Abstract
Purpose
Hypoparathyroidism is a rare endocrine disorder for which replacement therapy of the missing parathyroid hormone is not the standard therapeutic option. Current standard treatment consists of calcium and vitamin D supplementation. The intake of calcium and vitamin D supplementation can lead to complications and therefore might negatively influence patients’ quality of life.
Methods
A systematic literature review was performed to assess the current knowledge on the influence of hypoparathyroidism on patients’ quality of life. The literature search was conducted in PubMed and Web of Science; all relevant literature published by August 24, 2016, was included.
Results
In total 372 records were found. After title and abstract screening, 14 studies remained for a full-text screening. The full-text screening resulted in five studies which were included into the systematic review. Comparing the results with a norm-based reference population, three studies reported lower SF-36 scores for hypoparathyroidism patients. Two studies showed a reduced quality of life in hypoparathyroidism patients when their results were compared to control populations.
Conclusion
Most hypoparathyroidism patients receiving standard treatment show stable calcium and vitamin D levels. However, hypoparathyroidism patients still report reduced quality of life and experience physical, mental, and emotional symptoms. Therefore, it is assumed that the lack of parathyroid hormone directly influences the patients’ quality of life. This review indicates that patients with hypoparathyroidism have a reduced quality of life in comparison to norm-based populations or matched controls. Further studies are required to quantify the effect of hypoparathyroidism on patients’ quality of life using disease-specific questionnaires and controlling for the co-morbidities and etiologies of the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoparathyroidism (HPT) is an orphan endocrine disorder defined by hypocalcaemia and often hyperphosphatemia due to absent or low levels of parathyroid hormone (PTH). Frequent causes of HPT are surgical complications, genetic defects, or autoimmune disorders [1]. Thyroid surgery is the most common cause of HPT due to removal of the parathyroid glands or damage to them, in particular to glands’ blood supply [2, 3].
Little is known about HPT prevalence in the general population. A Danish registry study found a prevalence of 24 cases of HPT per 100,000 inhabitants, with 22 per 100,000 attributed to surgery and 2 per 100,000 occurring for non-surgical reasons [4, 5]. Other registry studies found a prevalence of 37 per 100,000 in the United States and 9.4 per 100,000 in Norway [6, 7]. Arlt et al. expect 500–1000 new postsurgical HPT patients per year in Germany [8].
HPT is the only endocrine disorder where replacing the missing hormone is not the standard therapeutic option. Studies evaluating the efficiency of synthetic PTH are currently underway, but therapies using PTH have only been approved for patients whose hypocalcemia can not be treated with calcium supplementation or active forms of vitamin D [9]. The current treatment consists of calcium and vitamin D supplementation, usually lifelong, with regular monitoring. However, the intake of calcium and vitamin D supplementation does not restore the physiological calcium/phosphorus homeostasis, and it is also associated with long-term complications like soft-tissue calcifications, kidney stones, nephrocalcinosis, and renal failure [1, 4]. In addition to these long-term complications, hypoparathyroid patients often report short-term complications like brain fog, tingling in the fingers, tetanies, and various other physical, cognitive, or emotional symptoms [10]. All those factors seem to have a negative impact on the patients’ quality of life (QOL).
Only a few studies, however, have analyzed the impact of HPT on patients’ QOL so far. The aim of this systematic review is to provide a current overview regarding the effects of HPT on QOL in patients receiving standard therapy with calcium and vitamin D supplementation.
Methods
Search strategy
An electronic literature search with the search engines Pubmed and Web of Science was performed. The search strategy consisted of the terms “hypoparathyroidism” or “hypocalcemia” or “hypocalcaemia” in combination with the terms “quality of life” or “qol” or “well-being”. The reference lists of all selected publications were checked for any other relevant publications. Due to the rarity of the disease, no further search restrictions were applied. The search was performed on August 24, 2016.
Eligibility criteria
Studies had to meet the following inclusion criteria: (a) adult patients with a diagnosis of HPT for more than 6 months, (b) patients needed to be on the current standard treatment consisting of calcium and/or vitamin D supplementation, (c) QOL had to be measured with a validated instrument, and (d) QOL outcome measure needed to be compared to a reference population or controls. Exclusion criteria were: (a) pediatric patients, (b) patients with untreated HPT, (c) case reports, (d) and review papers.
