Abstract
This study examined the association between hector battifora mesothelial antigen-1 (HBME-1) expression and papillary thyroid carcinoma (PTC). A total of 206 patients were enrolled in the current study including 96 PTC patients and 110 patients with benign thyroid nodules (BTN). Immunohistochemistry (Envision) were performed to assess the expression of HBME-1. Receiver operating characteristic curve (ROC) curves were applied to evaluate the diagnostic tumor node metastasis (TNM) value of HBME-1. Specimens from 96 patients with PTC and 110 patients with BTC were reviewed. HBME-1 was positively immunostained in PTC tissue, which was significantly higher than that in BTN tissues (77.1 vs. 5.77 %, P < 0.05). Immunohistochemistry also identified that HBME-1 expression did not show any statistically significant differences based on gender, age, tumor size, TNM stage, and lymph node metastasis (P > 0.05). Importantly, HBME-1 expression was correlated with infiltration levels and differential levels in PTC (both P < 0.05). HBME-1 was found to have high sensitivity (94.5 %) and specificity (77.08 %) for PTC diagnosis. Moreover, HBME-1 had a high specificity (83.33 %) at identifying the differential levels of PTC, but a low sensitivity (22.92 %). The sensitivity and specificity of HBME-1 identifying the infiltration levels of PTC were, respectively, 72.70 and 72.00 %. HBME-1 was highly expressed in PTC tissues, and HBME-1 can serve as a potential biomarker in the diagnosis of PTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is the most frequent subtype of thyroid malignancy, accounting for about 85–90 % cases of all malignant cases [1, 2]. The observed risk factors of this disease indicate that both environmental components, such as ionizing radiation, and genetic predisposition are implicated [3]. Moreover, both family history or dietary iodine deficiency and inflammation strongly contribute to the development and progression of PTC [4, 5]. Increasing evidence suggested that PTC occurs more frequently in women aged approximately 20–55 years [6]. Although surgical resection remains the prime strategy for PTC treatment, the recurrence of this disease is unpredictable [7]. In addition, the diagnosis of PTC is usually straightforward and challenging in specimens with low cellularity [8]. In this respect, the role of clinical biomarkers in the diagnosis of PTC cannot be underestimated and efforts have been made to identify diagnostic markers for thyroid cystic lesions by employing immunohistochemical markers for their potential in distinguishing PTC from other follicular lesions, including cytokeratin (CK)19, galectin-3 (GAL3), and hector battifora mesothelial antigen-1 (HBME-1) [9, 10].
HBME1 is a monoclonal antibody to an unknown microvillous surface antigen present on mesothelial cells and other epithelial cells [11]. HBME-1 is a useful marker of thyroid malignancy in fine-needle aspiration and tissue specimens, showing diffuse strong stained in the majority of malignant thyroid carcinomas [12]. Moreover, recent evidence shows that HBME-1 expression is linked with pathogenesis of PTC, implying that HBME-1 expression may have a significant diagnostic value in early diagnosis of PTC [8, 13]. The HBME-1 is one of the most reliable markers in understanding thyroid pathology, and it is a critical marker of thyroid follicular origin cancers, with greater affinity to malignant lesions compared to benign lesions [14]. Importantly, evidence supported that papillary carcinoma tissues presented with positive staining for HBME-1, while HBME-1 was expressed as negative in benign lesions [15, 16]. In a previous study, HBME-1 showed strong positive staining in majority of PTC tissues, while focal staining was found only in one third of the benign thyroid lesions [17]. In this regard, it is reasonable to hypothesize that the expression levels of HBME-1 might strongly influence the pathogenesis of PTC. Several studies have indicated that there is a close link between HBME-1 expression and the pathogenesis of PTC, suggesting that HBME-1 expression might be useful in diagnosis and monitoring of PTC patients [10, 17, 18].
However, few studies investigating the role of HBME-1 in diagnosing the infiltration degrees and differential levels in PTC. Therefore, we performed current study using immunohistochemical technique to identify whether HBME-1 expression can differentiate the infiltration degrees and differential levels in PTC.
