Abstract
A meta-analysis was conducted to evaluate the effect of treatment with angiotensin-converting enzyme inhibitors on the risk of fractures. All the included articleswere retrieved from MEDLINE, EMBASE and the Cochrane Database. Trial eligibility and methodological quality were assessed before data extraction. Relative risk (RR) with corresponding 95% confidence intervals (95% CI) were used to assess the effect. Six case-control studies with11,387,668 participants met the inclusion criteria and were included in the meta-analysis. A small but significant risk effect on fractures was shown in the overall analysis of angiotensin-converting enzyme inhibitor users compared with nonusers (Pooled RR 1.27; 95% CI 1.01–1.60), although a relatively high heterogeneity was found across studies. In the stratified analysis, therewas no statistically significant association in the subgroups of hip fracture (Pooled RR 1.14; 95% CI 0.73–1.76) and the study quality (Pooled RR 1.13; 95% CI 0.89–1.44), while the over 65-year-old angiotensin-converting enzyme inhibitor users showed a stronger risk effect on fractures (Pooled RR 2.06; 95% CI 1.53–3.17). Moreover, age was found to be contributed a large part of the high heterogeneity across the included studies. This study demonstrated that the use of angiotensin-converting enzyme inhibitors might have a small but significant risk effect on fractures, especially for the over 65-year-old users. These results should be interpreted with caution as the relatively high heterogeneity across studies. Additional multiple observational studies and high quality data from randomized controlled trials are needed to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone tissue leading to decreased bone strength and an increased risk of low-energy fractures [1]. Nowadays, Osteoporosis has become a serious problem throughout the world. For example, the prevalence rate of Osteoporosis in older adults was about 13–18 and 15.7% in the United States and the People’s Republic of China, respectively [2, 3]. The ratio is likely to increase significantly with the aging of the world’s population. As the primarily source of the clinical and public health importance of osteoporosis, fragility fracture is one of the most common causes of disability and a major contributor to costs of medical care in all regions of the world [4]. Clinical consequences of fracture include short and long-term morbidities characterized by pain, limitation of function, decreased health-related quality of life, and increased mortality. It was reported that mortality rates following hip fracture range from 10 to 45% in the first year [5]. In the year 2000, there was an estimated number of 9 million subjects with osteoporotic fractures world-wide and the total Disability Adjusted Life Years lost was 5.8 million accounting for 0.83 % of the global burden of non-communicable disease [6].
The pathogenesis of osteoporotic fracture is based on two key components: bone mass and falls, both of which were potentially affected by hypertension [7]. A large population based epidemiological study showed that approximately 50% hypertensive patients suffered from osteoporosis [8].Compared with normotensive controls, an increased 24 h urinary calcium excretion and a decreased Bone Mineral Density (BMD) had been found in patients with untreated essential hypertension [9]. Meanwhile, essential hypertension is associated with vitamin D insufficiency, which is an established risk factor for osteoporosis, falls and fractures [10]. Antihypertensive medications may have an effect on bone by virtue of the fact that they reduce blood pressure. For example, Thiazides appear to reduce the risk of hip fracture based on observationalstudies [11]. A previous meta-analysis examining the effects of antihypertensive drugs on fracture out comes involving eight observational studies on Beta-Adrenergic Blockers (BBs) from 1966 until December 2005 found that BBs provided astatistically significant protection against fractures (RR 0.86, 95% CI 0.76–0.98) [12].
The activation of the intrarenal renin-angiotensin system (RAS) is an important contributor to systemic hypertension, as the RAS axis plays a most vital role in regulating both sodium balance and blood pressure through its pleiotropic actions on multiple vascular, endocrine, and renal mechanisms [13]. Angiotensin-converting enzyme (ACE) inhibitors are reported to induce a lower level of tissue angiotensin II, which plays a key role in the RAS [14]. ACE inhibitors seemed so logical and appealing that seemingly beneficial changes in surrogate endpoints such as blood pressure, proteinuria, and endothelial dysfunction became accepted as afree pass for dual blockade having cardioprotective andnephroprotective effects. It was commonly used inpatients with hypertension and with diabetes or proteinuria, orboth and also to a lesser extent in those with heart failure resistant to treatment [15]. Some previous researches have showed that activation of the RAS induced an acceleration of bone resorption [16] and a decreased plasma ionic calcium level [17]. With the use of ACE inhibitors, some positive effects have been showed such as decreased fracture risk [18, 19] and improved BMD [20]. However, conflicting results have been reported. For example, in a population-based, self-controlled case series in Canada, ACE inhibitors were associated with an immediate increased hip fracture risk during the initiation of treatment in hypertensive elderly patients [21]. Meanwhile, an increasing bone loss in total hip and femoral neck was reported in continuous ACE inhibitor users [22]. Considering the undefined effects of ACE inhibitors on the risk of fractures, a systematic review is needed for the current available evidence.