Manuscript screening and data abstraction
The full texts for all eligible studies were acquired and reviewed. Extracted data was stored in an electronic file including: authors, year of publication, journal name, study design, sample population, reference population, and QOL instruments used. For intervention studies, only QOL data at baseline was extracted for reviewing the impact on QOL in patients receiving standard treatment.
Quality assessment
The quality assessment of the studies was conducted using the Newcastle–Ottawa Quality Assessment Scale adapted for cross-sectional studies proposed by Herzog et al. [11]. This assessment allows a scoring of the quality of cross sectional studies with a total sum of up to ten stars with ten stars reporting best quality. The results of the quality assessment can be found in Table 1. Even though two studies were not cross-sectional in their original design, they were assessed like cross-sectional studies because only baseline data were examined. Low quality of a study was not considered an exclusion criterion.
Results
Study selection
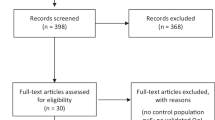
From the electronic literature search, 372 records were found. After title and abstract screening, 14 publications for full text screening remained. No additional records were found using hand search or reference screening.
Of the 14 studies, two were case reports, one study had no QOL comparison, three studies had not used validated QOL tools, and three studies presented only follow up information on populations that had already been published elsewhere, leaving five studies for the systematic review (Fig. 1).
Definition of HPT
Four of the studies gave detailed information regarding the definition of HPT used in their study. Arlt et al. [8] only stated that patients had an established diagnose of HPT without giving diagnostic criteria. The other four studies all defined HPT as low PTH levels in combination with hypocalcemia or inappropriately low calcium levels. Regarding the duration of the disease Cusano et al. [12] used a 2 year time frame for defining chronic HPT while the other three studies [7, 13, 14] defined 1 year as acceptable disease duration for the chronic state of HPT.
QOL in patients with HPT receiving standard treatment
Five studies were found that evaluated the impact of HPT on the patients’ QOL while receiving standard treatment with calcium and vitamin D supplementation (Table 2).
Of the five studies identified, three studies compared the QOL of HPT patients to a reference population.
Cusano et al. [12] describe the results of an open-label uncontrolled trial of the effect of PTH (1–84) on QOL in HPT patients. The study used the Rand 36 Item Health Survey (SF-36) SF-36 for the evaluation of baseline QOL of HPT patients. 54 individuals with chronic HPT for at least 2 years were included. Twenty-seven of whom had postsurgical HPT, 26 autoimmune HPT, and one had HPT due to DiGeorge syndrome. QOL was measured at baseline and compared with norm data from the United States. Patients with HPT scored significantly worse in all eight domains of the SF-36, with scores for physical functioning (PF) (66 ± 30), role-physical (RP) (46 ± 50), bodily pain (BP) (43 ± 30), general health (GH) (47 ± 30), vitality (VT) (33 ± 30), social functioning (SF) (60 ± 40), role-emotional (RE) (53 ± 60), and mental health (MH) (58 ± 20). Sikjaer et al. [13] performed a randomized controlled trial evaluating the effect of PTH (1–84) on QOL in patients with HPT using the SF-36 and the WHO-5 Well-Being Index Survey (WHO-5). They compared the patients’ data with those of the United States general population. 62 patients with chronic HPT were included, of whom 58 were postsurgical and 4 idiopathic. The patients’ QOL was significantly reduced in seven of the eight SF-36 domains, namely in PF (45.3 ± 9.2), RP (42.6 ± 11.7), BP (43.8 ± 10.9), GH (42.5 ± 10.6), VT (43.9 ± 11.9), RE (45.3 ± 11.2), and SF (47.3 ± 9.9, p < 0.05 for all), compared to a general US population with a mean score of 50 and standard deviation of 10 [15]. The physical component score (PCS) (42.8 ± 10.7, p < 0.001) was significantly reduced in HPT patients compared to the general population, but the mental component score (48.4 ± 10.1, p = 0.21) did not differ. In the WHO-5, six patients had a score indicating depression and 14 had scores indicating poor well-being, while