Methods and materials
Subjects
A total of 96 PTC patients, pathologically diagnosed within the period January 2012 to December 2013 at the Department of Liver and Gallbladder Surgery, First Affiliated Hospital of Xi’an Jiaotong University were included in this study. The PTC patients included 23 males and 73 females with a mean age of 38.6 ± 12.4 years (ranged from 13 to 56 years). According to differential levels, 96 PTC patients were classified into well-differentiated PTC (n = 46), moderately differentiated PTC (n = 34), and poorly differentiated PTC (n = 16) (Yunfei Lai, Quan Zhang, Fujin Chen. The correlation analysis on papillary thyroid carcinoma with different differential degrees [C]// 2005 International Conference: the 8th Head and neck neoplasm proceedings). The tumor size for PTC patients were 0.2~6.2 cm with mean size of 1.1 ± 0.7 cm. The inclusion criteria for PTC patients were as followed: (1) complete medical records, (2) pathological diagnosed as PTC, (3) no chronic diseases or malignant tumors, and (4) primary sites in thyroid. The exclusion criteria were: (1) uncompleted clinical materials; (2) any history of radiotherapy, chemotherapy, endocrine therapy or hormone therapy; and (3) complicated with other magnificent tumors. During the same period, 110 patients with benign thyroid nodules (BTN) (27 thyroid adenoma, 63 nodular goiter, and 20 Hashimoto’s thyroiditis) in our hospital were also enrolled in this study. Patients with BTN comprising of 26 males and 84 females had a mean age of 36.3 ± 12.0 years (ranged from 15 to 60 years). The baseline characters of PTC patients were comparable to patients with BTN (all P > 0.05). The current study was conducted strictly based on the Declaration of Helsinki [10] and was approved by the Ethics Committee of First Affiliated Hospital of Xi’an Jiaotong University. Written informed consents were obtained from each of patient prior to the study.
Immunohistochemistry
Surgical specimens were undergone for routine pathological examination and immunohistochemical staining (Envison method).The samples were dehydrated in gradient ethanol series from 70 to 100 %. Samples were cleared in xylene for 1 h. Then, excess xylene was removed by paraffin infiltration for 3 h and embedded in paraffin wax using an embedding machine (Sakura, Zoeterwoude, Netherlands). Paraffin sections were incubated in an oven at 67 °C for 2 h. After deparaffinization and hydration in a descending series of alcohol dilutions, the sections were washed in PBS for 3 × 3 min and then a microwave was applied to for antigen retrieval in citrate buffer (pH 6.0). The samples were rinsed in distilled water twice and followed by PBS wash for 2 × 3 min. Paraffin sections were added with 3 % hydrogen peroxide solution (H2O2) and incubated for 10 min to inactivate endogenous peroxidase. Then, the sections were washed in PBS for 3 × 3 min and were incubated with primary antibodies to HBME-1 (rat anti-human antibodies; used at 1:100 dilution; Long Island Biotech) overnight at 4 °C. Next, the sections were washed in PBS for 3 × 5 min after polyclonal goat anti-rat HBME-1 antibody (diluted at 1:50; Dako, Denmark) were added and incubated for 30 min at room temperature. Staining was performed using 3, 3′-diaminobenzidine (DAB) and was observed using a microscope. Then, the sections were washed under running water, followed by hematoxylin staining and 0.1 % hydrochloric acid. After washing in running water and PBS, the sections were subjected to ethanol dehydration and vitrification by dimethylbenzene and were then mounted by neutral gum. Reaction with PBS as first antibody was regarded as negative control.
A 0–4 scoring system was designed for sections according to previous descriptions [19]. The number of positive staining was determined for 5 × 400 fields. Inside these ×400 high power fields, the percentage of positive cells was categorized on a scale of 0 = no positive cells; 1 = 1~25 % positives, 2 = 26~50 % positives, 3 = 51~75 % positives, and 4 = 76~100 % positives. The intensity was scored on: 0 = absence of staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining. The final score for the staining was determined by adding the above two score together, namely 0 = negative (−), 2–3 = weak positive (+), 4–5 = moderate positive(++), 6–7 = strong positive (+++).The immunohistochemical staining analysis was performed by two observers blinded to the classification of two groups.
Statistical analysis
Statistical analyses were conducted by SPSS19.0 program (SPSS Inc., Chicago, IL, USA). Chi-square test to evaluate associations of categorical data presented as percentage. Continuous data were presented with mean ± standard deviation (SD) using t test for comparison. Receiver operating characteristic (ROC) curves were performed to identify the diagnostic value of HBME-1 in PTC diagnosis. Rank sum test was applied to examine the expression levels of HBME-1 between two groups. Sensitivity = true positive cells/ (true positive cells + false negative cells) × 100 %. Specificity = true positive cells/ (true negative cells + false positive cells) × 100 %. P values equal or less than 0.05 was used to determine the statistically significance.