Methods
We conducted a systematic computerized search in the following databases: MEDLINE, Embase, and the Cochrane central register of controlled trials (CENTRAL). Sources were searched from the earliest possible dates through October 2015. The following search terms were used: “bone,” “osteoporosis,” “fracture,” “ACE inhibitors” and “angiotensin-converting enzyme inhibitors,” with additional terms of individual ACE inhibitor agents (e.g., captopril, benazepril, enalapril, cilazapril, fosinopril, perindopril, ramipril, lisinopril, quinapril, trandolapril, delapril, imidapril, moexipril, spirapril, temocapril, zofenopril) to identify all potentially eligible studies. In view of the high likelihood of lack of such randomized controlled trials, we also searched for observational studies to be included in this review. The search was limited in human. Language was restricted in English. Ongoing clinical trials and unpublished studies were searched via the worldwide web on the following sites: http://www.controlled-trials.com, http://www.clinicaltrials.gov and http://www.centerwatch.com.
Selection criteria
Studies were regarded as eligible if they met the criteria listed below. The intervention of interest was the exposure of any ACE inhibitors. Only the comparison between ACE inhibitor users and nonusers was considered as an eligible one. As the outcome measurement, the incidence of fracture such as relative risks (RR) or odds ratios (OR) and 95% confidence intervals (95% CI) were presented or the study provided enough data for these values to be calculated. The exclusion of fractures related to pathological conditions or caused by high-impact traumatic injury was not deemed as selection criteria, considering the limited number of researches and relatively low proportion of the specific individuals. If two studies used the same study population during the same period of time, the study with stronger design would be selected based on the quality assessment analysis.
Data extraction
Two reviewers independently performed data extraction and the results were cross-checked by double-data entry. Disagreements were resolved by discussion and consensus. For this analysis we extracted study name, study design, year of publication, sample size, percentage of men, mean age, study duration, fracture site, RR or OR with 95% CI and confounders.
Quality assessment
The quality of the included observational studies was assessed by the Newcastle-Ottawa Scale (NOS). When considering comparability in NOS, we assessed whether ACE inhibitor users and nonusers were matched in the design and/or whether confounders were adjusted in the analysis. One star point was awarded if age was controlled by the study and another point was awarded if two or more factors were controlled. For cohort studies, selection of exposed and non-exposed cohort, comparability of cohorts, assessment of outcome, and adequacy of follow-up were addressed. For case-control studies, selection of cases and controls, comparability of cases and controls, and ascertainment of exposure were emphasized. A score of 7~9 represents high quality, a score of 5~6 represents moderate quality and a score of 0~4 represents as low quality [23].
Statistical analysis
Data entry and analyses were performed in STATA 12.1 (STATA Corp, College Station, Texas, USA). Furthermore, in those studies where multiple fracture sites and different age groups were analyzed, additional analyses were conducted separately on fracture sites and age subgroups. Applied formal meta-analytic techniques, the results were reported as pooled RR with corresponding 95% CI to assess the treatment effect of ACE inhibitors on the risk of fractures. Odds ratios were considered an approximation of relative risks. The random-effects models were used to calculate the pooled RR and 95% CI as this approach allows for heterogeneity across studies in meta-analysis [24]. Heterogeneity was assessed using the I 2 statistic [25]. I 2 < 25% was defined as low heterogeneity and I 2 > 75% as high heterogeneity. Sensitivity analysis was performed for safety outcomes in all meta-analysis estimates with each study omitted and subgroup analysis. Egger’s linear regression test and Begg’s rank correlation test were applied to check the publication bias. Regarding a high heterogeneity existing in these studies, meta-analysis regression was adapted to try to find the associated covariates contributing to the heterogeneity [26].