the rest had no signs of decreased well-being [13]. The largest study evaluating the effect of HPT on QOL to date is a cross-sectional Norwegian study by Astor et al. [7] with 283 respondents. Patients’ QOL was compared to a Norwegian reference population, matched for age and sex, using the SF-36. Emotional well-being was measured using the Hospital Anxiety and Depression scale (HADS) [16]. HPT patients scored worse in all eight SF-36 domains than the reference population for PF (74.2 ± 24.6 vs. 87.2 ± 18.7), RP (44.9 ± 43.8 vs. 77.9 ± 35.8), BP (58.1 ± 26.9 vs. 75.1 ± 26), GH (50.7 ± 27.2 vs. 76.8 ± 22), VT (42.2 ± 22.9 vs. 60.0 ± 20.8), SF (68.5 ± 27.3 vs. 85.5 ± 22.2), RE (65.1 ± 42.5 vs. 81.6 ± 32.4), and MH (70.5 ± 19.5 vs. 78.8 ± 16.5, p < 0.05 for all). HPT patients also had significantly increased anxiety (6.5 ± 4.4 vs. 4.2 ± 3.3) and depression (4.8 ± 4.1 vs. 3.4 ± 3.0), measured with the HADS, in comparison to the normative population. Surgical patients had worse QOL and emotional well-being than the non-surgical HPT patients. SF-36 scores for the RP (39.2 ± 43.1 vs. 58.6 ± 43.7, p = 0.002), BP (55.3 ± 26 vs. 63.8 ± 27.7, p = 0.03), and VT (40 ± 22.6 vs. 46.4 ± 23.3, p = 0.04) domains were decreased, and the depression (5.2 ± 4.0 vs. 4.0 ± 4.3) score of the HADS was increased.
Two studies used matched controls for investigating the impact of HPT on QOL. One study explored the impact of HPT on QOL using a cross-sectional design accounting for HPT but also hypothyroidism [14]. The study enrolled 66 subjects from three different groups, each with 22 individuals. The first group included patients with postsurgical HPT and well-substituted hypothyroidism. The second group included postsurgical patients without HPT and with well-substituted hypothyroidism. The third group included healthy controls with intact thyroid and parathyroid function. All groups were matched on gender and age. In addition, group 1 was matched with group 2 for the time interval between thyroid surgery and the survey. Compared to the healthy controls, patients with HPT had significantly decreased SF-36 scores in seven domains except the RE domain, and they had a lower PCS. In comparison to patients without HPT (group 2), patients with HPT had significantly decreased PF and RP, and the PCS was worse as well. In the WHO-5 well-being index, HPT patients, and patients with hypothyroidism scored significantly worse than the healthy control group. No differences were found between the HPT group and the hypothyroid patients for the WHO-5. These results did not change after adjusting for body weight, thyroid-stimulating hormone (TSH) levels, ionized calcium, 25 OH-vitamin D, and estimated glomerular filtration rate [14].
Arlt et al. [8] compared the QOL of 25 female HPT patients with a history of goiter surgery or hyperparathyroidism with 25 controls with normal parathyroid function matched for sex, age, subtotal thyroidectomy, and time since surgery using the Symptom Checklist 90, revised version (SCL-90-R), a list of complaints developed by Zerssen (B-L Zerssen), and the short form of the Giessen Complaint List (GBB-24). The SCL-90-R screens for psychological problems, the GBB-24 assesses physical symptoms and the B-L Zerssen is used to measure general well-being. In all three questionnaires, higher scores indicate an impairment in well-being.
Using the SCL-90-R, patients with HPT in comparison to controls had higher subscale scores for somatization (1.02 ± 0.69 vs. 0.65 ± 0.40, p = 0.023), depression (0.7 ± 0.46 vs. 0.42 ± 0.46, p = 0.037), anxiety (0.76 ± 0.12 vs. 0.33 ± 0.35, p = 0.003), hostility (0.44 ± 0.35 vs. 0.18 ± 0.23, p = 0.004), psychotic tendencies (0.28 ± 0.28 vs. 0.11 ± 0.2, p = 0.023), and global severity index (GSI) (0.67 ± 0.38 vs. 0.44 ± 0.36, p = 0.034). Additionally, standard T-scores showed pathologically elevated scores for HPT patients for anxiety, phobic anxiety, somatization, and GSI. In the GBB-24, HPT patients scored higher than the controls in exhaustion tendency (8.9 ± 5.6 vs. 5.6 ± 4.9, p = 0.027), pain in the limbs (10.1 ± 6.3 vs. 6.9 ± 4.4, p = 0.039), heart complaints (6.8 ± 5.2 vs. 3.8 ± 3.1, p = 0.021), and global score of discomfort (30.0 ± 17.5 vs. 18.9 ± 11.6, p = 0.11). The results from the B-L Zerssen support the finding that HPT patients have a higher global score (29.6 ± 14 vs. 18.7 ± 9.5, p = 0.002) than the controls.
