Abstract
The incidence of thyroid cancer has appeared as an increasing trend globally, especially in Asian countries. In this study, the expression of mucin-1 (MUC1) and Thomsen-Friedenreich antigen, Galβ1-3GalNAcα1-R (CD176) was investigated by immunohistochemistry in papillary thyroid carcinomas (PTCs), which accounts for approximately 80 % of all thyroid cancer. We found that 78 % of PTC overexpressed MUC1. Importantly, we observed firstly that CD176 was expressed in 63 % of PTC, but was faintly or not expressed in normal thyroid tissues and benign thyroid disease tissues, indicating that CD176 is also a tumour-associated antigen for PTCs. Moreover, expression of CD176 was strongly correlated with MUC1 by immunohistochemical staining in PTCs. Furthermore, we used the immunochemical method to confirm that MUC1 is a common and main carrier of CD176 in PTCs. Our data demonstrated that MUC1 and CD176 might be promising biomarkers for thyroid cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of thyroid carcinomas has doubled over the past three decades globally and continues to increase [1–4]. Thyroid malignancies are divided into papillary carcinomas (80 %), follicular carcinomas (10 %), medullary thyroid carcinomas (5–10 %), anaplastic carcinomas (1–2 %), primary thyroid lymphomas (rare) and primary thyroid sarcomas (rare). This disease usually manifests as a painless, palpable, solitary thyroid nodule. Patients or clinicians discover most of these nodules during routine palpation of the neck. Most of thyroid cancer patients have a good therapeutic effect through surgical operation compared with other types of cancer. However, some thyroid cancers have a poor prognosis, because sometimes, cancer cells have not been completely removed and these patients are insensitive to radiation therapy, hormone therapy and chemotherapy. Hence, the immunotherapy with higher specificity and affinity may be helpful for these patients. Studying differentially expressed molecules between cancer and normal tissues is crucial to identifying novel biomarkers for cancer immunotherapy.
Mucin-1 (MUC1) is an established tumour marker expressed on a variety of epithelial tumours, and has been used as a serum tumour marker, especially in the diagnosis of breast cancer patients [5, 6]. Overexpression of MUC1 oncoprotein is frequently observed in cancer and contributes to confer resistance to genotoxic agents [7]. MUC1 has attracted increasing attention as a potential target of tumour immunotherapies [8, 9]. Expression of MUC1 has been examined in thyroid carcinomas in several studies [Table 1, 7, 10–20].
MUC1 is a large, highly O-glycosylated transmembrane glycoprotein. The extra cellular portion consists of a variable number of 20–120 tandem repeats (TR). Each TR consists of 20 amino acids with five potential O-glycosylation sites. In normal secretory epithelial cells, MUC1 is expressed as a transmembrane glycoprotein that provides protection against pathogens and shows cell signalling ability [21]. Following synthesis as a single polypeptide and cleavage in the endoplasmic reticulum, MUC1 is expressed on the cell membrane as a heterodimer [22].An aberrant O-glycosylation in the tumour exposes new peptide epitopes on the MUC1 protein backbone and tumour-associated carbohydrate antigens (glycotope) such as the Thomsen-Friedenreich (TF) antigen [23, 24]. TF is a tumour-associated carbohydrate structure which is defined as the carbohydrate sequence Galβ1-3GalNAcα1-R [25], and it was assigned as CD176 during the Seventh Workshop and Conference on Human Leucocyte Differentiation [26]. In adult human normal and benign tissues, CD176 is masked by terminal sialylation [27], but it is exposed during tumorigenesis as a tumour-associated antigen [25, 28]. Approximately 70–80 % of carcinomas carry CD176 on their cell surface [25]. In addition, CD176 is expressed in some cancer stem cells [29] and it is functionally involved in the liver metastasis process of tumours [25, 30], the adhesion of cancer cells to the endothelium [31, 32]. CD176 may be a promising target for cancer immunotherapy [25, 33, 34]. However, expression of CD176 has not been investigated in papillary thyroid carcinomas (PTCs). Therefore, we studied the expression of CD176 in PTCs by immunohistochemistry. It is known that CD176 is mainly carried by MUC1 in epithelial tumours (Fig. 1). Thus, expression of MUC1 was also examined in the study. Furthermore, a new sandwich solid-phase enzyme-linked immunosorbent assay (ELISA) was used to investigate whether CD176 is carried directly by MUC1 in PTCs.
