Abstract
In patients with thyroid fine-needle aspiration (FNA) report of suspicious for malignancy (SFM), both lobectomy and thyroidectomy might be considered. BRAF mutation analysis could guide towards accurate surgical therapy. The primary outcome was the reliability of BRAF (V600E) in detecting malignancy in nodules with FNA reading of SFM. The secondary outcome was to analyze its positive predictive value (PPV) and negative predictive value (NPV) considering the surgical histology as gold standard. A literature search of online databases was performed in June 2019. BRAF prevalence among thyroid nodules with FNA read as SFM according to the most popular classification systems (i.e., Bethesda V, Thy4, TIR4 category) was searched. The random-effects model was used. Three hundred sixty original articles were identified and 34 were finally included in the study. There were 1428 thyroid nodules with FNA read as SFM and 1287 (90.1%) lesions underwent surgery with a cancer rate 89.6%. The pooled prevalence of BRAF (V600E) mutation among all nodules with SFM cytology was 47% (95% CI = 40 to 54, I2 = 85.5%). Pooled PPV and NPV of BRAF testing were 99% (95% CI, 97–99) and 24% (95% CI, 16–32), respectively. BRAF (V600E) mutation was found in about one in two nodules with thyroid FNA read as SFM, its PPV to detect cancers was excellent, and its NPV was very poor. The routine BRAF testing in FNA read as SFM cannot be recommended. BRAF (V600E) test may be useful to extend surgical approach in selected cases with further suspicious clinical/ultrasound features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fine-needle aspiration (FNA) for cytological evaluation is the pivotal tool for the management of thyroid nodule. The Bethesda System for Reporting Thyroid Cytopathology (TBRSTC) [1] is the most used system for classification of thyroid cytology worldwide, while the systems proposed by British Thyroid Association (BTA) [2] and Italian consensus for the classification and reporting of thyroid cytology (ICCRTC) [3] are used mainly in Europe. Theoretically, the aim of thyroid FNA is to discriminate cytologically benign nodules to be managed by clinical and ultrasound follow-up from those warranting thyroidectomy due to cytology consistent with malignancy. However, some limitations of thyroid cytological assessment exist, such as the inconclusive reports (i.e., indeterminate samples) and FNA suspicious for malignancy (SFM). The latter category, namely Bethesda V [1], Thy4 [2], and TIR4 [3], represents a condition in which the likelihood to confirm the cancer at histology ranges from 50 to 75% in TBRSTC [1], from 68 to 70% in BTA [2], and from 60 to 80% in ICCRTC [3]. Even if these systems have been published in updated versions over the time, no significant changes were present in the definition of the suspicious for malignancy category [4,5,6]. Furthermore, the risk of malignancy (ROM) of this FNA category decreases when considering the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTPs) as non-malignant entity [7]. As a consequence, there is no general consensus on the best therapeutic management of patients with SFM. In this context, the most recent American Thyroid Association (ATA) guidelines suggest to reduce both the extent of surgery and postoperative radioiodine treatment for many low-risk cancers with consequent raising possibility for lobectomy as initial surgical management [8]. Many factors could influence the decision of unilateral versus bilateral thyroid surgery in these patients and the molecular testing represents one major issue [1, 8].
BRAF (V600E) is the most frequent genetic mutation in papillary thyroid cancer (PTC) and has been reported as a predictor of poor prognosis of these patients. Being PTC the most frequent cancer type among nodules with preoperative FNA classified as Bethesda V [1], Thy4 [2], and TIR4 [3], there is a large literature on the use of BRAF status testing in this setting of patients. However, until now, there is not enough solid information on this topic.
This study aimed to obtain more robust information in this field by conducting a systematic review and meta-analysis. The primary outcome was the reliability of BRAF (V600E) testing in detecting malignancy before surgery in nodules with FNA reading of Bethesda V [1], Thy4 [2], and TIR4 [3]. The secondary outcome was to analyze both positive and negative predictive value (PPV and NPV, respectively) of BRAF test to detect malignancy considering the surgical histology as gold standard.
