Abstract
Purpose
Elevated serum levels of carbohydrate antigen 19.9 (CA19.9), a well-established tumor marker in pancreatic neoplasms, has been proposed as a prognostic marker of tumor aggressiveness in medullary thyroid carcinoma (MTC). A hypothesis of C-cell dedifferentiation has been raised. Here, we evaluated the expression of CA19.9 and CD133, a stem cell marker, in MTC tissues.
Methods
MTC samples from patients attending a university-based hospital were evaluated for CA19.9 and CD133 expression by immunohistochemistry. Clinical data were retrieved from medical records.
Results
Tumor specimens from 70 MTC patients (57.1% hereditary) were evaluated. The age at diagnosis was 36.1 ± 16.3 years, and 58.6% were female; 53% of patients had cervical and 20% distant metastases. CA19.9 staining was detected in 87% of the samples, but no association was observed with biochemical markers, tumor size, local or distant metastases (All P > 0.05). Remarkable, CA19.9 expression was higher in the metastasis than in primary tumor samples (P = 0.0002). CD133 was expressed in 90.5% samples, but no correlation was found with CA19.9. Interestingly, we identified three distinct expression patterns to CA19.9: individual, focal, and diffuse cells. Sporadic MTC was associated with the individual cell pattern (70.6%), while the hereditary form with the focal expression pattern (63.9%; P = 0.04). Remarkably, the diffuse pattern was associated with larger tumor size and distant metastases (P = 0.032).
Conclusions
The majority of samples stained for CA19.9, suggesting it is an MTC cell-intrinsic feature. Three distinct expression patterns were identified, which were associated with the hereditary or sporadic form, larger tumor size, and presence of metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medullary thyroid carcinoma (MTC) is a malignant neoplasm of C-cells of the thyroid, corresponding to ~2–3% of all malignant tumors of the thyroid but responsible for 13.5% of the tumor-related deaths [1, 2]. The survival rate of patients with MTC is 75% in 10 years [2, 3]; however, when distant metastases are present, the survival rate decreases to 42% in 5 years and 31% in 10 years [2]. MTC presents as sporadic (75–80%) or inherited tumors (20–25%). Germline activating mutations in the RET gene are responsible for hereditary MTC. The molecular mechanisms involved in sporadic MTC have not yet been clarified. Somatic RET or RAS mutations seem to represent alternative genetic events in sporadic MTC tumorigenesis [4].
Calcitonin is a specific and highly sensitive biomarker for MTC. Neoplastic C cells also produce the carcinoembryonic antigen (CEA). Both molecules are widely used as tumor markers of patients with MTC [5, 6]. Indeed, the calcitonin and CEA doubling-time are strong prognostic factors of disease progression and outcome [7].
The carbohydrate antigen 19.9 (CA19.9) is a mucinous glycoprotein of high molecular weight (>400 kD), which has a sialic structure originating from the Lewis blood group antigen [8]. CA19.9 is classically used as a tumor marker in pancreatic neoplasms [9,10,11]. However, although the maximum tissue expression of CA19.9 occurs in pancreatic cancer, it has also been observed in other malignant neoplasias, including the lung, colon, breast, ovary, and uterus [12].
Recently, two case reports describe elevated serum levels of CA19.9 antigen in MTC patients. In both cases, the patients had multiple distant metastases, low calcitonin, and increased CEA levels, suggesting CA19.9 as a prognostic marker of tumor aggressiveness and mortality in advanced MTC [13, 14]. A study by Elisei et al. evaluated CA19.9 serum levels in 100 patients with advanced MTC with persistent or recurrent disease. The authors observed increases levels of serum CA19.9 in patients with distant metastases. Remarkably, the higher serum Ca 19.9 level was an independent predictor of disease-related mortality [15]. The association between CA19.9 serum levels and outcomes was also recently investigated in 65 MTC patients. Similar to the findings described by Elisei et al. the authors observed higher serum CA19.9 levels in patients with disease progression or have died from MTC [16]. The CA19.9 expression was evaluated in MTC samples in a small pilot study, which has shown positive CA19.9 staining in 62.5% of the cases. However, the mechanism of the association between CA19.9 and advanced MTC remains mostly unknown.