Results
Study selection process
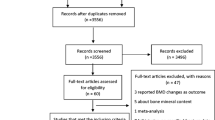
Through searching databases and the other sources, a total of 2113 articles were assessed for potential relevancy (Fig. 1). Among those, 2090 were excluded on basis of title, subject or abstract. The remaining 23 full text articles were further reviewed. Two of them were excluded for not being original investigations [27, 28]. Seven of them were excluded for ineligible comparisons or lack of data for calculation [29–35]. Eight of them were excluded for using bone mineral density rather than fracture as the outcome measure [14, 20, 22, 36–40] .
Description of studies
In final meta-analysis, six case-control studies, included a total of 11,387,668 participants from four countries, met the inclusion criteria [7, 18, 21, 41–43]. The studies were published between 2004 and 2015. The mean age of the participants is approximately over 58 years old and the mean follow-up time is 2.8 years. Characteristics of all the included studies were available in Table 1.
Among the six studies, two demonstrated statistically significant associations between use of ACE-inhibitor and reduction of the risk of fracture [18, 42], three showed increased risk of fracture [7, 21, 43] and remaining one found no statistically significant association [41]. Four studies attempted to control and adjust for potential confounding variables [18, 21, 41, 43], while the remaining two studies did not [7, 21]. Unfortunately, only one of the studies adjusted for age [43]. None of the studies was adjusted by the same covariates or conducted under similar conditions, which will cause a remarkable clinical heterogeneity across studies.
There were some noteworthy points in the included studies. The study conducted by Raymond et al. [42] was mainly focus on the effect of β-blockers, but the effect of longer-term current ACE inhibitors on fracture risk was also investigated in their study. Besides, one paper [18] contained subgroup data for various types of fracture, such as hip, spine and forearm, while the incidence of any fracture was provided at the same time. The three separate effect sizes and the overall effect size were simultaneously included in two independent meta-analyses. Another study conducted by Perez-Castrillon et al. [7] provided us the separate effect sizes from men and women rather than pooled data, so the effect in men and women were analyzed as two separate parts.
Quality assessment for the included studies
The median score (the number of stars awarded) was 6.5 (out of 9) for the 6 case-control studies with an overall range of 5–8 points. Three studies were deemed to be high quality and reached 7 or more star points [18, 21, 43] . The remainders were moderate quality and reached a range of 5–6 points (Table 2) [7, 41, 42].
Effects of interventions
There were six case-control studies included [7, 18, 21, 41–43] in the overall analysis of ACE inhibitor users compared nonusers resulting in a small but significant risk effect on fractures (Pooled RR 1.27; 95% CI 1.01–1.60), while a high-level heterogeneity was found (I 2 = 96.1%; p < 0.001). Forest plot is shown in Fig. 2a for the all studies included. With an overall effect size of Rejnmark et al. instead of the three separate effect sizes (details please refer to the section “Description of studies”), the meta-analysis gave a slightly rising risk effect (Pooled RR 1.43; 95% CI 1.06–1.92; I 2 = 97.1%; p for heterogeneity < 0.001).
Sensitivity analyses were performed for safety outcomes in all meta-analysis estimates with each study omitted and subgroup analysis. The order of magnitude of the pooled RR and 95% CI changed little with each study omitted, which indicated that the meta-analysis outcomes pooled the nine effect sizes were very stable (Fig. 3a). In the stratified analysis for the average age over 65-year-old subgroup, ACE inhibitor users were associated with a remarkable increasing risk of fractures (Pooled RR 2.06; 95% CI 1.53–3.17) compared with nonusers, while the under 65-year-old subgroup showed no statistically significant results (Pooled RR 1.02; 95% CI 0.84–1.25) (Fig. 3b). When we restricted the analysis to those studies which reported results specific to hip fractures, the results were not statistically significant (Pooled RR 1.14; 95% CI 0.73–1.76) (Fig. 3c). Furthermore, restricting the analysis to those 3 studies [18, 21, 43] that scored 7 points or higher on the quality scale also showed no significant effects (Pooled RR 1.13; 95% CI 0.89–1.44) (Fig. 3d).