Discussion
Patients with HPT receiving standard treatment show an impaired QOL compared to the general population and compared to other patients. Three studies found reduced QOL in comparison to a general population and two studies in comparison to matched patient controls.
In comparison to patients with other diseases, HPT patients have QOL scores similar to patients with chronic heart disease or diabetes. Postsurgical HPT patients have lower SF-36 scores than Norwegian patients with Addison’s disease in six of eight SF-36 domains or congenital adrenal hyperplasia in five out of eight SF-36 domains [17, 18].
Despite being on stable calcium and vitamin D supplementation, many HPT patients still experience physical, mental, or emotional symptoms on a regular basis [19]. This may indicate that it is not the disturbed calcium homeostasis but rather PTH deficiency that directly impairs QOL. PTH receptors have been found in several brain regions and the central nervous system [20, 21]. Missing PTH in these regions of the body might influence the regulation of fear and anxiety and might have an impact on the QOL domains related to mental health. Additionally, PTH receptors have been found in muscle cells where a lack of PTH might be the reason for physical symptoms and reduced QOL in physical domains [22, 23].
Studies exploring PTH replacement therapy have shown promising results in reducing symptoms and increasing the QOL of patients with HPT. However, not only medical conditions of HPT affect the patients’ QOL [24, 25]. A large web-based survey of 374 HPT patients revealed that healthcare providers often do not clearly understand the problems associated with HPT and that HPT patients feel that their condition is not fully understood and that they are lacking support [19]. Cho et al. showed that there is a difference in the perception of HPT on QOL between surgeons and postoperative HPT patients [26]. This information and empathy gap might have an additional negative impact on the patients’ well-being and therefore negatively impact their QOL.
Controversially discussed is the influence of daily medication on QOL. While studies have shown that the long-term intake of calcium and vitamin D might result in complications like soft-tissue calcifications, kidney stones, and nephrocalcinosis, it is not clear whether the chronic intake of medications as such and the constant monitoring might explain a negative impact on QOL [1]. In the PARADOX study, patients reported discomfort due to the constant monitoring. In contrast, other studies have shown no impairment of well-being in patients with more complex medication regimens [19, 27]. HPT seems to have a severe impact on QOL in various fields, but the exact impact of HPT on QOL is hard to quantify due to the small numbers of studies and the differences in the studies.
Only two of the studies investigated the influence of TSH levels and thyroid hormone replacement therapy on QOL [7, 14]. In the Astor study [7], TSH levels were normal, while in the Sikjear [14] study TSH levels of HPT patients were lower compared to the controls und to the well-substituted hypothyroid patients. The authors assumed that HPT patients received higher doses of levothyroxine because they reported complaints more often. As a consequence, their physicians might have tried to relive their symptoms by increasing the T4.
Regarding the effect of TSH supplementation on QOL, evidence is inconsistent [28, 29]. However, the possible impact of TSH supplementation on QOL should be kept in mind and evaluated in further studies.
Another aspect which needs careful consideration when evaluating the impact of postsurgical HPT are the different etiologies of the patients involved. Studies have shown that thyroid cancer itself has a negative impact on QOL and therefore needs to be considered as an aspect influencing postsurgical HPT patients separately from the HPT [30, 31]. Sikjaer et al. [14] reported that 23% of the patients in the postsurgical HPT group had a diagnosis of cancer compared to only 9% in the postsurgical hypothyroid group. Even though all cancers were diagnosed at least 5 years before the study, it can not be excluded that the malignant disease itself might have an impact on the patients’ QOL [14]. A second study reported that 20% of the study population underwent surgery due to thyroid cancer, but they found that etiology had no correlation with QOL [13]. In two studies, no differentiation of the surgical cause was made. It can therefore not be concluded whether QOL is reduced due to the malignant disease itself or to HPT.
All questionnaires used were validated, but all of them were unspecific regarding HPT and thyroid diseases. As a consequence, important QOL issues relevant for HPT might have been overlooked. To our knowledge, no HPT-specific questionnaire has been designed and validated to date.