The purpose of this study was to investigate the potential role of CA19.9 expression as a prognostic factor in MTC. First, we thought to determine whether the CA19.9 tissue expression was an MTC cell-intrinsic feature or related to the dedifferentiation of cancer cells. To further explore the hypothesis that higher CA19.9 expression could be associated with the dedifferentiation of the C cells, we evaluated the expression of CD133, cholesterol interacting penta-span transmembrane glycoprotein (120 kd), extensively used as a marker for the cancer stem cell in several carcinomas, including thyroid (19–24). In addition, we evaluated the association between tumor expression of CA19.9 and clinical presentation and outcome in a cohort of individuals with MTC.
Material and methods
Patients and study design
Patients with histopathological diagnosis of MTC followed at Thyroid Unit of Hospital de Clínicas de Porto Alegre, a university-based teaching hospital, with the primary tumor or metastasis samples paraffin-embedded available were included. Since 1997, our Unit has been a reference center for the molecular testing of RET germline mutations in Brazil. Therefore, patients are referred to us by other centers for molecular investigation. All patients or their legal guardians provided written informed consent by following the institutional Ethics Committee (CAAE 62017016300005327).
Clinical and histopathological data
Clinical and laboratory data were collected for each individual, and laboratory tests were performed as described previously [17]. The Institutional MTC treatment protocol follows the current guidelines [18]. Total thyroidectomy was performed in all patients with varying cervical neck dissection procedures. N0 status was determined by clinical examination of the neck or preoperative and postoperative neck ultrasound imaging or macroscopic analysis during surgery and pathological exam of patients with lymph node resection. Patients with suspicious distant metastases (i.e., the presence of lymph node metastases or serum calcitonin >150 pg/mL) underwent imaging exams (cervical, thoracic, and abdomen computed tomography or liver magnetic resonance) [19]. Tumor staging was performed according to the 8th edition of the TNM/AJCC (TNM8) staging system (I, II, III, or IV) [19]. The last medical visit was considered the final follow-up. Disease status was defined based on serum calcitonin levels and imaging exams. Patient status was classified as follows: no evidence of disease (undetectable calcitonin), persistent disease (detectable calcitonin or evidence of structural disease), or disease-related death.
RET germline mutation protocol
All patients with known hereditary MTC harbor a RET germline mutation. Our RET germline mutation protocol consists of performing mutational analysis using Sanger sequencing on exons 8, 10, 11, 13, 14, and 15 of this proto-oncogene. Standard procedures were described previously [17]. For those with sporadic MTC, the diagnosis was established based on the absence of known RET germline mutation, family history of MTC, or clinical phenotype of a specific syndrome.
Somatic M918T RET and RAS mutation analysis
The MTC samples were evaluated from paraffin-embedded formalin-fixed MTC blocks. Exon 16 of RET and exons 2 and 3 of H-RAS and K-RAS, and exon 3 of N-RAS was amplified by PCR using specific primers. Samples were sequenced using ABI 3500 Genetic Analyzer with 50 cm capillaries and POP7 polymer (Applied Biosystems). PCR products were labeled with 5.0 pmol of the specific primers and 1 μL of BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) in a final volume of 10 μL. Labeling reactions were performed in a Veriti1 96-Well Thermal Cycler (Applied Biosystems) with an initial denaturing step of 96°C for 1 min followed by 35 cycles of 96 °C for 15 sec, 50 °C for 15 sec and 60 °C for 4 min. Labeled samples were purified using BigDye XTerminator Purification Kit (Applied Biosystems) and electron injected in the automatic sequencer.