The heterogeneity continued to be very high after stratification by the quality of studies (I 2 = 95.7%, p < 0.001 for high quality subgroup), but subsided slightly when we restricted the analysis to age (I 2 = 82.4%, p = 0.003 for age over 65-year-old subgroup) [12] and fracture site (I 2 = 85.1%, p = 0.001 for hip fractures subgroup). Regarding the high heterogeneity existing in these studies, meta-analysis regression was adapted to try to find the associated covariates contributing to the heterogeneity, although the number of included studies was relatively small. In the regression analyses, age (below or above 65 year old), fracture site (hip or others) and study quality (high or moderate) were transferred to binary variables and considered as the potential confounders. The results showed that age contributed most (Adjusted R 2 = 56.56%, p-value = 0.016) to the high heterogeneity compared with the paper quality and the fracture site (both R 2 values are below 0 and p values are above 0.1). In addition, we intended to stratify subgroups and analyses performed based on gender and length of drug exposure, but we gave up as few studies reported stratified data.
No evidence of publication bias was suggested by Egger’s test (t = 1.92, 95% CI −1.25–12.09, p = 0.096 for all studies included analysis) and Begg’s Test (z = 1.56, P = 0.118 for all studies included analysis).
Discussion
Since Captopril, the first orally active ACE inhibitor, was approved by the United States Food and Drug Administration in 1981, ACE inhibitors have been amongst the most important drugs for the treatment of hypertension and congestive heart failure. Given the ubiquity of their use, side effect on fracture risk, whether harmful or protective, is likely to have a significant impact on public health. The present study evaluated the associations between treatment with ACE inhibitors and the risk of bone fractures. Our results suggested that ACE inhibitors were associated with a small but significant risk effect on fractures (Pooled RR = 1.27), especially for the over 65-year-old users (Pooled RR = 2.06). In fact, three previous papers performed the narrative review of the treatment effect of ACE inhibitors on the risk of fracture [12, 27, 44], but none of these studies carried out the meta-analysis. Among those, in 2009 Masunari et al. searched out the three case-control studies [7, 18, 42] of the six studies included in our study and reported that there was no consistent results of ACE inhibitors on bone fracture [44]. While Wiens et al. and Ilic et al. only searched out one [42] and two [18, 42] studies, repectively [12, 44].
Conflicting results of treatment effect of ACE inhibitors on BMD and fracture risk were reported previously [45, 46]. The risk effect might be related to the functional interaction between the two angiotensin II receptors (AT1 receptor and AT2 receptor) on the osteoblastic cell surface [16]. However, a cross-sectional study of elderly Chinese exploring the association between ACE inhibitor use and BMD was published [20] and demonstrated that ACE inhibitors might have possible benefits in BMD. The potential mechanisms of the beneficial effect of ACE inhibitors on bone metabolism were proposed in several researches. Since angiotensin II inhibited osteoblastic differentiation [47] and ACE is the major enzyme for the production of angiotensin II in humans, there are reasons to believe that ACE inhibitor is one of the regulators of bone metabolism through reducing the activity of ACE and then further blocking the conversion of angiotensin I to angiotensin II. The mechanisms by which ACE inhibitors influence bone metabolism are not fully elucidated and further studies are required to clarify this issue.
In the stratified analysis for age over 65 years, ACE inhibitor users were associated with a stronger risk effect on fractures compared with the results in overall analysis. Considering the number of included studies was only two, future research is very likely to have an important impact on our confidence in the estimate or even change the results. Nevertheless, our results, at least in some ways, showed a trend that the risk of fractures increased with the age in ACE inhibitor users. Older adults are more likely to experience side effects from medications. The risk of first-dose hypotension has been described with the use of specific ACE inhibitors [48, 49], which produce marked venous pooling with a consequent fall in cardiac output and profound hypotension [50]. This kind of hypotension is associated with an increased risk of falling or dizziness and adverse outcomes such as fractures, while the prevalence of hypotension increases with age [51]. This might be an explanation for the increased fracture risk in elderly ACE inhibitor users.