Most patients with HPT receiving standard treatment have calcium and vitamin D levels within the recommended ranges. However, HPT patients still report a reduced QOL in comparison to the general population and matched controls. It has been shown that it is not only the lack of calcium that influences the patients QOL, but that PTH levels also seem to have a direct influence in QOL in HPT patients. To date, all studies investigating the effect of HPT on patients’ QOL have used generic questionnaires which do not cover all symptoms and unmet needs of HPT patients. Therefore, further studies are required that quantify the effect of HPT on patients’ QOL using disease-specific questionnaires regarding etiology and other related factors like TSH levels and co-morbidities. Additionally, further studies need to be conducted that examine the effect of PTH treatment, in particular longitudinal studies. To help patients cope with their disease, more information and disease education regarding HPT must be made available to physicians and other health care providers.
References
D. Shoback, Clinical practice. Hypoparathyroidism. N. Engl. J. Med. 359(4), 391–403 (2008)
A. Bergenfelz, S. Jansson, A. Kristoffersson, H. Martensson, E. Reihner, G. Wallin et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3660 patients. Langenbecks Arch. Surg. 393(5), 667–673 (2008)
O. Thomusch, A. Machens, C. Sekulla, J. Ukkat, M. Brauckhoff, H. Dralle, The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 133(2), 180–185 (2003)
L. Underbjerg, T. Sikjaer, L. Mosekilde, L. Rejnmark, Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J. Bone Miner. Res. 28(11), 2277–2285 (2013)
L. Underbjerg, T. Sikjaer, L. Mosekilde, L. Rejnmark, The epidemiology of nonsurgical hypoparathyroidism in Denmark: a nationwide case finding study. J. Bone Miner. Res. 30(9), 1738–1744 (2015)
J. Powers, K. Joy, A. Ruscio, H. Lagast, Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J. Bone Miner. Res. 28(12), 2570–2576 (2013)
M.C. Astor, K. Lovas, A. Debowska, E.F. Eriksen, J.A. Evang, C. Fossum et al. Epidemiology and health-related quality of life in hypoparathyroidism in Norway. J. Clin. Endocrinol. Metab. 101(8), 3045–3053 (2016)
W. Arlt, C. Fremerey, F. Callies, M. Reincke, P. Schneider, W. Timmermann et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur. J. Endocrinol. 146(2), 215–222 (2002)
G. Marcucci, G. Della Pepa, M.L. Brandi, Natpara for the treatment of hypoparathyroidism. Expert. Opin. Biol. Ther. 16(11), 1417–1424 (2016)
J.P. Bilezikian, A. Khan, J.T. Potts Jr., M.L. Brandi, B.L. Clarke, D. Shoback et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J. Bone Miner. Res. 26(10), 2317–2337 (2011)
R. Herzog, M.J. Alvarez-Pasquin, C. Diaz, J.L. Del Barrio, J.M. Estrada, A. Gil, Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 13, 154 (2013)
N.E. Cusano, M.R. Rubin, D.J. McMahon, D. Irani, A. Tulley, J. Sliney Jr et al. The effect of PTH(1-84) on quality of life in hypoparathyroidism. J. Clin. Endocrinol. Metab. 98(6), 2356–2361 (2013)
T. Sikjaer, L. Rolighed, A. Hess, A. Fuglsang-Frederiksen, L. Mosekilde, L. Rejnmark, Effects of PTH(1-84) therapy on muscle function and quality of life in hypoparathyroidism: results from a randomized controlled trial. Osteoporos. Int. 25(6), 1717–1726 (2014)
T. Sikjaer, E. Moser, L. Rolighed, L. Underbjerg, L.S. Bislev, L. Mosekilde et al. Concurrent hypoparathyroidism is associated with impaired physical function and quality of life in hypothyroidism. J. Bone Miner. Res. 31(7), 1440–1448 (2016)
J.E. Ware Jr., C.D. Sherbourne, The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30(6), 473–483 (1992)
J.H. Loge, S. Kaasa, Short form 36 (SF-36) health survey: normative data from the general Norwegian population. Scand. J. Soc. Med. 26(4), 250–258 (1998)