Methods
Conduct of Review
The present systematic review and meta-analysis was conducted according to PRISMA guidelines [9].
Search Strategy
A comprehensive literature search was conducted using the online databases of PubMed/MEDLINE and Scopus. The search aimed to find data on the analysis of BRAF mutation performed on cytological samples of nodules cytologically classified as suspicious for malignancy (i.e., Bethesda V [1], Thy4 [2], and TIR4 [3]). The online search was conducted by the following algorithm: (suspicious for malignancy AND molecular analysis) OR (Bethesda V AND BRAF) OR (Thy4 and BRAF) OR (TIR4 AND BRAF) OR (suspicious thyroid lesions AND BRAF) OR (suspicious thyroid cancer AND BRAF) OR (suspicious for malignancy AND BRAF) OR (suspicious thyroid lesions AND molecular analysis) OR (suspicious thyroid cancer AND molecular analysis). A beginning date limit was not used. The search was updated until June 15, 2019, and no language restrictions were used. With an attempt to expand the search, references of the retrieved articles were also screened to identify additional studies.
Study selection
As the main inclusion criterion, only original articles reporting BRAF status analyzed on FNA specimens after a cytological diagnosis of suspicious for malignancy according to the above systems [1,2,3] were included. Exclusion criteria were absence/incompleteness of data of BRAF analysis, studies in which another classification system was used, BRAF test performed in non-cytological specimens, series comprising less than ten cases, series comprising only malignant post-surgical diagnosis, and articles with overlapping data on patients or nodules. Two researchers (PT, LS) independently reviewed titles and abstracts of the retrieved articles, applying the selection criteria; then, all authors independently reviewed the full text of the remaining articles to determine their final inclusion.
Data extraction
For each included study, the following information was extracted independently by two investigators (PT, LS) in a piloted form: [1] study data (authors, year and journal of publication, country of origin); [2] number of nodules with suspicious FNA; [3] cytological system used; [4] number of BRAF (V600E) mutated cases among nodules with suspicious FNA; [5] cytological preparation; [7] number of cancers and benign lesions at histology among patients operated upon. Data were cross-checked, and any discrepancies were discussed and mutually solved.
Study quality assessment
The risk of bias of included studies was assessed independently by two reviewers (PT, LS) through the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool for the following aspects: patient selection, index test, reference standard, flow, and timing. Risk of bias and concerns about applicability were rated as low, high, and unclear risk.
Statistical analysis
A proportion meta-analysis was performed to obtain the pooled rate of BRAF mutated cases on FNA samples among all suspicious cytologies, also separated in different subgroups. Moreover, a proportion meta-analysis was performed to obtain the pooled rate of BRAF mutated cases detected on FNA among all BRAF mutated cancers detected on histological specimens. Particularly, a lesion-based analysis was conducted. For statistical pooling of data, the DerSimonian and Laird method (random-effects model) was used [10]. Pooled data are presented with 95% confidence intervals (95% CI) and displayed using a forest plot. The I-square index was used to quantify the heterogeneity among the studies, and significant heterogeneity was defined as an I-square value > 50%. A funnel plot was carried out for any outcome and publication bias might be considered when smaller size studies had on average different results with respect to the larger ones. Statistical analyses were performed using the StatsDirect statistical software (StatsDirect Ltd; Cambridge, UK).
Results
Eligible articles
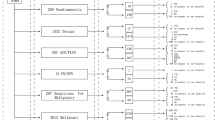
A number of 360 original articles were found by using the above algorithm. Applying the above selection criteria, a number of 34 studies were finally included in the review [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Figure 1 illustrates the flow of search.