Immunohistochemical (IHC) analysis
Paraffin sections (3 µm) of previously formalin-fixed and paraffin-embedded tissues were cut and prepared for IHC staining. The antibodies used were monoclonal mouse anti-CA 19.9 (Clone 1116-NS-19-9, M3517, DakoCytomation, Carpinteria, CA), and polyclonal rabbit CD133 antibody (CD133: orb18124, Biorbyt). Sections representing MTC were submitted to routine immunohistochemistry technique, which comprises deparaffination and rehydration, antigenic recovery, inactivation of endogenous peroxidase, and blockage of unspecific reactions. Primary antibodies were incubated overnight at a temperature of 4 °C, at dilutions of 1:800 (anti-CA19.9) and 1:300 (CD133), followed by the application of streptavidin-horseradish peroxidase conjugate (LSAB; DakoCytomation), and diaminobenzidine-tetrahydrochloride (Kit DAB; DakoCytomation). Sections of human tissue were used as a positive control (pancreatic tissue for CA19.9 and glioblastoma tissue for CD133), and the absence of the primary antibody as a negative control.
A pathologist (M.S.G.) analyzed the intensity of CA19.9 and CD133 staining by the h-score method. The h-score consists of the sum of the percent of tumor cells staining multiplied by an ordinal value corresponding to the intensity level (0 = none, 1 = weak, 2 = moderate, and 3 = strong). With four intensity levels, the resulting score ranges from 0 (no staining in the tumor) to 300 (diffuse intense staining of the tumor).
Statistical analysis
The clinical and laboratory data are reported as the mean ± standard deviation (SD) values or as the median and percentiles 25 and 75 (P25–75) for continuous variables or as absolute numbers and percentages for categorical variables. Comparative analyses were performed using an unpaired Student’s t test, Mann–Whitney U test, Kruskal–Wallis test, or χ2, as appropriate.
All tests were two-tailed, and all analyses were performed using the Statistical Package for Social Science Professional software version 20.0 (IBM Corp., Armonk, NY, USA). All tests were two-tailed, and a P < 0.05 was considered statistically significant.
Results
Patients
From a cohort of 369 MTC patients, 70 individuals were evaluated based on study inclusion criteria (Fig. 1). Clinical and oncological features at diagnosis of the patients included are described in Table 1. The mean age was 36.1 ± 16.3 years, and 41 (58.6%) were female. The median levels of calcitonin and CEA were 536 pg/mL (P25–75 49.35–1300.5) and 21.3 ng/mL (P25–75 3.6–52.6), respectively. At diagnosis, the median tumor size was 2.3 cm (P25–75 1.2–3.3); 37 (52.9%) patients had lymph node metastases, and 14 (20%) had distant metastases. The median follow-up time of 12.1 years (P25–75 7.5–16.5). Based on physical exam, calcitonin levels, and imaging exams, 42.6% of patients were considered free of disease, 45.9% with persistent disease, and 11.5% died due to the disease. The clinical and oncological characteristics of the 70 patients included in this study were similar to the whole cohort (all P > 0.05).
The hereditary and sporadic forms of MTC were also analyzed separately (Table 1). Our sample comprised 57.1% sporadic MTC and 42.9% of hereditary MTC. The majority of hereditary patients harbor codon mutation 634 (83.3%). Of the 40 sporadic MTC samples, 35 were evaluated for M918T somatic mutation, and 9 (25.7%) were positive. RAS mutations were evaluated in 19 out of 26 samples negative for M918T mutation, and one sample was positive (H-RAS, exon 2, G13R).
Expression of CA19.9 and CD133 in MTC
IHC analysis was performed in 78 tissues (64 primary and 13 metastatic tissue samples; of them, 7 were patient-matched samples). CA19.9 immunoreaction was detectable in the cytoplasm of the cells, and the vast majority (87.5%) of primary tumor samples had some level of CA19.9 staining. The median of the h-score was 20 (3–31.5). Of interest, seven of the eight samples negative for CA19.9 were sporadic MTC. We observed no differences in the expression of CA19.9 on gender, age, calcitonin or CEA levels, lymph node or distant metastases (all P > 0.05). The metastasis samples analyzed were as follows: lymph node (7), lung (4), bone (1), and liver (1). All metastases were present at diagnosis, but only two of them were obtained at this time. The remaining 11 samples were collected during follow-up to confirm the diagnosis or as part of the treatment strategy. CA19.9 was expressed in 69.2% of the metastasis samples. Remarkably, the median of the CA19.9 h-score observed in the metastasis samples was significantly higher than in the primary tumor (67.5 (3.75–202), P = 0.0002; Fig. 2a). Of interest, when analyzing the seven primary tumor-metastasis matched samples, we observed a concordance on the presence or absence of CA19.9 staining. In those samples with CA 19.9 positive staining, the h-score in the metastasis was higher than that found in primary tumors (Fig. 2b). We found no differences on the tissue CA19.9 h-score among patients free of disease, with a persistent disease or those who died due to the disease (21.5 vs. 3 vs. 14, respectively, P = 0.1).