Materials and Methods
Antibodies
Antibodies applied in this study were anti-MUC1 monoclonal antibody (mAb) PankoMab (Glycotope, Berlin, Germany), E-29 (anti-MUC1 mAb, which recognizes the peptide epitope APDTRP of the MUC1 tandem repeat; Proteintech Group, Wuhan, China) and CD176 mAb (NM-TF2; Glycotope).
Tissues
Human samples were taken from a total of 90 resected thyroids. They include 60 PTC tissues, 10 normal thyroid tissues and 20 benign thyroid disease tissues as well. In 25 cases of PTCs, adjacent non-malignant thyroid tissues were also studied. All specimens were immediately frozen, transferred to Kunming Institute of Zoology, and then fixed in 10 % buffered formalin and embedded in paraffin. Paraffin sections were cut at 4-μm thickness. The pathological diagnosis was made and the histological types were classified on the basis of finding in haematoxylin and eosin-stained sections. All PTCs used in the study are classic PTC.
All samples were fully encoded to protect patient confidentiality and were approved by the local research ethics committees at all participating sites.
Immunohistochemistry
Paraffin sections, 4-μm thick, were deparaffinised. For immunoperoxidase staining, the tissue sections were treated with 3 % H2O2 for 30 min to block endogenous peroxidases, then washed three times with phosphate-buffered saline (PBS) and blocked with 2 % bovine serum albumin (BSA). Afterwards, they were incubated with the primary antibody, and thereafter treated with peroxidase-labelled goat anti-mouse immunoglobulin antiserum (Dako, Copenhagen, Denmark). Negative controls were performed with 2 % BSA in PBS instead of the mAbs. The MUC1 mAbs and anti-CD176 mAb were used in breast carcinoma specimens and KG1 cells (human acute myelogenous leukaemia cell line, which is positive for CD176 [26]) as positive controls in all batches, respectively. Colour was developed with the peroxidase substrate 3,3-diaminobenzidine. Counterstaining was performed with haematoxylin. Cell numbers were counted at ×200 magnification with a microscope.
Sandwich ELISA
The 10 PTC tissues which expressed MUC1 and CD176 by immunohistochemistry, as well as 5 normal thyroid tissues, were dissolved to radioimmunoprecipitation assay (RIPA) containing a mixture of protease inhibitors (#539134; Calbiochem, Darmstadt, Germany), and homogenized with oscillation at 4 °C for 30 min. After centrifugation for 30 min at 12,000g, the supernatants were taken. Ninety-six-well polystyrene microtest plates were coated with the capture antibody against MUC1 at a concentration of 1 μg⁄mL in coating buffer at 4 °C for overnight. After blocking the remaining protein-binding sites with 5 % BSA, 50 μL of supernatants of tissue lysates were added to the wells and incubated at room temperature for 2 h. Then, the plates were incubated with the anti-CD176 mAb (NM-TF2, IgM) followed by peroxidase-labelled goat anti-mouse IgM antibody (μ-chain specific) (SouthernBiotech, Birmingham, AL, USA). The colour reaction was developed with o-phenylenediamine dihydrochloride (OPD)⁄H2O2 solution at room temperature, and stopped with 2.5 M sulphuric acid. Negative controls were performed with 2 % BSA in PBS instead of the coating antibody, the protein extracts or the detecting antibody. The optical density of each well was determined within 30 min using a microplate reader (Bio-Rad, Hercules, CA, USA) at 490 nm.
Statistical Analysis
All data were analysed using the SPSS 17.0 software package (Chicago, IL, USA). The data of the immunochemical staining were analysed with either the chi-square test or the Fisher’s exact probability test (two-tailed). The relationship between the expression of MUC1 and CD176 was determined using Spearman analysis method. A p value of <0.05 was considered statistically significant.
Results
Expressions of MUC1 and CD176 in Different Thyroid Tissues
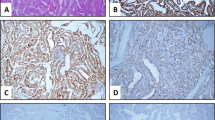
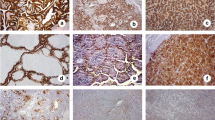
We found that 47 cases of PTC (78.3 %) were positive for MUC1. MUC1 was located on the membrane and cytoplasm of cancer cells (Fig. 2). The three cases of benign thyroid disease (benign thyroid adenoma) were also stained by the MUC1 antibodies. MUC1 was mainly localized at the top surface of epithelial cells and showed the polarized distribution in benign thyroid diseases (Fig. 2). MUC1 was not stained in normal thyroid tissues (Fig. 2). The percentage of cases positive for MUC1 in PTCs was significantly higher than in benign thyroid diseases and normal thyroid tissues (p < 0.05, Table 2), but there was no statistically significant difference between benign thyroid diseases and normal thyroid tissues (p > 0.05, Table 1).