Qualitative analysis (systematic review)
The 34 studies included were published by authors from 12 different countries during the period from 2009 and 2019. An overall number of 1428 nodules with FNA report of Bethesda V, Thy4, or TIR4 was found. Of these, a subgroup of 1287 (90.1%) lesions underwent surgery and 1153/1287 (89.6%) of these were histologically proven to be malignant. The majority of studies (25/34) performed only BRAF test, while the other ones performed one or more further genetic analyses alongside with BRAF. The analysis of BRAF (V600E) status was always performed by commercialized PCR kits. The larger part of studies classified nodules according to TBRSTC. Data on size of nodules were usually missing. Study sample size ranged from 11 to 142 nodules. Table 1 shows in detail the main characteristics of the studies included. Figure 2 illustrates schematically the available data from the included studies. Notably, there were 20 studies (58.8%) reporting histologically proven diagnosis for both cancers and benign lesions.
Study quality assessment
As summarized in Supplemental Table 1, in many of the 34 studies, the overall risk of bias and the concerns about applicability were considered to be low. However, high risk of bias and concerns regarding applicability about the patient selection (and also the flow and timing item) were present in five studies [18, 26, 39, 40, 43], where BRAF mutational analysis was not routinely and consecutively performed in all cytological suspicious for malignancy nodules but only in selected cases. Risk of bias and debatable feasibility of index test were rated as high in two articles [20, 42], since palpation-guided technique for FNA was adopted for part of the nodules. Moreover, although a high risk of bias for reference standard belonged to six studies [21, 22, 27, 30, 33, 44], the preoperative FNA accuracy in predicting malignant histology was within the ranges specified in the cytological classification systems [1,2,3]. Lastly, unclear judgment to the items of QUADAS-2 was given when studies showed approximated or missing data.
Quantitative analysis (meta-analysis)
The meta-analysis of the prevalence of BRAF (V600E) mutation among nodules with SFM FNA (Bethesda V, Thy4, TIR4) was conducted by pooling the results of 1428 lesions of the 34 studies (Table 2). The rate of positive BRAF ranged from 15 to 85% in the different series. The pooled rate was 47% (95% CI = 40 to 54). There was inconsistency (I [2] = 85.5%) (Fig. 3a). As shown in funnel plot, a significant publication bias was not to be considered (Fig. 3b). In view of the heterogeneity found among all articles, we performed a subgroup analysis considering only studies with more than 50 nodules. However, the pooled prevalence did not change (50%, 95% CI = 36 to 64) and there was again inconsistency among studies (I [2] = 93%).
Both PPV and NPV were calculated considering only 20 articles [13, 14, 18,19,20,21,22, 25, 26, 29, 31, 33, 34, 37,38,39,40,41, 43, 44] in which histological diagnosis of both cancers and benign lesions were detailed according to BRAF status. The overall number of these nodules was 903, being mutated and wild-type BRAF (V600E) 505 and 398, respectively. Among these nodules, 812 were PTC (of which 501 mutated BRAF and 311 wild-type BRAF) and 91 were benign. Pooled PPV and NPV of BRAF testing were 99% (95% CI, 97–99) and 24% (95% CI, 16–32), respectively. A significant publication bias was not to be considered (Table 3).
Discussion
Whether BRAF (V600E) testing could be useful to improve the decision of surgical approach in patients with SFM thyroid FNA is still unclear. To date, four meta-analyses have been reported on this topic [45,46,47,48]. All these reviews included data published up to 2014 from studies on indeterminate nodules (i.e., Bethesda III, Bethesda IV, Bethesda V). Jia et al. [45] did not report specific data on SFM FNA category. Fnais et al. [46] included a limited number of studies and reported BRAF status only in histologically proven PTC. Su et al. (47) included as reference standard clinical plus cytological follow alongside with histology. Jinih et al. [48] did not detail results of SFM. Also, these studies did not consider the most recent classification for thyroid FNA [1,2,3] as the only standard to identify SFM. Considering these study designs, the present meta-analysis is fully different and the present results are quite new.
