Abstract
Purpose
This study aimed to evaluate the real-life use of BRAF-V600E mutation analysis in washout liquid from thyroid nodule fine needle aspiration (FNA), and the consequences of genetic result on clinical decision-making.
Methods
We retrospectively considered subjects tested for BRAF-V600E among those attending the Endocrinology Unit of Modena for FNA between 2014 and 2018. Washing fluid was collected together with cytological sample and stored at −20 °C. If the clinician deemed it necessary, the sample was thawed, DNA extracted, and genetic test performed by high-resolution melting technique. We collected data on cytology according to the Italian Consensus for the cytological classification of thyroid nodules, type of surgery (when performed), histology, and adverse events.
Results
Out of 7112 subjects submitted to FNA, BRAF analysis was requested for 683 (9.6%). Overall, 896 nodules were analyzed: 74% were indeterminate at cytology, mainly TIR3A (low risk). Twenty-two nodules were mutant (BRAF+). Only 2% of indeterminate, mainly TIR3B, were BRAF+. Based on final histological diagnosis, BRAF test had high specificity (100%) but poor sensitivity (21%), also in indeterminate nodules. Mutant subjects underwent more extensive surgery compared to wild type (p = 0.000), with frequent prophylactic central lymph node dissection. One third had local metastases. Higher prevalence of hypoparathyroidism was found in BRAF+ compared to wild type (p = 0.018).
Conclusions
The analysis of BRAF-V600E outside of gene panels has low sensitivity, especially in indeterminate nodules, and a positive result could lead to more extensive surgery with greater risk of hypoparathyroidism and questionable clinical utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiated thyroid carcinomas are constantly increasing worldwide, with a clear prevalence of small tumors, with overall excellent prognosis thanks to early diagnosis [1]. In order to avoid overtreatment, efforts have been multiplied to refine diagnostic tools and to pick out those with good prognosis or, conversely, the ones requiring more aggressive treatment and tighter follow-up. Ultrasound and cytology certainly have the main role in thyroid nodule investigation [2]. However, ultrasound is operator-dependent and limited by the lack of categorizations able to definitively rule in or rule out cancer [3]. Cytology certainly allows for higher accuracy, but is heavily limited by indeterminate categories. Indeed, up to 30% of nodules are reported as indeterminate [4], representing a significant challenge for clinicians. Moreover, another problem still to be solved is the possibility of discriminating between malignant lesions with better prognosis and those with worse prognosis, which also are on the rise [5].
Since there are gene mutations specific to malignant lesions, genetic testing may be helpful in investigating suspicious thyroid nodules. For instance, the thymine to adenine substitution at nucleotide position 1799 (rs113488022, c.1799T>A) of the BRAF gene, resulting in a valine-to-glutamine replacement at residue 600 (p.V600E), is the most common genetic mutation in papillary thyroid carcinomas [6]. B-Raf proto-oncogene serine/threonine kinase (BRAF) is an enzyme that after being bound and activated by the rat sarcoma GTPase (RAS) is translocated to the cell membrane and drive the phosphorylation and activation of mitogen-activated protein kinase (MAPK) and the related signaling pathways.
Several molecular testing for BRAF mutation alone or in panel have been studied so far, both on cytological samples and on washing liquid from fine needle aspiration (wFNA) [6]. However, the use of gene panels in clinical practice is limited by costs, feasibility and by the need for second ad hoc sampling. Even the latest international guidelines weakly recommend the use of molecular testing because of moderate-quality evidence [2].
In this study, we aimed to evaluate the real-life use of BRAF mutation analysis by endocrinologists in a second-level reference center and the impact of the mutational status knowledge on subsequent clinical decision-making. Indeed, in our center, we previously validated a BRAF-V600E search method based on High-Resolution Melting (HRM) analysis [7]. It has the advantage of being sensitive, cost-effective, time-saving and based on the use of wFNA, collected at the same time as the collection of cytological material, and stored until clinician demands for genetic analysis.