Results
Immunohistochemical findings
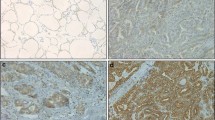
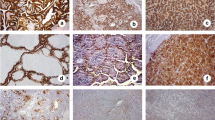
The HBME-1 positive staining was mainly expressed at the cell membrane or the glandular cavity. As shown in Fig. 1a, HBME-1-positive staining were expressed as moderate positive or strong positive in PTC while in patients with BTN, HBME-1-positive staining was mainly presented with negative or weak positive (Fig. 1b). Of 96 PTC patients, 76 patients (77.1 %) showed positive staining with HBME-1. Frequencies of positive staining were lower for HBME-1 in 110 patients with BTN, with HBME-1 showing positive staining in 6 patients (5.77 %). Positive immunostaining was observed in a higher percentage of PTCs than in patients with BTN for HBME-1 (P < 0.05) (Table 1).
HBME-1 expression and clinical pathological characteristics
As presented in Table 2, the clinicopathological features in patients with PTC and HBME-1 expression were not significant related to sex, age, tumor size, lymph node metastasis, and TNM stage (all, P > 0.05). However, infiltration degrees and differential levels were found to be positively associated with HBME-1 expressions (both P < 0.05). PTC patients with infiltration breakthrough the capsule had a decreased HBME-1 expression level than those without capsular infiltration (89.83 vs. 56.76 % ). Furthermore, poorly differentiated PTC patients presented with increased HBME-1 expression compared with moderately or well-differentiated PTC patients (94.00 vs. 85.29 %/62.21 %).
Diagnostic value of HBME-1
HBME-1 diagnosing PTC is highly in sensitivity (94.5 %) but low in specificity (77.08 %) with the area under the curve (AUC) of 0.879 (P < 0.001). HBME-1 had a high specificity (83.33 %) at identifying the differentiated levels of PTC, but a low sensitivity (22.92 %) and AUC was 0.7159 with 95 % confidence interval (CI) of 0.641–0.791 (P = 0.13). The sensitivity and specificity of HBME-1 identifying the infiltration levels of PTC were, respectively, 72.70 and 72.00 % with AUC of 0.722 (95 % CI, 0.598–0.845) (P = 0.02) (Table 3 and Fig. 2).
The diagnostic value of HBME-1 in PTC (a HEME-1 expression in diagnosing PTC and benign thyroid nodules, b HEME-1 expression in identifying the differential levels in PTC, c HEME-1 expression in identifying the infiltration levels in PTC). PTC papillary thyroid carcinoma, BTN benign thyroid nodule, HBME-1 hector battifora mesothelial antigen-1
Discussion
The result of our study showed a strong link between high expression levels of HBME-1 and the risk of developing PTC. Thyroid cancers are the most frequent endocrine tumors with more than 40 % of the population aged 30~60 years have benign thyroid nodules [20, 21]. The diagnosis on follicular thyroid lesions, obtained through fine-needle aspiration and cytology examination, are difficult, and moreover, histological evaluation of resected follicular thyroid lesions could be challenging and lack biomarkers [22, 23]. Many authors have also explored immunohistochemistry as a complementary method to morphological criteria for diagnosis of well-differentiated thyroid tumors [24, 25]. Our results demonstrated that PTC patients presented with a high expression levels of HBME-1 than BTN patients, which was in agreement with other studies, suggesting HBME-1 is a promising biomarker in thyroid pathology and is referred to a universal marker of malignancy due to its high expression in several aggressive tumors [26, 27]. As a monoclonal antibody against malignant epithelial mesothelioma cell suspension, anti-HBME-1 reacts with the micro-villous surface protein of mesothelial cells, with exhibits mostly membranous staining pattern and some positive staining in the cytoplasm, and is used in detection of mesothelium-derived tumors [28, 29].