Heterogeneity was fairly high in overall analysis and was still observed when stratified by fracture site, age and study quality. It is vital to explore possible reasons for heterogeneity. The results showed that age contributed most (Adjusted R 2 = 56.56%) to the high heterogeneity compared with the quality of studies and the fracture site. Obviously, the causes of heterogeneity were not limited to these three aspects. These considerable variation sample sizes different from 1538 to 8,315,709 might contribute to the source of heterogeneity. Duration of ACE inhibitors exposure was likely to be one of causes. Furthermore, the other confounding factors, such as study location, study design, gender, available description of ACE inhibitors, dosage of ACE inhibitors and individual biological characteristics, concomitant treatments, comorbidities, life habits and culture might also be the causes. Each of the factors could be identified as a potential source of heterogeneity among case-control studies. To reduce the heterogeneity, more targeted randomized clinical trials are needed.
In addition to the high heterogeneity, our study was subject to some other limitations. First, as the retrospective nature of the study design, recall bias could be a potential limitation. However, most of the included studies in this meta-analysis used medical records or pharmacy database to ascertain ACE inhibitors use as well as outcome of fractures, which should reduce the potential for exposure misclassification. Nonetheless, since the source of information on drug use was a database, some problems remained in data accuracy about compliance of drug use. Second, the sample size in this meta-analysis was small, which limited the statistical power. Only a number of six studies were included, even less in the subgroup analysis. In order to better decipher our results, more studies with larger sample sizes are needed in the future. Third, adjustments on confounding factors did not conducted in all studies or under similar conditions. Moreover, it was difficult to adjust for certain clinical differences such as estrogen levels, dietary habits, exercise and other lifestyle factors, which were considered effective influencing factors in bone health. Fourth, language was restricted to English in this meta-analysis, which might lead to a potential language bias. Finally, we were unable to analyze the association between the duration of ACE inhibitors use and the risk of fractures for the limited number of studies available, which might affect the final results partly.
Conclusion
The present study indicated that ACE inhibitors might be associated with a small but significant risk effect on fractures in overall and even stronger when specific to over 65-year-old users, although a relatively high heterogeneity was found across studies. Our results suggested physicians should consider this potentially undesirable effects of ACE inhibitors in treating hypertensive individuals with high risk of fractures. Extra cautions are advised when initiating antihypertensive drugs in the elderly. Additional multiple observational studies and high quality data from randomized controlled trials are needed to confirm these potentially important findings.
References
B.C. Lupsa, K. Insogna, Bone health and osteoporosis. Endocrinol. Metab. Clin. North. Am. 44(3), 517–530 (2015). doi:10.1016/j.ecl.2015.05.002
X. Lin, D. Xiong, Y.Q. Peng, Z.F. Sheng, X.Y. Wu, X.P. Wu, F. Wu, L.Q. Yuan, E.Y. Liao, Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin. Interv. Aging. 10, 1017–1033 (2015). doi:10.2147/cia.s54613
A.C. Looker, E.S. Orwoll, C.J. Johnston, R.L. Lindsay, H.W. Wahner, W.L. Dunn, M.S. Calvo, T.B. Harris, S.P. Heyse, Prevalence of low femoral bone density in older U.S. adults from NHANES III. J. Bone. Miner. Res. 12(11), 1761–1768 (1997). doi:10.1359/jbmr.1997.12.11.1761
J.A. Cauley, N.S. Wampler, J.M. Barnhart, L. Wu, M. Allison, Z. Chen, S. Hendrix, J. Robbins, R.D. Jackson, Incidence of fractures compared to cardiovascular disease and breast cancer: the women’s health initiative observational study. Osteoporos. Int. 19(12), 1717–1723 (2008). doi:10.1007/s00198-008-0634-y
G.G. Teng, J.R. Curtis, K.G. Saag, Mortality and osteoporotic fractures: is the link causal, and is it modifiable? Clin. Exp. Rheumatol. 26(5 Suppl 51), S125–137 (2008)
O. Johnell, J.A. Kanis, An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17(12), 1726–1733 (2006). doi:10.1007/s00198-006-0172-4
J.L. Perez-Castrillon, J.C. Martin-Escudero, M.P. Alvarez, S.R. Cortes, Z.S. Iglesias, A.M. Garcia, Hypertension as a risk factor for hip fracture. Am. J. Hypertens. 18(1), 146–147 (2005). doi:10.1016/j.amjhyper.2004.08.016
J. Woo, T. Kwok, J. Leung, N. Tang, Dietary intake, blood pressure and osteoporosis. J. Hum. Hypertens. 23(7), 451–455 (2009). doi:10.1038/jhh.2008.156
K. Tsuda, I. Nishio, Y. Masuyama, Bone mineral density in women with essential hypertension. Am. J. Hypertens. 14(7 Pt 1), 704–707 (2001)
L. Mosekilde, Vitamin D and the elderly. Clin. Endocrinol. 62(3), 265–281 (2005). doi:10.1111/j.1365-2265.2005.02226.x
K. Aung, T. Htay, Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst. Rev. (10), CD005185 (2011). doi:10.1002/14651858.CD005185.pub2.