Our systematic review enrolled a large number of studies evaluating the BRAF testing in a selected sample of nodules with FNA read as SFM. Importantly, these studies performed BRAF mutation analysis on cytological samples. Following our algorithm of search, we found online a significant number of articles reporting data on the use of BRAF (V600E) test in FNA classified according to TBRSTC [1], BTA [2], or ICCRTC [3]. The overall number of nodules (i.e., 1428) undergone BRAF test was relevant, and a 90.1% of these had histological follow-up. The pooled rate of mutated BRAF cases was 47% and a publication bias should be not considered (i.e., the rate of BRAF mutation was not influenced by sample size of the studies). These results did not change when we considered only the subgroup of articles with larger sample. As a major result, even if PPV of BRAF test was excellent (99%), its NPV was very poor (24%). These findings achieve some relevance for clinical practice.
The expected cancer rate of nodules with FNA of suspicious for malignancy ranges from 50 to 80% according to available guidelines [1,2,3]. International guidelines [2, 8, 49,50,51] agree with the surgical indication of these patients but what should be considered the optimal approach is widely debated. The Recommendation 20 of ATA guidelines [8] strongly indicates total thyroidectomy for nodules cytologically classified as SFM having BRAF (V600E), RET/PTC, and PAX8/PPAR mutations. However, these guidelines state that mutational analysis of BRAF or the 7-gene mutation marker panel might be considered if such data would be expected to alter surgical decision-making (R17B, weak recommendation, and moderate-quality evidence). Moreover, ATA guidelines [8] claim that thyroid lobectomy alone is sufficient for < 1 cm, unifocal, intrathyroidal carcinomas in the absence of prior head and neck irradiation, familial thyroid carcinoma, or clinically detectable cervical nodal metastases and as the initial procedure for thyroid cancer > 1 cm and < 4 cm without extrathyroidal extension and without clinical evidence of any lymph node metastases (R35, strong recommendation, and moderate-quality evidence). In this context, AACE/ACE/AME Task Force on Thyroid Nodules [49] recommend to use BRAF mutational analysis or intraoperative histological examination as a guide for the extent of surgery, without specifying the type of surgery and if some clinical data have to be considered for the surgical decision. The BTA 2014 guidelines [2] recommend to perform diagnostic hemithyroidectomy for Thy4 and they only mention molecular tests as a tool to assist in stratification of risk, without providing details about genes. NCCN guidelines [50] state that total thyroidectomy or lobectomy with isthmusectomy should be indicated on the basis of clinical and morphological criteria and not on the basis of molecular diagnostics which may be performed in Bethesda III and IV results. Lastly, European Thyroid Association (ETA) guidelines [51] report that selected mutations, such as BRAF, TERT, and TP53, need to be further investigated; nonetheless, in the main text of these guidelines [51], it is asserted that the detection of BRAF (and, probably, also of TERT promoter and TP53 mutations) may drive towards total thyroidectomy if the clinico-pathological setting is appropriate and that the identification of specific mutations, such as BRAF (V600E) and TERT in thyroid nodules > 1 cm, justifies a total thyroidectomy and possibly prophylactic central lymph node dissection.
The present results from our meta-analysis demonstrated that a routine use of BRAF test is not indicated in all FNA read as SFM. In fact, available literature suggests that this approach is likely not cost-effective being positive test present in less than 50% of these cases. However, when managing patients with clinico-pathological features prompting to extend surgery, BRAF may be reasonably used as a rule-in test because its positivity can strongly indicate the presence of cancer. On the contrary, in patients without further suspicious features, BRAF test is not supported to corroborate the clinical indication to non-extended surgery (i.e., rule-out use of BRAF). In example, in a patient with single nodule cytologically SFM with no evidence of extra-thyroid extension at ultrasound, a lobectomy may be considered and this clinical decision cannot be significantly influenced by BRAF testing due to its poor NPV. According to the present results, R17 of ATA should be considered with high-quality evidence and probably the grade of recommendation might be revised.