K. Lovas, J.H. Loge, E.S. Husebye, Subjective health status in Norwegian patients with Addison’s disease. Clin. Endocrinol. 56(5), 581–588 (2002)
I. Nermoen, E.S. Husebye, J. Svartberg, K. Lovas, Subjective health status in men and women with congenital adrenal hyperplasia: a population-based survey in Norway. Eur. J. Endocrinol. 163(3), 453–459 (2010)
N. Hadker, J. Egan, J. Sanders, H. Lagast, B.L. Clarke, Understanding the burden of illness associated with hypoparathyroidism reported among patients in the PARADOX study. Endocr. Pract. 20(7), 671–679 (2014)
A.G. Bago, E. Dimitrov, R. Saunders, L. Seress, M. Palkovits, T.B. Usdin et al. Parathyroid hormone 2 receptor and its endogenous ligand tuberoinfundibular peptide of 39 residues are concentrated in endocrine, viscerosensory and auditory brain regions in macaque and human. Neuroscience 162(1), 128–147 (2009)
S. Balabanov, U. Tollner, H.P. Richter, F. Pohlandt, G. Gaedicke, W.M. Teller, Immunoreactive parathyroid hormone, calcium, and magnesium in human cerebrospinal fluid. Acta Endocrinol. 106(2), 227–233 (1984)
P. Divieti, N. Inomata, K. Chapin, R. Singh, H. Juppner, F.R. Bringhurst, Receptors for the carboxyl-terminal region of pth(1-84) are highly expressed in osteocytic cells. Endocrinology 142(2), 916–925 (2001)
T.B. Usdin, T.I. Bonner, S.R. Hoare, The parathyroid hormone 2 (PTH2) receptor. Recept. Channels 8(3-4), 211–218 (2002)
N.E. Cusano, M.R. Rubin, D.J. McMahon, D. Irani, L. Anderson, E. Levy et al. PTH(1-84) is associated with improved quality of life in hypoparathyroidism through 5 years of therapy. J. Clin. Endocrinol. Metab. 99(10), 3694–3699 (2014)
A. Santonati, A. Palermo, E. Maddaloni, D. Bosco, A. Spada, F. Grimaldi et al. PTH(1-34) for surgical hypoparathyroidism: A prospective, open-label investigation of efficacy and quality of life. J. Clin. Endocrinol. Metab. 100(9), 3590–3597 (2015)
N.L. Cho, J. Moalem, L. Chen, C.C. Lubitz, F.D. Moore Jr., D.T. Ruan, Surgeons and patients disagree on the potential consequences from hypoparathyroidism. Endocr. Pract. 20(5), 427–446 (2014)
W. Arlt, F. Callies, J.C. van Vlijmen, I. Koehler, M. Reincke, M. Bidlingmaier et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N. Engl. J. Med. 341(14), 1013–1020 (1999)
P. Saravanan, W.F. Chau, N. Roberts, K. Vedhara, R. Greenwood, C.M. Dayan, Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin. Endocrinol. 57(5), 577–585 (2002)
O. Husson, H.R. Haak, W.A. Oranje, F. Mols, P.H. Reemst, L.V. van de Poll-Franse, Health-related quality of life among thyroid cancer survivors: a systematic review. Clin. Endocrinol. 75(4), 544–554 (2011)
H.C. Hoftijzer, K.A. Heemstra, E.P. Corssmit, A.A. van der Klaauw, J.A. Romijn, J.W. Smit, Quality of life in cured patients with differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 93(1), 200–203 (2008)
S. Singer, T. Lincke, E. Gamper, K. Bhaskaran, S. Schreiber, A. Hinz et al. Quality of life in patients with thyroid cancer compared with the general population. Thyroid 22(2), 117–124 (2012)
Acknowledgements
Authors’ roles: All authors contributed to the conception and design of the study. All authors contributed to the acquisition, analysis and interpretation of the data. All authors participated in drafting or critically revising the manuscript and all authors’ approved the final version of the manuscript for submission.
Funding
This study did not receive any funding or grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Matthias Buettner has nothing to disclose. Thomas Musholt has nothing to disclose. Susanne Singer reports grants from Pfitzer, outside the submitted work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Büttner, M., Musholt, T.J. & Singer, S. Quality of life in patients with hypoparathyroidism receiving standard treatment: a systematic review. Endocrine 58, 14–20 (2017). https://doi.org/10.1007/s12020-017-1377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1377-3