M. Wiens, M. Etminan, S.S. Gill, B. Takkouche, Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J. Intern. Med. 260(4), 350–362 (2006). doi:10.1111/j.1365-2796.2006.01695.x
L.G. Navar, Counterpoint: activation of the intrarenal renin-angiotensin system is the dominant contributor to systemic hypertension. J. Appl. Physiol. 109(6), 1998–2000 (2010). doi:10.1152/japplphysiol.00182.2010a. (1985)discussion 2015
T. Kwok, J. Leung, Y.F. Zhang, D. Bauer, K.E. Ensrud, E. Barrett-Connor, P.C. Leung, Does the use of ACE inhibitors or angiotensin receptor blockers affect bone loss in older men?. Osteoporos. Int. 23(8), 2159–2167 (2012). doi:10.1007/s00198-011-1831-7
H. Makani, S. Bangalore, K.A. Desouza, A. Shah, F.H. Messerli, Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 346, f360 (2013)
Y. Asaba, M. Ito, T. Fumoto, K. Watanabe, R. Fukuhara, S. Takeshita, Y. Nimura, J. Ishida, A. Fukamizu, K. Ikeda, Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J. Bone. Miner. Res. 24(2), 241–250 (2009). doi:10.1359/jbmr.081006
F.D. Grant, S.J. Mandel, E.M. Brown, G.H. Williams, E.W. Seely, Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J. Clin. Endocrinol. Metab. 75(4), 988–992 (1992). doi:10.1210/jcem.75.4.1400892
L. Rejnmark, P. Vestergaard, L. Mosekilde, Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J. Hypertens. 24(3), 581–589 (2006). doi:10.1097/01.hjh.0000203845.26690.cb
S. Yamamoto, R. Kido, Y. Onishi, S. Fukuma, T. Akizawa, M. Fukagawa, J.J. Kazama, I. Narita, S. Fukuhara, Use of renin-angiotensin system inhibitors is associated with reduction of fracture risk in hemodialysis patients. PLoS. One. 10(4), e0122691 (2015). doi:10.1371/journal.pone.0122691
H. Lynn, T. Kwok, S.Y. Wong, J. Woo, P.C. Leung, Angiotensin converting enzyme inhibitor use is associated with higher bone mineral density in elderly Chinese. Bone 38(4), 584–588 (2006). doi:10.1016/j.bone.2005.09.011
D.A. Butt, M. Mamdani, P.C. Austin, K. Tu, T. Gomes, R.H. Glazier, The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch. Intern. Med. 172(22), 1739–1744 (2012). doi:10.1001/2013.jamainternmed.469
Y.F. Zhang, L. Qin, P.C. Leung, T.C. Kwok, The effect of angiotensin-converting enzyme inhibitor use on bone loss in elderly Chinese. J. Bone. Miner. Metab. 30(6), 666–673 (2012). doi:10.1007/s00774-012-0363-3
G.A. Wells, B. Shea, D. O’Connell, J. Peterson, V. Welch, The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2004), http://www.ohri.ca/programs/clinicalepidemiology/oxford.htm. Accessed 5 July 2004.