The expression of CD176 was found in 38 cases of PTC (63.3 %). CD176 showed membrane and cytoplasmic staining in PTCs (Fig. 2). We did not detect CD176 in benign thyroid diseases and normal thyroid tissues (Fig. 2). The expression rate of CD176 in PTCs was significantly higher than in benign thyroid diseases and normal thyroid tissues (p < 0.05, Table 2).
Correlations of MUC1 and CD176 Expressions with Pathological Parameters in PTCs
No significant correlations were found between the expression of MUC1 and CD176 as well as patient gender and age, and tumour size (Table 3). We found that the expression of MUC1 and CD176 was closely related to the lymph node metastasis (p < 0.05, Table 3).
Correlation Between MUC1 and CD176 Expressions in PTCs
A majority of PTC (63 %, 38/60) expressed MUC1 and CD176 simultaneously. The 38 cases of PTC that were positive for CD176 also expressed MUC1. Otherwise, among the 22 PTC tissues with negative staining for CD176, the 9 cases were positively stained with the MUC1 antibody. In general, there was a correlation between both antigens with respect to the intensity of expression (tumours strongly positive for CD176 were also intensely stained for MUC1 and vice versa).
The correlation between MUC1 and CD176 expressions was determined using Spearman analysis method. There was a high correlation between to MUC1 and CD176 expression in PTCs (γ = 0.691, p = 0.000, Fig. 3).
Detection of MUC1 as a Carrier Protein of CD176
The potential carrier molecular of CD176 was analysed by a sandwich ELISA in the 10 cases of PTC. We used polystyrene microplates coated with anti-MUC1 antibody to capture MUC1 glycoprotein from tissue lysates. The plates were then incubated with the anti-CD176 mAb as detection antibody. As shown in Table 4, the captured MUC1 was found to react with the anti-CD176 mAb in all PTC tissues. These results indicated that MUC1 is a common and main carrier of CD176 in PTCs.
Discussion
Thyroid cancer patients have generally good prognoses for surgical therapy compared with other cancers. However, the effect of surgical treatment and even radiation therapy, hormone therapy as well as chemotherapy is not good in some thyroid cancers. Therefore, development of novel immunotherapy is valuable for these patients. Since MUC1 is localized exclusively at the luminal membrane in normal epithelial cells, which is inaccessible to antibodies present in the blood and to active immunocytes, but it is strongly overexpressed in cancer cells, MUC1 is a potential target for cancer immunotherapy. For examples, the immunotherapy based on MUC1 vaccine (TG4010) and a humanized antibody (hPankoMab) has entered clinical trials [17, 34, 35]. In this study, we observed that 78 % cases of PTC overexpressed MUC1 similar to previous studies [12, 17]. Interestingly, a previous study demonstrated that MUC1 expression was correlated with the lymph node metastasis and the presence of the BRAF (V600E) mutation in PTCs [19]. We also observed that PTCs with the lymph node metastasis were positive for MUC1 in a significantly higher percentage than PTCs without the lymph node metastasis. Importantly, the distribution of MUC1 in PTCs was found at the apical and basolateral membrane, as well as in the cytoplasm of cancer cells. In general, the pattern of PTC expressing MUC1 resembled that of other carcinomas such as breast, colon and lung cancer. This indicated that the immunotherapy and diagnosis based on MUC1 for other carcinomas could also be used for PTCs. This deserves further study.
CD176 is determined to be expressed on the surface of various cancer cells, such as breast carcinomas [25], lung cancer [29], colorectal carcinomas [30], hepatocellular carcinomas [36], and leukaemia [37]. CD176 is present on the surface of cancer cells and virtually absent from normal adult human tissues [27]. Thus, it is reasonable to assume that CD176 is a suitable target for cancer biotherapy. In the present study, we used a highly specific mAb to investigate the expression of CD176 in PTCs. We found firstly that CD176 was expressed in 63 % of PTC, but was faintly or not expressed in normal thyroid tissues, adjacent non-malignant thyroid tissues and benign thyroid disease tissues. Furthermore, CD176 expression was found to correlate with the TNM stage and the lymph node metastasis. Our data demonstrated that CD176 is also a tumour-associated antigen for PTCs. Compared with expressions of other biomarkers such as HBME-1, galectin-3 and cytokeratin 19 in PTCs [38], CD176 showed higher specificity than galectin-3 and cytokeratin 19, but lower specificity than HBME-1. However, immunotherapy trials of CD176 vaccines and antibodies have been performed on experimental animals and patients [25, 28, 34]. These researches focused on breast carcinomas, ovarian carcinomas, prostate carcinomas and leukaemia. We think that CD176 vaccines and antibodies may also be used for PTCs. Considering 63 % cases of PTC expressing CD176 and thyroid carcinomas quickly increasing in the past decades, studying clinical significances of CD176 expression in thyroid carcinomas is very valuable.