CD133 staining was positive in 90.5% of the samples analyzed, with a median of the h-score of 40 (1.5–110). We found no difference in the h-score of CD133 on age, sex, serum calcitonin or CEA levels, tumor size, lymph node, or distant metastases (All P > 0.05). Despite the positivity in most samples, there was no correlation between the h-score of CA19.9 and CD133 (r = −0.09; P = 0.74).
Next, we thought to investigate whether CA19.9 tissue expression would differ between patients with a hereditary or sporadic form of MTC. Interestingly, we observed that hereditary MTC tumors had a higher h-score of CA19.9 than those of sporadic form (median h-score 23.5 vs. 3.5; P = 0.018). Of note, patients harboring codon 634 mutations have a higher h-score than those with less-aggressive mutations (median h-score 25 vs. 1.5; P = 0.007). The MTC sample with M918T mutation displayed a high h-score value (Table 2). In contrast, we found no significant differences in the h-score between samples with or without somatic mutations in the sporadic group (Table 2).
IHC staining patterns
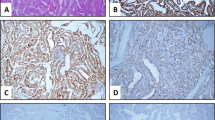
When analyzing the samples positive for CA19.9 (n = 56), our attention was caught by the presence of three distinct IHC staining patterns: individual cell, focal, and diffuse pattern (Fig. 3). The majority of the samples presented a focal pattern (n = 36, 64.3%). Individual cell pattern was observed in 17 (30.3%) samples, and the diffuse pattern only in 3 (5.36%). The sporadic MTC samples were associated with the individual cell pattern (70.6%), while the hereditary form was associated with the focal expression pattern (63.9%) (P = 0.04) (Table 3).
To further explore whether the distinct CA19.9 expression patterns were associated with the disease presentation, we compare them with the clinical and oncological characteristics of the patients. Interestingly, we observed a trend toward an association between the individual cell pattern and the presence of lymph node metastases (73.3%; P = 0.056). On the other hand, the diffuse pattern was associated with larger tumor size and distant metastases at diagnosis (66.7%; P = 0.032) (Table 3).
Discussion
Increased levels of serum CA19.9, a well-established tumor marker in pancreatic neoplasms, has been proposed as a prognostic marker of aggressiveness and mortality in individuals with advanced MTC. However, the relationship between CA19.9 expression and MTC remains unclear. Here we show that CA19.9 IHC staining is present in the vast majority of MTC samples, even in small tumors at the early stages of the disease. No association was observed between CA19.9 tissue expression and oncological features, but hereditary samples have higher levels of CA19.9 expression than the sporadic form. Interestingly, we observed three distinct patterns of expression of CA19.9, which were associated with the hereditary or sporadic form, larger tumor size, and metastatic disease at diagnosis.
Serum calcitonin and CEA are the classical markers used for diagnosis, prognosis, and follow-up of MTC patients [18, 20]. More recently, the CA19.9 has also been advocated as a potential MTC marker. The first case reporting increased of serum levels CA19.9 expression associated with aggressiveness in patients with MTC was described in 2011 [14]. The patient presented with an extensive metastatic spread of MTC to the lungs and liver. Nevertheless, the calcitonin levels were relatively low for the amount of disease, contrasting with the high levels of serum CEA. The CA19.9 levels were measured to exclude other malignancies, revealing an impressive concentration of 39,334 U/mL (reference range <35.1 U/mL). The patient died, and the autopsy confirmed MTC in the metastatic lesions. A similar case was described 2 years later in a patient with multiples metastases in lymph nodes, liver, and bones with rapid clinical worsening and death. Serum CEA and CA19.9 levels were increased while the calcitonin remained stable at low high levels [13]. Posteriorly, the prognostic value of serum levels of CA19.9 was investigated in a group of selected 100 advanced/metastatic MTC patients, which showed a weak but significant correlation between serum CA19.9 and calcitonin/CEA levels. Moreover, distant metastases were more frequent in patients with elevated CA19.9 levels [15].