T.A. Trikalinos, G. Salanti, E. Zintzaras, J.P Ioannidis, Meta-analysis methods. Adv. Genet. 60, 311–334 (2008). doi:10.1016/s0065-2660(07)00413-0
J.P. Higgins, S.G. Thompson, J.J. Deeks, D.G. Altman, Measuring inconsistency in meta-analyses. BMJ. 327(7414), 557–560 (2003). doi:10.1136/bmj.327.7414.557
J.P. Higgins, S.G. Thompson, Controlling the risk of spurious findings from meta-regression. Stat. Med. 23(11), 1663–1682 (2004). doi:10.1002/sim.1752
M. Naomi, F. Saeko, Impact of antihypertensive drug use on bone miner density and osteoporotic fracture-from an epidemiological perspective. Recent Pat Endocr Metab Immune Drug Discov 4(1), 15–33 (2010)
B. Medhi, A. Prakash, S. Kaur, K. Bhagwat, S. Chaudhary, V. Goni, Angiotensin Converting Enzyme Inhibition: Therapeutic Implications in Fracture Healing. JK Science 13(2), 107–108 (2011)
J.R. Curtis, H. Yun, J.L. Lange, R. Matthews, P. Sharma, K.G. Saag, E. Delzell, Does medication adherence itself confer fracture protection? An investigation of the healthy adherer effect in observational data. Arthritis Care Res 64(12), 1855–1863 (2012). doi:10.1002/acr.21759
C.J. Bulpitt, R. Peters, J.A. Staessen, L. Thijs, M.C. De Vernejoul, A.E. Fletcher, N.S. Beckett, Fracture risk and the use of a diuretic (indapamide SR) +/- perindopril: a substudy of the hypertension in the very elderly trial (HYVET). Trials. 7, 33 (2006). doi:10.1186/1745-6215-7-33
C. Bulpitt, A. Fletcher, N. Beckett, J. Coope, B. Gil-Extremera, F. Forette, C. Nachev, J. Potter, P. Sever, J. Staessen, C. Swift, J. Tuomilehto, Hypertension in the very elderly trial (HYVET): protocol for the main trial. Drugs. Aging. 18(3), 151–164 (2001)
D.H. Solomon, H. Mogun, K. Garneau, M.A. Fischer, Risk of fractures in older adults using antihypertensive medications. J. Bone. Miner. Res. 26(7), 1561–1567 (2011). doi:10.1002/jbmr.356
D.A. Butt, M. Mamdani, T. Gomes, L. Lix, H. Lu, K. Tu, Risk of osteoporotic fractures with angiotensin II receptor blockers versus angiotensin-converting enzyme inhibitors in hypertensive community-dwelling elderly. J. Bone. Miner. Res. 29(11), 2483–2488 (2014). doi:10.1002/jbmr.2271
R. Peters, N. Beckett, L. Burch, M.C. de Vernejoul, L. Liu, J. Duggan, C. Swift, B. Gil-Extremera, A. Fletcher, C. Bulpitt, The effect of treatment based on a diuretic (indapamide) +/− ACE inhibitor (perindopril) on fractures in the hypertension in the very elderly trial (HYVET). Age. Ageing. 39(5), 609–616 (2010). doi:10.1093/ageing/afq071
R. Peters, N. Beckett, T. McCormack, R. Fagard, A. Fletcher, C. Bulpitt, Treating hypertension in the very elderly-benefits, risks, and future directions, a focus on the hypertension in the very elderly trial. Eur. Heart. J. 35(26), 1712–1718 (2014). doi:10.1093/eurheartj/eht464
A. Garcia-Testal, A. Monzo, G. Rabanaque, A. Gonzalez, A. Romeu, Evolution of the bone mass of hypertense menopausal women in treatment with fosinopril. Med. Clin. 127(18), 692–694 (2006)
J.L. Perez-Castrillon, J. Silva, I. Justo, A. Sanz, M. Martin-Luquero, R. Igea, P. Escudero, C. Pueyo, C. Diaz, G. Hernandez, A. Duenas, Effect of quinapril, quinapril-hydrochlorothiazide, and enalapril on the bone mass of hypertensive subjects: relationship with angiotensin converting enzyme polymorphisms. Am. J. Hypertens. 16(6), 453–459 (2003)
N. Masunari, S. Fujiwara, Y. Nakata, K. Furukawa, F. Kasagi, Effect of angiotensin converting enzyme inhibitor and benzodiazepine intake on bone loss in older Japanese. Hiroshima. J. Med. Sci. 57(1), 17–25 (2008)