In our pooled series, four BRAF mutated cases were false positive (i.e., benign at histology). This is a critical issue, since BRAF mutation is considered pathognomonic of PTC and decision to move to total thyroidectomy plus lymphadenectomy may be based on this finding. One BRAF mutated case on cytology was an oncocytic adenoma [18] at histology. The other three cases showed a chronic lymphocytic thyroiditis, a multinodular hyperplasia [20], and an undefined benign lesion (thyroiditis or nodular hyperplasia) [39]. It is known that some highly sensitive BRAF detection method can cause false positive results by detecting mutation when this is present in only 2% of cells. It should be reminded that BRAF is frequently mutated in other more common neoplastic conditions, such as melanoma, colorectal carcinoma, lung carcinoma, and leukemia, that can affect the thyroid as metastasis. Kuhn et al. [52] detected the presence of BRAF mutated cells in a FNA of the thyroid classified as SFM that turned out at histology to be primary Langerhans cell histiocytosis of the thyroid. In the latter examples, BRAF mutation cannot be considered a false positive result; however, management could be different from that of a primary thyroid tumor.
Some limitations of the present findings should be mentioned. First, we have no data on the impact of BRAF test in the surgical decision-making of patients. All included studies are retrospective while there is not a randomized controlled trial on this topic. Then, whether BRAF status would have influenced the surgical approach is not known. Second, high heterogeneity was present probably due to the high number of studies included. The strength of the present study was its design which allowed to obtain clear data differently from the previous reviews [45,46,47,48].
In conclusion, the present meta-analysis showed that BRAF (V600E) mutation was found in about one in two nodules with thyroid FNA read as suspicious for malignancy according to the most recent classification systems. PPV of BRAF test to predict malignant lesions was excellent, while its NPV was very poor. Considering these evidence-based data, the routinely BRAF testing in FNA suspicious for malignancy cannot be recommended. In the setting of patients with FNA report of Bethesda V, Thy4, or TIR4, BRAF may be useful to support a more extended surgical approach in selected cases with further suspicious clinical/ultrasound features. A role of BRAF testing to guide to less extensive surgery is not supported.
References
The Bethesda System for Reporting Thyroid Cytopathology (2018) Eds. Ali SZ, Cibas ES. Springer International Publishing AG
Perros P, Boelaert K, Colley S, et al. (2014) British Thyroid Association. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 81 Suppl 1:1-122
Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, Palombini L, Papini E, Zini M, Pontecorvi A, Vitti P (2014) Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 37:593-599
Cibas ES, Ali SZ (2009) The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19:1159-1165
Guidelines for the management of thyroid cancer (second edition) (2007) Royal College of Physicians of London. The Lavenham Press, Suffolk
Fadda G, Basolo F, Bondi A, et al. (2010) SIAPEC-IAP Italian Consensus Working Group. Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica 102(5):405-408
Bongiovanni M, Giovanella L, Romanelli F & Trimboli P (2018) Cytological diagnoses associated with non-invasive follicular thyroid neoplasms with papillary-like nuclear features, (NIFTP) according to the Bethesda System for Reporting Thyroid Cytopathology: a systematic review and meta-analysis. Thyroid 29(2):222-228