Here, we evaluated CA19.9 tissue expression in MTC. We observed the presence of this marker in the majority of MTC samples. Remarkably, CA19.9 staining was detected even in less than 1 cm MTC tumors, suggesting that CA19.9 expression is an intrinsic MTC cell property. In contrast with two previous studies [15, 21], we were unable to demonstrate an association between the tissue CA19.9 expression and clinical or oncological features. However, the characteristics of the study populations might potentially explain these conflicting findings. In the study conducted by Elisei et al. IHC analysis was performed only in samples of patients with advanced MTC disease [15]. The second study was a pilot study that included only 16 MTC samples, narrowing the conclusions [21]. Of interest, however, we found that the median of the CA19.9 h-score was significantly higher in the metastasis samples than in the primary tumor (Fig. 2a). These observations support the hypothesis that serum CA19.9 levels are dependent on the tumoral mass and might explain the increased levels of serum CA19.9 in patients with advanced MTC, particularly those with structural metastatic disease [15, 16].
Surprisingly, the CA19.9 expression levels were higher in the hereditary than in sporadic MTC samples. In hereditary samples, we observed an association between the intensity of CA19.9 staining and the germline RET codon mutation. Patients harboring codon 634 mutations displayed a higher h-score than those with less-aggressive mutations (Table 2). In line with previous studies, we found no differences in the CA19.9 h-score between samples with or without somatic mutations [15].
We also evaluated whether the CA19.9 expression would be associated with C-cell dedifferentiation by analyzing the expression of CD133, a widely used marker for identifying tumor stem cells in cell dedifferentiation. The presence of cancer stem cells in well-characterized MTC cell lines and MTC samples has been demonstrated, but the role of CD133 in MTC is still unclear [22]. Here, we observed that the majority of MTC samples presented CD133 staining (90.5%). However, we did not find any correlation between CA19.9 and CD133 h-scores. Moreover, the levels of expression of CD133 did not correlate with any clinical or laboratory characteristics studied [23]. The expression profile of the CD44, another tumor dedifferentiated marker, is similar to that of CD133 in MTC samples [23].
Confirming previous findings described by Milman et al. [21] we observed three distinct patterns of CA19.9 expression in MTC, namely individual cells, focal, and diffuse. Noteworthy, we found an association between the expression pattern and MTC features. The sporadic MTC was associated with the individual cell pattern, while the hereditary form was associated with the focal expression pattern. The uncommon diffuse pattern was associated with larger tumor sizes and distant metastases.
It should be noted some flaws in our study. First, it is a retrospective analysis in a cohort being followed prospectively. Another limitation is the absence of serum levels of CA19.9 data. On the other hand, the study has several strengths. It included a large number of patients of a relatively rare disease, at different tumor stages. In addition, all patients undergo surgery and are followed at a single institution, which ensures a similar therapeutic approach and follow-up strategy, thereby enhancing our data’s validity.
In conclusion, we demonstrate that CA19.9 is expressed in the vast majority of MTC samples, including small tumors in the early stages of the disease. Accordingly, CA19.9 expression was not associated with cell dedifferentiation or advanced MTC, indicating that CA19.9 expression is an intrinsic feature of MTC cells. Noteworthy, higher levels of CA19.9 expression were observed in metastatic samples compared with that of the primary tumor, providing a potential explanation for the increased levels of serum CA 19.9 in patients with advanced MTC. Three distinct patterns of expression were identified, which were associated with the hereditary or sporadic form, larger tumor size, and distant metastases.