CD176 (Galβ1-3GalNAcα1-R) is a ubiquitous core structure (core-1) found in a cryptic manner in many membrane glycoproteins, and sialic acid is one of the most important molecules for the masking of CD176. CD176 is exposed during tumorigenesis by an aberrant O-glycosylation due to the defect of glycotransferases [25]. The exposed CD176 in cancer cells was carried by various glycoproteins. A previous study found that CD176 was mainly carried by MUC1 in colorectal carcinomas [39]. In the study, the carrier protein of CD176 in PTCs was studied. Firstly, we observed that the expression of CD176 was strongly correlated with MUC1 by immunohistochemical staining in PTCs. Furthermore, we used the immunochemical method to confirm that MUC1 is a common and main carrier of CD176 in PTCs. Thus, we put forward the hypothesis that the up-regulated MUC1 protein in PTCs could carry more CD176 glycotope, so the expressions of both MUC1 and CD176 reveal a high correlation. However, CD176 showed higher specificity and lower sensitivity than MUC1 in PTCs. In clinical application, the over-expressions of both MUC1 and CD176 in cancer cells may be beneficial to immunotherapy and diagnosis.
In conclusion, more than 60 % PTCs expressed MUC1 and CD176. MUC1 is a common and main carrier of CD176 in PTCs. MUC1 and CD176 are promising biomarkers for PTCs. As a pilot study, our data demonstrated that the clinical significances of MUC1 and CD176 on PTCs should be further studied.
References
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167, 2006.
Hodgson NC, Button J, Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol 11:1093–1097, 2004.
Haselkorn T, Bernstein L, Preston-Martin S, Cozen W, Mack WJ. Descriptive epidemiology of thyroid cancer in Los Angeles County, 1972–1995. Cancer Causes Control 11:163–170, 2000.
Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid 16:47–53, 2006.
Hayes DF, Zurawski VR, Kufe DW. Comparison of circulating CA15-3 and carcinoembryonic antigen in patients with breast cancer. J Clin Oncol 4:1542–1550, 1986.
Safi F, Kohler I, Rottinger E, Berger H-G. The value of the tumour marker CA153-3 in diagnosing and monitoring breast cancer. Cancer 68:574–582, 1991.
Siragusa M, Zerilli M, Iovino F, et al. MUC1 oncoprotein promotes refractoriness to chemotherapy in thyroid cancer cells. Cancer Res 67:5522–5530, 2007.
Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther 6: 481–486, 2007.
Beatty PL, Finn OJ. Preventing cancer by targeting abnormally expressed self-antigens: MUC1 vaccines for prevention of epithelial adenocarcinomas. Ann N Y Acad Sci 1284:52–56, 2013.
Wreesmann VB, Sieczka EM, Socci ND, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res 64: 3780–3789, 2004.
Patel KN, Maghami E, Wreesmann VB, Shaha AR, Shah JP. Ghossein R, Singh B. MUC1 plays a role in tumor maintenance in aggressive thyroid carcinomas. Surgery 138: 994–1001, 2005.
Morari EC, Silva JR, Guilhen AC, et al. Muc-1 expression may help characterize thyroid nodules but does not predict patients’ outcome. Endocr Pathol 21: 242–249, 2010.
Baek SK, Woo JS, Kwon SY, Lee SH, Chae YS, Jung KY. Prognostic significance of the MUC1 and MUC4 expressions in thyroid papillary carcinoma. Laryngoscope 117: 911–916, 2007.
Weiss M, Baruch A, Keydar I, Wreschner DH. Preoperative diagnosis of thyroid papillary carcinoma by reverse transcriptase polymerase chain reaction of the MUC1 gene. Int J Cancer 66:55–59, 1996.
Abrosimov A, Saenko V, Meirmanov S, et al. The cytoplasmic expression of MUC1 in papillary thyroid carcinoma of different histologicalvariants and its correlation with cyclin D1 overexpression. Endocr Pathol 18: 68–75, 2007.