R. Agarwal, A.D. Sinha, M.K. Pappas, T.N. Abraham, G.G. Tegegne, Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol. Dial. Transplant. 29(3), 672–681 (2014). doi:10.1093/ndt/gft515
M. Kinjo, S. Setoguchi, D.H. Solomon, Anti-Hypertensive therapy and bone mineral density: analysis in a population-based U.S. sample. Arthritis. Rheum. 62(suppl 10), 956 (2010). doi:10.1002/art.26725
S.D. Berry, Y. Zhu, H. Choi, D.P. Kiel, Y. Zhang, Diuretic initiation and the acute risk of hip fracture. Osteoporos. Int. 24(2), 689–695 (2013). doi:10.1007/s00198-012-2053-3
R.G. Schlienger, M.E. Kraenzlin, S.S. Jick, C.R. Meier, Use of beta-blockers and risk of fractures. JAMA. 292(11), 1326–1332 (2004). doi:10.1001/jama.292.11.1326
H.J. Choi, C. Park, Y.K. Lee, Y.C. Ha, S. Jang, C.S. Shin, Risk of fractures in subjects with antihypertensive medications: a nationwide claim study. Int. J. Cardiol. 184, 62–67 (2015). doi:10.1016/j.ijcard.2015.01.072
K. Ilic, N. Obradovic, N. Vujasinovic-Stupar, The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue. Int. 92(3), 217–227 (2013). doi:10.1007/s00223-012-9671-9
T.Y. Diao, H. Pan, S.S. Gu, X. Chen, F.Y. Zhang, M.S. Wong, Y. Zhang, Effects of angiotensin-converting enzyme inhibitor, captopril, on bone of mice with streptozotocin-induced type 1 diabetes. J. Bone. Miner. Metab. 32(3), 261–270 (2014). doi:10.1007/s00774-013-0500-7
K.Y. Kang, Y. Kang, M. Kim, Y. Kim, H. Yi, J. Kim, H.R. Jung, S.H. Park, H.Y. Kim, J.H. Ju, Y.S. Hong, The effects of antihypertensive drugs on bone mineral density in ovariectomized mice. J. Korean. Med. Sci. 28(8), 1139–1144 (2013). doi:10.3346/jkms.2013.28.8.1139
H. Hagiwara, Y. Hiruma, A. Inoue, A. Yamaguchi, S. Hirose, Deceleration by angiotensin II of the differentiation and bone formation of rat calvarial osteoblastic cells. J. Endocrinol. 156(3), 543–550 (1998)
L. Burton, M. Norton, J.L. Newton, Are some antihypertensives more prone to induce hypotensive side effects than others?. Age. Ageing. 33(6), 626–628 (2004). doi:10.1093/ageing/afh196
I. Slavachevsky, R. Rachmani, Z. Levi, D. Brosh, M. Lidar, M. Ravid, Effect of enalapril and nifedipine on orthostatic hypotension in older hypertensive patients. J. Am. Geriatr. Soc. 48(7), 807–810 (2000)
S. Capewell, A. Capewell, ‘First dose’ hypotension and venodilatation. Br. J. Clin. Pharmacol. 31(2), 213–215 (1991)
L.J. Benvenuto, L.R. Krakoff, Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am. J. Hypertens. 24(2), 135–144 (2011). doi:10.1038/ajh.2010.146
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81270966, No.81500679), the Natural Science Foundation of Guangdong Province, China (Grant No. S2012010009494, 2014A030310036 and 2014A030310472), Science and technology plan of Guangdong province (Grant No. 2016A020215097) and the Natural Science Foundation of Southern Medical University, Guangdong Province, China (Grant No. PY2013N050).
Author contributions
Y. Cheng and Z. Huang wrote the main manuscript text, Z. Shen, H. Wu, J. Peng performed data extraction and quality assessment, M. Waye, S. Rao and L. Yang performed statistical analysis and overall design of the meta-analysis. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. They have full control of all primary data and agree to allow the journal to review their data if requested.
Additional information
Yan-Zhen Cheng and Zhen-Zi Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cheng, YZ., Huang, ZZ., Shen, ZF. et al. ACE inhibitors and the risk of fractures: a meta-analysis of observational studies. Endocrine 55, 732–740 (2017). https://doi.org/10.1007/s12020-016-1201-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1201-5