References
L. Davies, H.G. Welch, Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 295(18), 2164–2167 (2006). https://doi.org/10.1001/jama.295.18.2164
S.A. Hundahl, I.D. Fleming, A.M. Fremgen, H.R. Menck, A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer 83(12), 2638–2648 (1998). 10.1002/(SICI)1097-0142(19981215)83:12<2638::AID-CNCR31>3.0.CO;2-1 [pii]
M.R. Pelizzo, I.M. Boschin, P. Bernante, A. Toniato, A. Piotto, C. Pagetta, O. Nibale, L. Rampin, P.C. Muzzio, D. Rubello, Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur. J. Surg. Oncol. 33(4), 493–497 (2007). https://doi.org/10.1016/j.ejso.2006.10.021
A.L. Maia, S.M. Wajner, C.V. Vargas, Advances and controversies in the management of medullary thyroid carcinoma. Curr. Opin. Oncol. 29(1), 25–32 (2017). https://doi.org/10.1097/CCO.0000000000000340
A. Laure Giraudet, A. Al Ghulzan, A. Auperin, S. Leboulleux, A. Chehboun, F. Troalen, C. Dromain, J. Lumbroso, E. Baudin, M. Schlumberger, Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur. J. Endocrinol. 158(2), 239–246 (2008). https://doi.org/10.1530/EJE-07-0667
J.A. Meijer, S. le Cessie, W.B. van den Hout, J. Kievit, J.W. Schoones, J.A. Romijn, J.W. Smit, Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin. Endocrinol. 72(4), 534–542 (2010). https://doi.org/10.1111/j.1365-2265.2009.03666.x
J. Barbet, L. Campion, F. Kraeber-Bodere, J.F. Chatal, Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 90(11), 6077–6084 (2005). https://doi.org/10.1210/jc.2005-0044
R. Lamerz, Role of tumour markers, cytogenetics. Ann. Oncol. 10(Suppl 4), 145–149 (1999).
V. Hess, B. Glimelius, P. Grawe, D. Dietrich, G. Bodoky, T. Ruhstaller, E. Bajetta, P. Saletti, A. Figer, W. Scheithauer, R. Herrmann, CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 9(2), 132–138 (2008). https://doi.org/10.1016/S1470-2045(08)70001-9
N.R. Maisey, A.R. Norman, A. Hill, A. Massey, J. Oates, D. Cunningham, CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br. J. Cancer 93(7), 740–743 (2005). https://doi.org/10.1038/sj.bjc.6602760
O. Micke, F. Bruns, R. Kurowski, E. Horst, A.F. deVries, J.W. Hausler, N. Willich, U. Schafer, Predictive value of carbohydrate antigen 19-9 in pancreatic cancer treated with radiochemotherapy. Int J. Radiat. Oncol. Biol. Phys. 57(1), 90–97 (2003). S0360301603005248 [pii]
R. Passerini, M.C. Cassatella, S. Boveri, M. Salvatici, D. Radice, L. Zorzino, C. Galli, M.T. Sandri, The pitfalls of CA19-9: routine testing and comparison of two automated immunoassays in a reference oncology center. Am. J. Clin. Pathol. 138(2), 281–287 (2012). https://doi.org/10.1309/AJCPOPNPLLCYR07H
R. Elisei, L. Lorusso, C. Romei, V. Bottici, S. Mazzeo, C. Giani, E. Fiore, L. Torregrossa, A.C. Insilla, F. Basolo, A. Guerini, A. Menghi, A. Poletti, L. Cugudda, P. Vitti, Medullary thyroid cancer secreting carbohydrate antigen 19-9 (Ca 19-9): a fatal case report. J. Clin. Endocrinol. Metab. 98(9), 3550–3554 (2013). https://doi.org/10.1210/jc.2013-1940
S. Milman, K.D. Whitney, N. Fleischer, Metastatic medullary thyroid cancer presenting with elevated levels of CA 19-9 and CA 125. Thyroid 21(8), 913–916 (2011). https://doi.org/10.1089/thy.2010.0425
R. Elisei, L. Lorusso, P. Piaggi, L. Torregrossa, G. Pellegrini, E. Molinaro, L. Agate, V. Bottici, F. Pani, A. Cacciato Insilla, F. Casella, R. Ciampi, I. Tognetti, G. Materazzi, F. Basolo, C. Romei, Elevated level of serum carbohydrate antigen 19.9 as predictor of mortality in patients with advanced medullary thyroid cancer. Eur. J. Endocrinol. 173(3), 297–304 (2015). https://doi.org/10.1530/EJE-15-0304