Materials and methods
Subjects
This single-center, retrospective, real-world evidence, observational study was performed on a large cohort of subjects attending the Endocrinology Unit of Modena for nodular goiter. In particular, we considered data of subjects who underwent fine needle aspiration (FNA) from January 2014 to November 2018. Each patient may have undergone FNA more than once because of non-diagnostic result and/or changes in the sonographic pattern. Moreover, some subjects had multinodular goiter and each aspirated nodule was considered separately in the analyzes. FNA performed on lymph nodes were excluded. Among the aspirated nodules, we then considered those for which molecular investigation was requested. In our center, we perform the test for BRAF mutation on washing liquid whenever considered useful. It is mainly requested in case of indeterminate cytological outcome or, occasionally, and at the discretion of the clinician, in case of ultrasonographically suspicious nodules with any cytological outcome.
All patients signed written informed consent for FNA, for washing liquid storage and for performance of molecular tests in case of clinical need. The study protocol was approved by the local Ethics Committee of Modena (reference number 122/08).
Fine needle aspiration procedure
Ultrasound-guided FNA was performed with 23- to 27-gauge needle. FNA samples were expelled onto glass slides, smeared, fixed and stained according to standard procedures. The wFNA samples were obtained washing out the needle with 1 ml of sterile saline solution and collecting the remaining material into a 2 ml sterile tube, as previously described [7]. This material was stored at −20 °C for future molecular analyses, required by the clinician when necessary.
Mutation analysis
Somatic DNA was extracted from wFNA samples thanks to a lysis buffer, containing Tris-HCl 50 mM at pH 8.5, EDTA 1 mM, Tween 20 0.5% and 200 µg of proteinase K (Merck KGaA, Darmstadt, Germany), as previously described [7], before quantification by NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
The region containing the BRAF c.1799 site was amplified according to Marino et al. [7]. Briefly, 15 µl of reaction mix, containing 20 ng of DNA, 7.5 µl of the SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories Inc., Hercules, CA, USA), primers (forward 5′-AGGTGATTTTGGTCTAGC-3′; reverse 5′-ATCCAGACAACTGTTCAA-3′) at the final concentration of 500 nM and sterile water, were prepared. HRM analysis was performed using the protocol previously described [7]. Samples resulted indeterminate at HRM underwent direct sequencing and, if necessary, were confirmed by pyrosequencing, as previously described [7].
As positive controls were used both the 50% BRAF-V600E Reference Standard (Diatech Pharmacogenetics, Jesi, Italy) and the DNAs of patients previously confirmed positive for BRAF-V600E mutation by direct sanger sequencing and pyrosequencing [7]. DNAs from healthy patients, previously screened for BRAF-V600E mutation in our laboratory and resulted wild-type (WT), were used as negative controls.
All reactions were carried out in duplicate and performed on the CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories Inc.). Raw data were analyzed using the software CFX Manager and Precision Melt Analysis software (Bio-Rad Laboratories Inc.).
Clinical data collection
In addition to genetic data, we collected the following information about nodules: cytology classification according to the Italian Consensus for the cytological classification of thyroid nodules [8]; details about surgical treatment when performed (considering central and lateral neck lymph node dissection as “more extensive surgery”); histology, classified according to the 8th edition of the AJCC/TNM staging system of thyroid cancer [9]; surgical adverse events (hypoparathyroidism, recurrent laryngeal nerve injury). Hypoparathyroidism was defined as biochemical hypocalcemia and/or PTH levels below normal ranges and need for calcium medication 1 month after surgery (transient) or more than 6 months after surgery (permanent) [10]. All surgical treatments were performed by surgeons expert in endocrine surgery.
Statistics
Statistical analysis was performed using SPSS v.26.0 (IBM Corp., Armonk, NY, USA).
Analyses of genetics, cytology and histology were performed by separately evaluating each nodule subjected to FNA. The number of nodules is greater than the number of subjects since some subjects had more than one nodule. The impact of knowledge of the mutational status on clinical decision-making was analyzed considering subjects, instead of nodules.