Our results also demonstrated that the HBME-1 expression was significant associated with the infiltration levels and differential levels of PTC, revealing that HBME-1 may be correlated with the progression of this disease. In contrast to our results, Cui et al. declared that no significant association was found between the expression of HBME-1 and focal lymphocytic infiltration in thyroid nodules [14]. The discrepancy may be explained by the small sample size that Cui included, which only enroll a small sample size with 150 cases of benign and malignant thyroid lesions. Furthermore, we employed the expression of HBME-1 to differentiate distinguish PTC from BTN, as well as to distinguish the infiltration degrees and differential levels of PTC. Our results manifested that HBME-1 was found to have a high degree of both sensitivity and specificity for PTC. Consistent with our results, previous studies also identified that HBME-1 expression was effective in distinguishing BTN from PTC, presenting with a high sensitivity and specificity [8, 30]. Multiple studies showed that high expression of HBME-1 can be a reliable marker alone for PTC or can be used in combination with other biomarkers such as CK19, Galectin-3, CD56, and p63 [24, 31, 32]. Nga et al. reported that the combination of HBME-1 and CK-19 in the diagnosis of PTC improved the sensitivity and specificity both to 100 % [13]. However, another study also showed that the combination of HBME-1 and CK-19 failed to contribute to the accuracy of PTC diagnosis with sensitivity and specificity of, respectively, 62 and 95 % [8]. Moreover, Saleh et al. also reported that the combination of two or more markers unable to improve the PTC diagnostic value [29]. Our results also identified that HBME-1 plays an important role in differentiate the progression in PTC by presenting with a reliable ROC area. However, this conclusion still requires further confirmation.
To summarize, we reported that HBME-1 expression differing between PTC and BTN based on immunohistochemical technique. We also identified that HBME-1 expression was implicated with the disease progression and could be considered as a diagnostic marker of PTC. The role of HBME-1 protein in differentiating the progression of PTC needs further double investigation and confirmation in a large sample sized and well-designed study.
References
Dinets A, Pernemalm M, Kjellin H, Sviatoha V, Sofiadis A, Juhlin CC, et al. Differential protein expression profiles of cyst fluid from papillary thyroid carcinoma and benign thyroid lesions. PLoS One. 2015;10(5):e0126472.
Grant CS. Papillary thyroid cancer: Strategies for optimal individualized surgical management. Clin Ther. 2014;36(7):1117–26.
Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, Takigawa-Imamura H, et al. The foxe1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum Mol Genet. 2010;19(12):2516–23.
Kim SK, Park HJ, Hong IK, Chung JH, Eun YG. A missense polymorphism (rs11466653, met326thr) of toll-like receptor 10 (tlr10) is associated with tumor size of papillary thyroid carcinoma in the Korean population. Endocrine. 2013;43(1):161–9.
Boufraqech M, Fassassi C, Kebebew E. Tlr-10 polymorphism and papillary thyroid cancer: one more snp to consider? Endocrine. 2013;43(1):10–1.
Dinets A, Hulchiy M, Sofiadis A, Ghaderi M, Hoog A, Larsson C, et al. Clinical, genetic, and immunohistochemical characterization of 70 Ukrainian adult cases with post-Chernobyl papillary thyroid carcinoma. Eur J Endocrinol. 2012;166(6):1049–60.
Owonikoko TK, Hossain MS, Bhimani C, Chen Z, Kim S, Ramalingam SS, et al. Soluble fas ligand as a biomarker of disease recurrence in differentiated thyroid cancer. Cancer. 2013;119(8):1503–11.
Schmitt AC, Cohen C, Siddiqui MT. Paired box gene 8, hbme-1, and cytokeratin 19 expression in preoperative fine-needle aspiration of papillary thyroid carcinoma: diagnostic utility. Cancer Cytopathology. 2010;118(4):196–202.
Guerra A, Marotta V, Deandrea M, Motta M, Limone PP, Caleo A, et al. Braf (v600e) associates with cytoplasmatic localization of p27kip1 and higher cytokeratin 19 expression in papillary thyroid carcinoma. Endocrine. 2013;44(1):165–71.
de Matos LL, Del Giglio AB, Matsubayashi CO, de Lima FM, Del Giglio A, da Silva Pinhal MA. Expression of ck-19, galectin-3 and hbme-1 in the differentiation of thyroid lesions: Systematic review and diagnostic meta-analysis. Diagn Pathol. 2012;7:97.
Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of hbme1, galectin-3, ck19, and cited1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol. 2006;126(5):700–8.
de Matos PS, Ferreira AP, de Oliveira FF, Assumpcao LV, Metze K, Ward LS. Usefulness of hbme-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology. 2005;47(4):391–401.
Nga ME, Lim GS, Soh CH, Kumarasinghe MP. Hbme-1 and ck19 are highly discriminatory in the cytological diagnosis of papillary thyroid carcinoma. Diagn Cytopathol. 2008;36(8):550–6.