Magro G, Schiappacassi M, Perissinotto D, Corsaro A, et al. Differential expression of mucins 1–6 in papillary thyroid carcinoma: evidence for transformation-dependent post-translational modifications of MUC1 in situ. J Pathol 200: 357–369, 2003.
Fan XN, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol Res Pract 206:585–589, 2010.
He F, Li H, Li WS, Dong XH. Expression of mucin-l and beta-catenin in papillary thyroid carcinoma and the clinical significance thereof. Zhong Hua Yi Xue Za Zhi 82:257–261, 2002.
Renaud F, Gnemmi V, Devos P, Aubert S, et al. MUC1 expression in papillary thyroid carcinoma is associated with BRAF mutation and lymph node metastasis; the latter is the most important risk factor of relapse. Thyroid 24: 1375–1384, 2014.
Bieche I, Ruffet E, Zweibaum Abet et al. MUC1 mucin gene, transcripts, and protein in adenomas and papillary carcinomas of the thyroid. Thyroid 7: 725–731, 1997.
Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 6:339–53, 2001.
Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem 267: 6171–6177, 1992.
Cao Y, Blohm D, Ghadimi BM, Karsten U et al. Mucins (MUC1 and MUC3) of gastrointestinal and breast epithelia reveal different and heterologous tumour associated aberrations in glycosylation. J Histochem Cytochem 45:1547–1557, 1997.
Cao Y, Schlag PM, Karsten U. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation. Virchows Arch 431:159–166, 1997.
Goletz S, Cao Y, Danielcyk A, Ravn P, Schoeber U and Karsten U. Thomsen-Friedenreich antigen: the ‘hidden’ tumour antigen. Adv Exp Med Biol 535: 147–162, 2003.
Cao Y, Merling A, Karsten U, Schwartz-Albiez R. Expression of Thomsen-Friedenreich-related carbohydrate antigens on human leukemia cells. In: Leucocyte Typing VII (eds. D. Mason et al.), Oxford University Press, Oxford, 204–205, 2002.
Cao Y, Stosiek P, Springer GF and Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol 106:197–207, 1996.
Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med 75: 594–602, 1997.
Lin WM, Karsten U, Goletz S, Cheng RC, Cao Y. Expression of CD176 (Thomsen-Friedenreich antigen) on lung, breast and liver cancer-initiating cells. Int J Exp Pathol 92:97–105, 2011.
Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF and Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas, a reevaluation. Cancer 76: 1700–1708, 1995.
Glinsky VV, Glinsky GV, Rittenhouse-Olson K, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res 61:4851–4857, 2001.
Heimburg J, Yan J, Morey S, et al. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia 8: 939–948, 2006.
Yi B, Zhang M, Schwartz-Albiez R, Cao Y. Mechanisms of the apoptosis induced by CD176 antibody on human leukemic cells. Int J Oncol 38:1565–1573, 2011.
Yi B, Zhang Z, Zhang M, Schwartz-Albiez R, Cao Y. CD176 (Thomsen-Friedenreich antigen) anti-serum treatment leads to a therapeutic response in a murine model of leukemia. Oncol Rep 30:1841–1847, 2013.
Watson T. Outlook lung cancer: immunotherapy—chemical tricks. Nature 513:S10–S11, 2014.
Cao Y, Karsten U, Otto G, Bannasch P. Expression of MUC1, Thomsen-Friedenreich antigen, Tn, sialosyl-Tn, and 2,6-linked sialic acid in hepatocellular carcinomas and preneoplastic hepatocellular lesions. Virchows Arch 434:503–509, 1999.
Cao Y, Merling A, Karsten U, Goletz S, Punzel M, Butschak G, Schwartz-Albiez R. Expression of CD175 (Tn), CD175s (sialosyl-Tn), and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. Int J Cancer 123: 89–99, 2008.
Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of HBME-1, galectin-3, CK19 and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol 126:700–708, 2006.
Baldus SE, Hanisch F-G, Kotlarek GM, Zirbes TK, Thiele J, Isenberg J, Karsten UR, Devine PL, Dienes HP. Coexpression of MUC1 mucin peptide core and the Thomsen-Friedenreich antigen in colorectal neoplasms. Cancer 82: 1019–1027, 1998.
Acknowledgments
This work was financially supported by the grants from National Natural Science Foundation of China (No. 81072563).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhan, Xx., Zhao, B., Diao, C. et al. Expression of MUC1 and CD176 (Thomsen-Friedenreich antigen) in Papillary Thyroid Carcinomas. Endocr Pathol 26, 21–26 (2015). https://doi.org/10.1007/s12022-015-9356-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9356-9