Comparisons among nodules or subjects were performed by the nonparametric Mann–Whitney’s U test, since variables were not normally distributed at Kolmogorov–Smirnov test. Chi-square analysis was performed to evaluate for frequency differences. Spearman’s rank correlation tests were performed to verify correlations between adverse events and extent of surgery. P was considered significant when <0.05. Sensitivity and specificity of BRAF mutation test were calculated considering final histological diagnosis.
Results
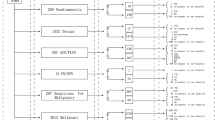
Among 7112 subjects submitted to FNA between January 2014 and November 2018, BRAF mutation analysis was requested in 683 (9.6%) (Fig. 1). Mutation analyses in two washout liquids from lateral neck- lymph nodes were performed and they were excluded from subsequent analyses. Most of the studied subjects were females (487/681; 71.5%) and their mean age was 55 ± 13 years (min 18-max 85). Each patient could have undergone FNA repeatedly because of the presence of more than one suspicious nodule or because of changes in the sonographic features of a previously analyzed nodule. Finally, the total number of nodules tested for BRAF was 896. All the results presented below will refer to nodules.
The vast majority of BRAF tests were performed because of indeterminate result at cytology: 65% were low-risk indeterminate lesions (TIR3A), 8% were high-risk indeterminate lesions (TIR3B) and 1% were indeterminate without sub-classification (TIR3). Surprisingly, 15% of the BRAF tests concerned cytologically benign nodules (TIR2), suggesting that the request for molecular analysis derived from other clinical suspicions (e.g., ultrasound appearance). A small group (10%) underwent the genetic test for nodules with non-diagnostic cytological result. In only 1% of cases, the genetic test was requested on nodules cytologically suspicious (TIR4) or diagnostic for malignancy (TIR5).
Analysis of BRAF-V600E genotyping
Initially, only 20 nodules were positive (BRAF+) while 869 nodules were WT for BRAF V600E mutation. Seven samples were not determinable by HRM analysis, possibly due to low DNA quality, resulting in weakly interpretable melting curves. These indeterminate samples were further analyzed with direct sequencing and, if necessary, with pyrosequencing, as previously described [7], documenting 5 WT nodules and 2 BRAF+. After these additional analyses, a total of 874 nodules (97.5%) were WT while 22 (2.5%) were mutants.
WT and BRAF+ subjects did not differ for age (p = 0.306) or sex (p = 0.767). Female sex was prevalent in both groups, reflecting higher prevalence of thyroid nodules in women.
Prevalence of BRAF mutation among cytological classes
The prevalence of BRAF mutation in each class was as follows: 0% in TIR1 and TIR1C, 1% in TIR2, 0% in TIR3 not further sub-classified, 1% in TIR3A, 9% in TIR3B, 55% in TIR4, and 67% in TIR5 (Table 1). Overall, only 2% of indeterminate nodules were BRAF+.
Histological results
Of the 267 WT removed nodules, 74 (28%) were malignant at the histological examination: 57 (77%) papillary thyroid cancer (PTC) and 17 (23%) follicular thyroid cancer. All the BRAF+ nodules confirmed to be PTC at histological examination. Histology distribution among cytological classes is shown in Table 1.
As expected, the vast majority of PTC in the BRAF+ group had a classic papillary pattern, while follicular was the most common variant of PTC in the WT group.
Based on the final histological diagnosis, BRAF-V600E analysis resulted to have very high specificity (100%), but poor sensitivity (21%) in the diagnosis of differentiated thyroid cancer. Even considering separately indeterminate nodules sent to surgery (149 TIR3A and 52 TIR3B), HRM BRAF test confirmed to be highly specific (100%) but, again, poorly sensitive (16% for TIR3A and 26% for TIR3B).
