, Ribom D, Smits A (2011) PROX1 is a predictor of survival for gliomas WHO grade II. Br J Cancer 104:1747–1754
Miettinen M, Wang ZF (2012) Prox1 transcription factor as a marker for vascular tumors-evaluation of 314 vascular endothelial and 1086 nonvascular tumors. Am J Surg Pathol 36:351–359
Conflict of interests
The authors declare that they have no competing interest.
Funding
This research received funding from The Sigrid Jusélius Foundation, the Finnish Cancer Foundation and Helsinki University Hospital Research Funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Johanna Arola and Caj Haglund contributed equally to this work
Rights and permissions
About this article
Cite this article
Jernman, J., Kallio, P., Hagström, J. et al. PROX1 is involved in progression of rectal neuroendocrine tumors, NETs. Virchows Arch 467, 279–284 (2015). https://doi.org/10.1007/s00428-015-1795-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1795-7