Haugen BR, Alexander EK, Bible KC, et al. (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26: 1-133
McInnes MDF, Moher D, Thombs BD, McGrath T, Bossuyt PM, the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 319:388-396
DerSimonian R & Laird (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45:139-145
Adeniran AJ, Theoharis C, Hui P, Prasad ML, Hammers L, Carling T, Udelsman R, Chhieng DC (2011) Reflex BRAF testing in thyroid fine-needle aspiration biopsy with equivocal and positiveinterpretation: a prospective study. Thyroid 21:717-723
Beiša A, Kvietkauskas M, Beiša V, Stoškus M, Ostanevičiūtė E, Jasiūnas E, Griškevičius L, Strupas K (2017) The utility of the Bethesda category and its association with BRAF mutation in the prediction of papillary thyroid cancer stage. Langenbecks Arch Surg 402:227-234
Capelli L, Marfisi C, Puccetti M, Saragoni L, de Paola F, Zaccaroni A, Chiadini E, Gagliardi L, Ferretti G, Zoli W, Ulivi P (2015) Role of BRAF molecular analysis in the management of papillary thyroid carcinoma: analysis of cytological and histological samples. Cytopathology 26:297-302
Collet JF, Lacave R, Hugonin S, Poulot V, Tassart M, Fajac A (2016) BRAF V600E detection in cytological thyroid samples: A key component of the decisiontree for surgical treatment of papillary thyroid carcinoma. Head Neck 38:1017-1021
Danilovic DL, Lima EU, Domingues RB, et al. (2013) Pre-operative role of BRAF in the guidance of the surgical approach and prognosis of differentiated thyroid carcinoma. Eur J Endocrinol 170:619-625
Decaussin-Petrucci M, Descotes F, Depaepe L, Lapras V, Denier ML, Borson-Chazot F, Lifante JC, Lopez J (2017) Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathology 28:482-487
Dhir M, McCoy KL, Ohori NP, Adkisson CD, LeBeau S, Carty SE, Yip L (2018) Correct extent of thyroidectomy is poorly predicted preoperatively by the guidelines of the American Thyroid Association for low and intermediate risk thyroid cancers. Surgery 163:81-87
Eszlinger M, Böhme K, Ullmann M, et al. (2017) Evaluation of a Two-Year Routine Application of Molecular Testing of Thyroid Fine-Needle Aspirations Using a Seven-Gene Panel in a Primary Referral Setting in Germany. Thyroid 27:402-411
Hemalatha R, Pai R, Manipadam MT, Rebekah G, Cherian AJ, Abraham DT, Rajaratnam S, Thomas N, Ramakant P, Jacob PM (2018) Presurgical Screening of Fine Needle Aspirates from Thyroid Nodules for BRAFMutations: A Prospective Single Center Experience. Indian J Endocrinol Metab 22:785-792
Jara SM, Bhatnagar R, Guan H, Gocke CD, Ali SZ, Tufano RP (2015) Utility of BRAF mutation detection in fine-needle aspiration biopsy samples read as “suspicious for papillary thyroid carcinoma”. Head Neck 37:1788-1793
Johnson SJ, Hardy SA, Roberts C, et al. (2014) Pilot of BRAF mutation analysis in indeterminate, suspicious and malignant thyroid FNAcytology. Cytopathology 25:146-154
Kim SK, Hwang TS, Yoo YB, Han HS, Kim DL, Song KH, Lim SD, Kim WS, Paik NS (2011) Surgical results of thyroid nodules according to a management guideline based on the BRAF(V600E) mutation status. J Clin Endocrinol Metab 96:658-664
Jieun Koh, Jong Rak Choi, Kyung Hwa Han, et al. (2013) Proper Indication of BRAFV600E Mutation Testing in Fine- Needle Aspirates of Thyroid Nodules. PLoS One 8:e64505
Krane JF, Cibas ES, Alexander EK, et al. (2015) Molecular analysis of residual ThinPrep material from thyroid FNAs increasesdiagnostic sensitivity. Cancer Cytopathol 123:356-361