Impact of knowledge of the mutational status on clinical decision-making
All the results presented below - referred to subjects instead to nodules - are summarized in Table 2. Two hundred and twelve subjects were addressed to surgery: 190 were WT but with suspicious cytology and/or other clinical risk factors; 14 were both BRAF+ and cytologically suspicious; only 8 were BRAF+ but cytologically unsuspicious. Since 64% of BRAF+ were TIR3B-4-5 at cytology, they had surgical indication, for thyroidectomy, even before the genetic test. Two BRAF+ subjects refused to undergo surgery and were lost at follow-up. At the end, 210 subjects (for a total of 287 nodules) were surgically treated (Fig. 1).
Then, we analyzed the type of surgery performed. Subjects in the BRAF+ group were treated with more extensive surgery compared to WT (p = 0.000) (Table 2). Only few subjects had pre-surgical suspicious lymph nodes before central dissection (13% among WT and 14% among BRAF+), all histologically confirmed. Among those without suspicious pre-surgical lymph nodes, 45% of the WT and 33% of the BRAF+ had metastases to the central compartment.
Consequences of greater surgical invasiveness were significantly different (Table 2): 55% of BRAF+ subjects developed post-surgical hypoparathyroidism (25% transient and 30% permanent), against 26% (11% transient and 15% permanent) in WT (p = 0.018). All the BRAF+ with permanent hypoparathyroidism were treated with central neck dissection. No significant correlation between the extent of surgery and hypoparathyroidism onset was found both in the BRAF+ group (rs = 0.630, p = 0.115), and in the whole group, BRAF+ and WT taken together (rs = 0.021, p = 0.769).
No differences were found regarding the recurrent laryngeal nerve injury between BRAF+ and WT (p = 0.577).
Discussion
This single-center study confirms that clinicians rely on molecular tests especially after indeterminate cytology. However, genetic testing for the BRAF gene alone has questionable utility because of its low sensitivity and the consequent risk of missing thyroid carcinomas harboring mutations in other genes [2]. In our real-life series, only 2% of indeterminate nodules were BRAF+, despite 22–50% malignancy rate in WT, resulting in a sensitivity of only 16% for TIR3A and 26% for TIR3B. These results fit well in a literature studded with heterogeneous data, regarding prevalence of BRAF mutation in indeterminate nodules – ranging between 0 and 48% – and, consequently, its reported sensitivity [11]. Such low sensitivity could be due to the presence of follicular, rather than papillary, neoplasm in many indeterminate nodules, supported by nucleotide changes in genes other than BRAF, e.g., RAS [12, 13]. Moreover, recent metanalytic studies demonstrate a limited role of BRAF testing in both indeterminate [14] and suspicious for malignancy nodules [15].
Our data also demonstrate that molecular testing is requested mainly but not only in nodules with indeterminate cytology. In fact, clinicians deemed it necessary also in: benign nodules (TIR2), because of suspicious ultrasound appearance; nodules with non-diagnostic cytology (TIR1), to look for diagnostic clues other than cytology; albeit rarely, even in nodules already suspicious for (TIR4) or diagnostic for malignancy (TIR5), to have diagnostic confirmation and/or prognostic information. Thus, we demonstrated that 26% of the requests for genetic screening occurring in real-life are out of guideline indications, with questionable clinical benefit. Indeed, BRAF analysis unmasked only one malignant nodule, falsely determined as benign by cytological examination.
Most BRAF+ subjects would already have had indication for surgery on the basis of cytology, even without the genetic information. In a previous smaller series, BRAF mutation confirmed to be highly specific but not able to improve cytological diagnosis, since mainly diagnosed in already suspicious or malignant nodules [16].
It is worth of note that preoperative identification of BRAF mutation was related to a more invasive surgical approach: mutant subjects were preferentially treated with total thyroidectomy plus central lymph nodes dissection, even in the absence of preoperatively suspicious lymphadenopathies - only 13–14% of the subjects treated with central dissection had pre-surgical suspicious lymph nodes. Our data cast doubts on the efficacy of this choice, since there was no significant difference between WT and BRAF+ in terms of histological finding of lymph node metastases. Several studies, mainly retrospective, suggested the association of BRAF mutation and lymph node metastasis [17]. However, long-term effectiveness of prophylactic central lymphadenectomy compared to thyroidectomy alone is questionable, regardless of BRAF state [18]. However, it is undeniable that the prophylactic removal of level VI lymph nodes allows to identify some patients with metastatic disease. In our series, one third of the sonographically unsuspicious lymph nodes were metastases, which would have escaped without prophylactic removal, both in the BRAF+ and in the WT group. Nevertheless, the direct effect on long-term outcome is questionable and probably clinically irrelevant [2, 18, 19]. International guidelines state that BRAF status in the primary tumor should not impact on the decision for prophylactic central neck dissection [2].