R. Alencar, D.B. Kendler, F. Andrade, C. Nava, D. Bulzico, C.C.D. Pessoa, R. Corbo, F. Vaisman, CA19-9 as a Predictor of Worse Clinical Outcome in Medullary Thyroid Carcinoma. Eur. Thyroid J. 8(4), 186–191 (2019). https://doi.org/10.1159/000497201
D.R. Siqueira, M. Romitti, A.P. da Rocha, L. Ceolin, C. Meotti, A. Estivalet, M.K. Punales, A.L. Maia, The RET polymorphic allele S836S is associated with early metastatic disease in patients with hereditary or sporadic medullary thyroid carcinoma. Endocr. Relat. Cancer 17(4), 953–963 (2010). https://doi.org/10.1677/ERC-09-0312
S.A. Wells Jr., S.L. Asa, H. Dralle, R. Elisei, D.B. Evans, R.F. Gagel, N. Lee, A. Machens, J.F. Moley, F. Pacini, F. Raue, K. Frank-Raue, B. Robinson, M.S. Rosenthal, M. Santoro, M. Schlumberger, M. Shah, S.G. Waguespack, Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25(6), 567–610 (2015). https://doi.org/10.1089/thy.2014.0335
M.B. Amin, S. Edge, F. Greene, D.R. Byrd, R.K. Brookland, M.K. Washington, J.E. Gershenwald, C.C. Compton, K.R. Hess, D.C. Sullivan, J.M. Jessup, J.D. Brierley, L.E. Gaspar, R.L. Schilsky, C.M. Balch, D.P. Winchester, E.A. Asare, M. Madera, D.M. Gress, L.R. Meyer (Eds.), AJCC Cancer Staging Manual (8th edition) (Springer International Publishing, American Joint Commission on Cancer, 2017)
A.L. Maia, D.R. Siqueira, M.A. Kulcsar, A.J. Tincani, G.M. Mazeto, L.M. Maciel, Diagnosis, treatment, and follow-up of medullary thyroid carcinoma: recommendations by the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism. Arq. Bras. Endocrinol. Metab. 58(7), 667–700 (2014)
S. Milman, J.L. Arnold, M. Price, A. Negassa, M.I. Surks, N. Fleischer, K.D. Whitney, Medullary thyroid cancer that stains negative for Ca 19-9 has decreased metastatic potential. Endocr. Pr. 21(6), 590–594 (2015). https://doi.org/10.4158/EP14357.OR
W. Zhu, T. Hai, L. Ye, G.J. Cote, Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J. Clin. Endocrinol. Metab. 95(1), 439–444 (2010). https://doi.org/10.1210/jc.2009-1485
Y. Bi, Y. Meng, H. Wu, Q. Cui, Y. Luo, X. Xue, Expression of the potential cancer stem cell markers CD133 and CD44 in medullary thyroid carcinoma: A ten-year follow-up and prognostic analysis. J. Surg. Oncol. 113(2), 144–151 (2016). https://doi.org/10.1002/jso.24124
Funding
This work has been made possible by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundo de Incentivo a Pesquisa (FIPE), and Programa de Apoio a Núcleos de Excelência (PRONEX)/Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Contributions
Conception and design: C.V.F.V., A.L.M. Provision of study materials or patients: A.L.M. Collection and assembly of data: all authors. Data analysis and interpretation: C.V.F.V., L.C., R.S.S., A.L.M. Manuscript writing: C.V.F., L.C., R.S.S., and A.L.M. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients or their legal guardians provided written informed consent by following the institutional Ethics Committee (CAAE 62017016300005327).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vargas, C.V.F., Ceolin, L., Scheffel, R.S. et al. The tissue expression pattern of CA 19.9 is associated with oncological features in medullary thyroid carcinoma. Endocrine 70, 544–551 (2020). https://doi.org/10.1007/s12020-020-02377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02377-3