Kwon HJ, Kimc E, Kwakc JY (2015) Cytomorphologic features in thyroid nodules read as “suspicious for malignancy” on cytology may predict thyroid cancers with the BRAF mutation. Pathol Res Pract 211:671-676
Liu S, Gao A, Zhang B, Zhang Z, Zhao Y, Chen P, Ji M, Hou P, Shi B (2014) Assessment of molecular testing in fine-needle aspiration biopsy samples: an experience in a Chinese population. Exp Mol Pathol 97:292-297
Macerola E, Rago T, Proietti A, Basolo F, Vitti P (2019) The mutational analysis in the diagnostic work-up of thyroid nodules: the real impact in a center with large experience in thyroid cytopathology. J Endocrinol Invest 42:157-166
Marchetti I, Lessi F, Mazzanti CM, Bertacca G, Elisei R, Coscio GD, Pinchera A, Bevilacqua G (2009) A morpho-molecular diagnosis of papillary thyroid carcinoma: BRAF V600E detection as an important tool in preoperative evaluation of fine-needle aspirates. Thyroid 19:837-842
Meng Z, Lu J, Wu H, et al. (2016) Mutant-specific BRAF and CD117 immunocytochemistry potentially facilitate riskstratification for papillary thyroid carcinoma in fine-needle aspiration biopsy specimens. Tumour Biol 37:611-618
Monti E, Bovero M, Mortara L, et al. (2015) BRAF Mutations in an Italian Regional Population: Implications for the Therapy of Thyroid Cancer. Int J Endocrinol 2015:138734
Moon HJ, Kwak JY, Kim EK, et al. (2009) The role of BRAFV600E mutation and ultrasonography for the surgical management of a thyroid nodule suspicious for papillary thyroid carcinoma on cytology. Ann Surg Oncol 16:3125-3131
Moses W, Weng J, Sansano I, Peng M, Khanafshar E, Ljung BM, Duh QY, Clark OH, Kebebew E (2010) Molecular testing for somatic mutations improves the accuracy of thyroid fine-needleaspiration biopsy. World J Surg 34:2589-2594
Park KS, Oh YL, Ki CS, Kim JW (2015) Evaluation of the Real-Q BRAF V600E Detection Assay in Fine-Needle AspirationSamples of Thyroid Nodules. J Mol Diagn 17:431-437
Pelizzo MR, Boschin IM, Barollo S, Pennelli G, Toniato A, Zambonin L, Vianello F, Piotto A, Ide EC, Pagetta C, Sorgato N, Torresan F, Girelli ME, Nacamulli D, Mantero F, Mian C (2011) BRAF analysis by fine needle aspiration biopsy of thyroid nodules improvespreoperative identification of papillary thyroid carcinoma and represents a prognosticfactor. A mono-institutional experience. Clin Chem Lab Med 49:325-329
Poller DN, Glaysher S, Agrawal A, Caldera S, Kim D, Yiangou C (2014) BRAF V600 co-testing in thyroid FNA cytology: short-term experience in a large cancercentre in the UK. J Clin Pathol 67:684-689
Pongsapich W, Chongkolwatana C, Poungvarin N, Amornpichetkul K, Piyawattayakorn N, Vejvisithsakul P, Maneeprasopchoke P (2019) BRAF mutation in cytologically indeterminate thyroid nodules: after reclassification of a variant thyroid carcinoma. Onco Targets Ther 12:1465-1473
Rossi ED, Martini M, Capodimonti S, Straccia P, Cenci T, Lombardi CP, Pontecorvi A, Larocca LM, Fadda G (2013) Diagnostic and prognostic value of immunocytochemistry and BRAF mutation analysison liquid-based biopsies of thyroid neoplasms suspicious for carcinoma. Eur J Endocrinol 168:853-859
Seo JY, Kim EK, Kwak JY (2014) Additional BRAF mutation analysis may have additional diagnostic value in thyroid nodules with “suspicious for malignant” cytology alone even when the nodules do not show suspicious US features. Endocrine 47:283-289
Wu Y, Xu T, Cao X, et al. (2019) BRAF V600E vs. TIRADS in predicting papillary thyroid cancers in Bethesda system I, III, and V nodules. Cancer Biol Med 16:131-138