In the risk/benefit balance it is essential to assess whether the greater extent of surgery increases the risk of adverse events. Our data show that, in the hands of experienced endocrine surgeons, recurrent laryngeal nerve injury is rare both in BRAF+ and in WT subjects, regardless of the surgical approach. However, we found higher prevalence of post-surgical hypoparathyroidism in BRAF+ subjects. Since subjects with BRAF+ nodules were more frequently treated with extensive surgery compared to WT, more complications are expected in this group. In our series, all the BRAF+ patients with permanent hypoparathyroidism were treated with central neck dissection, confirming the correlation between surgery extent and hypoparathyroidism [10]. Post-surgical hypoparathyroidism can lead to severe hypocalcemia, an urgent life-threatening situation [10]. After passing urgency, chronicity has heavy consequences for the patient, including potentially lifelong supplementation, gastrointestinal or renal complications, frequent laboratory testing, potential hospital admissions, and an overall reduced quality of life [20]. Thus, we conclude that extending surgery on the basis of molecular test of the single BRAF gene may expose the patient to more invasive surgery (i.e., central neck dissection) and, thus, greater risk of hypoparathyroidism, not justified by substantial oncological benefit. Obviously this study does not aim to demonstrate a causal link between the molecular test and the onset of hypoparathyroidism. However, our results raise a critical issue that would require further investigation with prospective studies.
Although the cost of the BRAF gene mutation analysis is increasingly affordable (between 9 and 123 dollars per tested sample [11]), cost-effectiveness for both the economic and clinical benefits is worth of careful consideration. This clearly goes beyond the aim of this study, limited by the lack of long-term follow-up data and the small size of the mutant group. If, on the one hand, it is true that a positive BRAF result should avoid two-step surgery [21], on the other hand we must also consider the long-term consequences of more extensive initial surgery on patient’s quality of life. In general, BRAF mutant subjects seem to have higher risk of recurrence and decreased survival at long follow-up [22], likely due to radioactive iodine resistance [23, 24]. In this contest, optimizing initial surgery could be relevant, balancing oncological and surgical risks. However, the most recent guidelines state that the BRAF status taken in isolation has a limited role for guiding patient management both in the diagnostic phase for nodules with indeterminate cytology and in the prognostic evaluation of micro-PTC [2].
Our results confirm that the analysis of BRAF-V600E outside of gene panels has low sensitivity, especially in indeterminate nodules, and that in real life practice a positive result could lead to more extensive therapeutic choices with potentially increased side effects without real oncological benefit. Future prospective studies are necessary to understand the consequences of the result of the analysis of the BRAF gene alone, which in real life is used alone and not as part of gene panels.