Cui W, Sang W, Zheng S, Ma Y, Liu X, Zhang W. Usefulness of cytokeratin-19, galectin-3, and hector battifora mesothelial-1 in the diagnosis of benign and malignant thyroid nodules. Clin Lab. 2012;58(7-8):673–80.
Fadda G, Rossi ED, Raffaelli M, Pontecorvi A, Sioletic S, Morassi F, et al. Follicular thyroid neoplasms can be classified as low- and high-risk according to hbme-1 and galectin-3 expression on liquid-based fine-needle cytology. Eur J Endocrinol. 2011;165(3):447–53.
Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001;14(4):338–42.
Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, Tanaka Y, et al. Hbme-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J. 2003;50(2):173–7.
Chung SY, Park ES, Park SY, Song JY, Ryu HS. Cxc motif ligand 12 as a novel diagnostic marker for papillary thyroid carcinoma. Head Neck. 2014;36(7):1005–12.
Beesley MF, McLaren KM. Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology. 2002;41(3):236–43.
Zintzaras E, Ioannidis JP. Hegesma: Genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3.
Carling T, Ocal IT, Udelsman R. Special variants of differentiated thyroid cancer: does it alter the extent of surgery versus well-differentiated thyroid cancer? World J Surg. 2007;31(5):916–23.
Faquin WC, Baloch ZW. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to national cancer institute (nci) recommendations. Diagn Cytopathol. 2010;38(10):731–9.
Ohori NP, Wolfe J, Hodak SP, LeBeau SO, Yip L, Carty SE, et al. "Colloid-rich" follicular neoplasm/suspicious for follicular neoplasm thyroid fine-needle aspiration specimens: cytologic, histologic, and molecular basis for considering an alternate view. Cancer Cytopathology. 2013;121(12):718–28.
Nasr MR, Mukhopadhyay S, Zhang S, Katzenstein AL. Immunohistochemical markers in diagnosis of papillary thyroid carcinoma: utility of hbme1 combined with ck19 immunostaining. Mod Pathol. 2006;19(12):1631–7.
Lansoy-Kuhn C, Picquenot JM, Edet-Sanson A, Mechken F, Laberge-Le Couteulx S, Cornic M, et al. Relationship between the immunohistochemistry of the primary tumour and 18f-fdg-pet/ct at recurrence in patients with well-differentiated thyroid carcinoma. Nucl Med Commun. 2013;34(4):340–6.
Barut F, Onak Kandemir N, Bektas S, Bahadir B, Keser S, Ozdamar SO. Universal markers of thyroid malignancies: Galectin-3, hbme-1, and cytokeratin-19. Endocr Pathol. 2010;21(2):80–9.
Paunovic I, Isic T, Havelka M, Tatic S, Cvejic D, Savin S. Combined immunohistochemistry for thyroid peroxidase, galectin-3, ck19 and hbme-1 in differential diagnosis of thyroid tumors. APMIS. 2012;120(5):368–79.
Wu G, Wang J, Zhou Z, Li T, Tang F. Combined staining for immunohistochemical markers in the diagnosis of papillary thyroid carcinoma: Improvement in the sensitivity or specificity? J Int Med Res. 2013;41(4):975–83.
Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol. 2010;5:9.
Zhu X, Sun T, Lu H, Zhou X, Lu Y, Cai X, et al. Diagnostic significance of ck19, ret, galectin-3 and hbme-1 expression for papillary thyroid carcinoma. J Clin Pathol. 2010;63(9):786–9.
Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Borson-Chazot F, Lifante JC, et al. Immunohistochemical markers in the diagnosis of papillary thyroid carcinomas: The promising role of combined immunostaining using hbme-1 and cd56. Pathol Res Pract. 2013;209(9):585–92.
Casey MB, Lohse CM, Lloyd RV. Distinction between papillary thyroid hyperplasia and papillary thyroid carcinoma by immunohistochemical staining for cytokeratin 19, galectin-3, and hbme-1. Endocr Pathol. 2003;14(1):55–60.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Chen, YJ., Zhao, RM., Zhao, Q. et al. Diagnostic significance of elevated expression of HBME-1 in papillary thyroid carcinoma. Tumor Biol. 37, 8715–8720 (2016). https://doi.org/10.1007/s13277-015-4169-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4169-5