Ye W, Hannigan B, Zalles S, Mehrotra M, Barkoh BA, Williams MD, Cabanillas ME, Edeiken-Monroe B, Hu P, Duose D, Wistuba II, Medeiros LJ, Stewart J, Luthra R, Roy-Chowdhuri S (2019) Centrifuged supernatants from FNA provide a liquid biopsy option for clinical next-generation sequencing of thyroid nodules. Cancer Cytopathol 127:146-160
Yeo MK, Liang ZL, Oh T, Moon Y, An S, Kim MK, Kim KS, Shong M, Kim JM, Jo YS (2011) Pyrosequencing cut-off value identifying BRAFV600E mutation in fine needle aspirationsamples of thyroid nodules. Clin Endocrinol (Oxf) 75:555-560
Zarkesh M, Zadeh-Vakili A, Akbarzadeh M, Nozhat Z, Fanaei SA, Hedayati M, Azizi F (2019) BRAF V600E mutation and microRNAs are helpful in distinguishing papillary thyroidmalignant lesions: Tissues and fine needle aspiration cytology cases. Life Sci 223:166-173
Zatelli MC, Trasforini G, Leoni S, et al. (2009) BRAF V600E mutation analysis increases diagnostic accuracy for papillary thyroid carcinoma in fine-needle aspiration biopsies. Eur J Endocrinol 161:467-473
Zheng B, Zarka MA, Chen C, You J, Sun L, Chen L (2018) The largest CAP-certified Chinese reference laboratory experience with the Bethesdasystem for reporting thyroid cytopathology: correlation with histologic and BRAF data. J Am Soc Cytopathol 7:16-21
Jia Y, Yu Y, Li X, et al. (2014) Diagnostic value of B-RAF(V600E) in difficult-to-diagnose thyroid nodules using fine-needle aspiration: systematic review and meta-analysis. Diagn Cytopathol 42:94-101
Fnais N, Soobiah C, Al-Qahtani K, et al. (2015) Diagnostic value of fine needle aspiration BRAF(V600E) mutation analysis in papillary thyroid cancer: a systematic review and meta-analysis. Hum Pathol 46:1443-1454
Su X, Jiang X, Xu X, et al. (2016) Diagnostic value of BRAF (V600E)-mutation analysis in fine-needle aspiration of thyroid nodules: a meta-analysis. Onco Targets Ther 9:2495-2509
Jinih M, Foley N, Osho O, et al. (2016) BRAFV600E mutation as a predictor of thyroid malignancy in indeterminate nodules: A systematic review and meta-analysis. Eur J Surg Oncol 43:1219-1227
Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, Paschke R, Valcavi R, Vitti P, AACE/ACE/AME Task Force on Thyroid Nodules (2016) American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocrine Practice 22:622–639
Haddad R, Nasr C, Bischoff L, et al. (2018) NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018 J Natl Compr Canc Netw 16:1429-1440
Paschke R, Cantara S, Crescenzi A, Jarzab B, Musholt TJ, Sobrinho Simoes M (2017) European Thyroid Association Guidelines regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur Thyroid J 6:115-129
Kuhn E, Ragazzi M, Zini M, Giordano D, Nicoli D, Piana S (2016) Critical Pitfalls in the use of BRAF Mutation as a Diagnostic Tool in Thyroid Nodules: a Case Report. Endocr Pathol 27:220-223
Acknowledgments
L.S. contributed to this paper as a recipient of an internal grant (“programma STAR”) by the University of Federico II (Naples, Italy) and as a fellow in Department of Nuclear Medicine and Thyroid Centre, Ente Ospedaliero Cantonale (Bellinzona, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Trimboli, P., Scappaticcio, L., Treglia, G. et al. Testing for BRAF (V600E) Mutation in Thyroid Nodules with Fine-Needle Aspiration (FNA) Read as Suspicious for Malignancy (Bethesda V, Thy4, TIR4): a Systematic Review and Meta-analysis. Endocr Pathol 31, 57–66 (2020). https://doi.org/10.1007/s12022-019-09596-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-019-09596-z