Availability of data and material
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
S. Vaccarella, S. Franceschi, F. Bray, C.P. Wild, M. Plummer, L. Dal Maso, Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 375(7), 614–617 (2016). https://doi.org/10.1056/NEJMp1604412
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. Off. J. Am. Thyroid. Assoc. 26(1), 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
B. Madeo, G. Brigante, A. Ansaloni, E. Taliani, S. Kaleci, M.L. Monzani, M. Simoni, V. Rochira, The added value of operator’s judgement in thyroid nodule ultrasound classification arising from histologically based comparison of different risk stratification systems. Front Endocrinol. 11(434), (2020). https://doi.org/10.3389/fendo.2020.00434
C. Durante, G. Grani, L. Lamartina, S. Filetti, S.J. Mandel, D.S. Cooper, The diagnosis and management of thyroid nodules: a review. JAMA 319(9), 914–924 (2018). https://doi.org/10.1001/jama.2018.0898
K.L. Yan, S. Li, C.H. Tseng, J., Kim, D.T. Nguyen, N.B. Dawood, M.J. Livhits, M.W. Yeh, A.M. Leung, Rising incidence and incidence-based mortality of thyroid cancer in California, 2000-2017. J. Clin Endocrinol. Metabol. 105(6) (2020). https://doi.org/10.1210/clinem/dgaa121
Y.E. Nikiforov, M.N. Nikiforova, Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 7(10), 569–580 (2011). https://doi.org/10.1038/nrendo.2011.142
M. Marino, M.L. Monzani, G. Brigante, K. Cioni, B. Madeo, D. Santi, A. Maiorana, S. Bettelli, V. Moriondo, E. Pignatti, L. Bonacini, C. Carani, V. Rochira, M. Simoni, High-resolution melting is a sensitive, cost-effective, time-saving technique for braf v600e detection in thyroid fnab washing liquid: a prospective cohort study. Eur. thyroid J. 4(2), 73–81 (2015). https://doi.org/10.1159/000430092
G. Fadda, F. B, A. Bondi et al. Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica 102(5), 405–408 (2010)
M. Tuttle, L. Morris, B. Haugen, J. Shah, J. Sosa, E. Rohren, R. Subramaniam, J. Hunt, N. Perrier, M. Amin, S. Edge, F. Greene, D. Byrd, R. Brookland, M. Washington, C. Compton, K. Hess, D. Sullivan, J. Jessup, Thyroid-differentiated and anaplastic carcinoma (Chapter 73) (Springer International Publishing, New York, 2017)
H.S. Kazaure, J.A. Sosa, Surgical hypoparathyroidism. Endocrinol. Metab. Clin. North Am. 47(4), 783–796 (2018). https://doi.org/10.1016/j.ecl.2018.07.005
E.J. de Koster, L.F. de Geus-Oei, O.M. Dekkers, I. van Engen-van Grunsven, J. Hamming, E.P.M. Corssmit, H. Morreau, A. Schepers, J. Smit, W.J.G. Oyen, D: Vriens, Diagnostic utility of molecular and imaging biomarkers in cytological indeterminate thyroid nodules. Endocr. Rev. 39(2), 154–191 (2018). https://doi.org/10.1210/er.2017-00133
R.T. Kloos, J.D. Reynolds, P.S. Walsh, J.I. Wilde, E.Y. Tom, M. Pagan, C. Barbacioru, D.I. Chudova, M. Wong, L. Friedman, V.A. LiVolsi, J. Rosai, R.B. Lanman, G.C. Kennedy, Does addition of BRAF V600E mutation testing modify sensitivity or specificity of the Afirma Gene Expression Classifier in cytologically indeterminate thyroid nodules? J. Clin. Endocrinol. Metab. 98(4), E761–E768 (2013). https://doi.org/10.1210/jc.2012-3762
N.P. Ohori, J. Wolfe, S.P. Hodak, S.O. LeBeau, L. Yip, S.E. Carty, U. Duvvuri, K.E. Schoedel, M.N. Nikiforova, Y.E. Nikiforov, “Colloid-rich” follicular neoplasm/suspicious for follicular neoplasm thyroid fine-needle aspiration specimens: cytologic, histologic, and molecular basis for considering an alternate view. Cancer Cytopathol. 121(12), 718–728 (2013). https://doi.org/10.1002/cncy.21333
P. Trimboli, G. Treglia, E. Condorelli, F. Romanelli, A. Crescenzi, M. Bongiovanni, L. Giovanella, BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: a systematic review and meta-analysis. Clin. Endocrinol. 84(3), 315–320 (2016). https://doi.org/10.1111/cen.12806
P. Trimboli, L. Scappaticcio, G. Treglia, L. Guidobaldi, M. Bongiovanni, L. Giovanella, Testing for BRAF (V600E) mutation in thyroid nodules with fine-needle aspiration (FNA) read as suspicious for malignancy (Bethesda V, Thy4, TIR4): a systematic review and meta-analysis. Endocr. Pathol. 31(1), 57–66 (2020). https://doi.org/10.1007/s12022-019-09596-z
E. Macerola, T. Rago, A. Proietti, F. Basolo, P. Vitti, The mutational analysis in the diagnostic work-up of thyroid nodules: the real impact in a center with large experience in thyroid cytopathology. J. Endocrinological Investig. 42(2), 157–166 (2019). https://doi.org/10.1007/s40618-018-0895-z
A.L. Melck, L. Yip, S.E. Carty, The utility of BRAF testing in the management of papillary thyroid cancer. oncologist 15(12), 1285–1293 (2010). https://doi.org/10.1634/theoncologist.2010-0156
T.S. Wang, K. Cheung, F. Farrokhyar, S.A. Roman, J.A. Sosa, A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann. surgical Oncol. 20(11), 3477–3483 (2013). https://doi.org/10.1245/s10434-013-3125-0
G.W. Randolph, Q.Y. Duh, K.S. Heller, V.A. LiVolsi, S.J. Mandel, D.L. Steward, R.P. Tufano, R.M. Tuttle, The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. Off. J. Am. Thyroid. Assoc. 22(11), 1144–1152 (2012). https://doi.org/10.1089/thy.2012.0043
M. Buttner, T.J. Musholt, S. Singer, Quality of life in patients with hypoparathyroidism receiving standard treatment: a systematic review. Endocrine 58(1), 14–20 (2017). https://doi.org/10.1007/s12020-017-1377-3
L. Yip, M.N. Nikiforova, S.E. Carty, J.H. Yim, M.T. Stang, M.J. Tublin, S.O. Lebeau, S.P. Hodak, J.B. Ogilvie, Y.E. Nikiforov, Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery 146(6), 1215–1223 (2009). https://doi.org/10.1016/j.surg.2009.09.011
R. Elisei, C. Ugolini, D. Viola, C. Lupi, A. Biagini, R. Giannini, C. Romei, P. Miccoli, A. Pinchera, F. Basolo, BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J. Clin. Endocrinol. Metab. 93(10), 3943–3949 (2008). https://doi.org/10.1210/jc.2008-0607
G. Oler, J.M. Cerutti, High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer 115(5), 972–980 (2009). https://doi.org/10.1002/cncr.24118
C. Romei, R. Ciampi, P. Faviana, L. Agate, E. Molinaro, V. Bottici, F. Basolo, P. Miccoli, F. Pacini, A. Pinchera, R. Elisei, BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr. Relat. Cancer 15(2), 511–520 (2008). https://doi.org/10.1677/erc-07-0130
Acknowledgements
Authors are grateful to Katia Cioni, Chiara Diazzi, Alessandro Guidi, Bruno Madeo, Daniele Santi, Erica Taliani and Lucia Zirilli, who performed some fine needle aspirations at the Unit of Endocrinology, Department of Medical Specialties, Azienda Ospedaliero-Universitaria of Modena, Modena, Italy. The study was supported by IBSA Institut Biochimique SA. IBSA had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; nor any restrictions regarding the submission of the report for publication.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by G.B. and M.S. Material preparation and data collection were performed by G.B., A.C., E.P., M.M., G.B., G.M., M.I., L.C., S.S., and V.R. Data analysis was performed by G.B. and A.C. The first draft of the manuscript was written by G.B., M.L.M., S.D.V., V.R., and M.S. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Subjects have given their written informed consent.
Consent for publication
All the authors gave their consent for publication.
Ethical approval
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study protocol was approved by the institute’s committee on human research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brigante, G., Craparo, A., Pignatti, E. et al. Real-life use of BRAF-V600E mutation analysis in thyroid nodule fine needle aspiration: consequences on clinical decision-making. Endocrine 73, 625–632 (2021). https://doi.org/10.1007/s12020-021-02693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02693-2