Abstract
Ischemic stroke is one of the leading causes of death and disability worldwide. Although miR-149-5p downregulation is observed in rats after ischemia/reperfusion (I/R) injury, its function and role in ischemic stroke remain unclear. This study aimed to investigate the roles of miR-149-5p in I/R injury. The results showed that miR-149-5p was significantly downregulated in brain tissues of rats subjected to middle cerebral artery occlusion (MCAO) and primary cortical neurons subject to oxygen and glucose deprivation (OGD). MiR-149-5p overexpression effectively reduced MCAO/R-induced infarct volume, neurological score, and brain water content as well as OGD/R-induced cortical neurons apoptosis and OGD/R-induced expression of TNF-α, IL-4, IL-6, IL-1β, and COX-2. Moreover, Notch2 was identified as a target of miR-149-5p and Notch2 overexpression significantly attenuated the inhibitory effects of miR-149-5p mimics on inflammation and apoptosis. Taken together, our study revealed that miR-149-5p overexpression protects the rat brain against I/R injury by regulating Notch2-mediated inflammation and apoptosis pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is becoming a major leading cause of long-term disability in developed countries and approximately 9% of deaths worldwide (Powers, 2020). In the past decades, the therapeutic strategies for acute stroke include rapid neurological evaluation and imaging to initiate effective reperfusion treatment with intravenous thrombolysis and mechanical thrombectomy (Barthels & Das, 1866; Phipps & Cronin, 2020). However, reperfusion treatment may lead to reperfusion injury, also known as ischemia/reperfusion (I/R) injury (Wang et al., 2017). Despite increasing efforts, dealing with cerebral I/R injury remains a challenge in current treatments (Granger & Kvietys, 2015; Prabhakaran et al., 2015). Therefore, understanding the specific molecular mechanisms involved in cerebral I/R injury will help develop new and effective therapeutic targets for ischemic stroke.
MicroRNAs (miRNAs) have emerged as a highly conserved class of small non-coding RNAs with approximately 20–22 nucleotides in length (Lu & Rothenberg, 2018). Currently, more and more miRNAs have been identified in eukaryotic cells (Chen et al., 2019). Increasing studies have demonstrated that miRNAs can regulate diverse biological processes such as cell proliferation, apoptosis, and neuroinflammation by directly binding to the 3′-untranslated region (3′-UTR) of their target mRNAs (Ghafouri-Fard et al., 2020). Recently, the functions of miRNAs involved in cerebral I/R injury have been well investigated. For example, downregulation of miR-19a-3p reduces cerebral I/R injury by targeting IGFBP3 (Chai et al., 2020). MiR-193b-3p alleviates cerebral I/R injury in rats by downregulating the expression of 5-lipoxygenase (Chen et al., 2020a). The miR-211 level is highly downregulated during ischemia injury in vivo and in vitro, and miR-211 overexpression protects cerebral I/R injury by suppressing cell apoptosis (Liu et al., 2020). MiR-149-5p exerts essential roles in different types of human cancers such as osteosarcoma (Xu et al., 2018), gastric cancer (Zhang et al., 2019), and medullary thyroid carcinoma (Ye & Chen, 2019). Recently, Teertam et al. found that miR-149-5p level is downregulated in rats after I/R injury, and resveratrol treatment increases miR-149-5p activity and protects the brain against ischemia injury by targeting p53 (Teertam et al., 2020). However, the potential mechanisms of miR-149-5p involved in inflammation and apoptosis during cerebral I/R injury remain unclear.
Notch signal is a highly conserved pathway, and notch homolog protein (Notch) 1–4 are four receptors for Notch pathway in mammals (D’Souza et al., 2008, 2010). Notch1–4 can be bound by a ligand to activate Notch signaling in the canonical pathways (Pan & Rubin, 1997). It has been reported that Notch pathway is associated with many cellular processes such as cell differentiation, proliferation, apoptosis, and regeneration (Siebel & Lendahl, 2017). In addition, Notch signaling also regulates the inflammatory responses during cerebral I/R injury. Li et al. revealed that Notch1 upregulation participates in inflammatory responses after cerebral I/R injury by increasing inflammatory cytokine release (Li et al., 2019a). In cerebral I/R injury, oxygen and glucose deprivation/reoxygenation (OGD)/R treatment enhances Notch2 expression, and miR-485-5p overexpression effectively reduces OGD/R-induced Notch2 upregulation, enhances cell viability, and decreases oxidative stress and apoptotic rate in SH-SY5Y cells (Chen et al., 2020a). These results indicate that Notch2 may be a direct target of miRNAs to affect the development of cerebral I/R injury.
In the present study, we confirmed that miR-149-5p was downregulated during cerebral I/R injury, both in vitro and in vivo. Overexpression of miR-149-5p effectively attenuated middle cerebral artery occlusion (MCAO)-induced brain damages in rats and OGD-induced cortical neurons, as well as the secretion of pro-inflammatory cytokines TNF-α, IL-4, IL-6, IL-1β, and COX-2. Meanwhile, Notch2 was identified as a target of miR-149-5p and Notch2 overexpression reduced OGD-induced cortical neurons in vitro. Taken together, our study revealed a novel mechanism of miR-149-5p in cerebral I/R injury, indicating that miR-149-5p might be an effective therapeutic target for ischemic stroke.
Materials and Methods
Animal Model
A total of 24 male Sprague–Dawley (SD) rats (10–12 weeks, weighing 260–320 g) were purchased from the Laboratory Animal Center of School of Basic Medical Sciences, Chongqing Medical University and kept in a pathogen-free condition (23 ± 2 °C, 55% ± 5% humidity) on a 12-h light/12-h dark cycle. All animal procedures were approved by the Animal Care and Use Committee of Chongqing Medical University. Rats with I/R injury were induced by the MCAO method as previously described (Kuts et al., 2019). Rats were divided into four groups, namely I/R group, sham group, I/R + control mimics group and I/R + miR-149-5p mimics group with six rats in each group. Rats in the I/R group underwent 2 h ischemia followed by 24 h reperfusion. Rats in the sham group underwent the same procedure without occluding. Rats in the I/R + control mimics group underwent 2 h ischemia, and afterward were injected with 20 μΜ scramble miRNA (miR-NC)/mouse through intracerebroventricular injection, followed by 24 h reperfusion. Rats in the I/R + miR-149-5p mimics group underwent 2 h ischemia, and then were injected with 20 μΜ miR-149-5p mimics/mouse through the intracerebroventricular injection, followed by 24 h reperfusion. After reperfusion, all rats were anesthetized by intraperitoneally injecting 100 mg/kg sodium pentobarbital and sacrificed by cervical dislocation. The brains were then collected for subsequent experiments.
Neurological Deficit
After 24 h reperfusion, the neurological deficit of rats were evaluated using the ZeaLong scoring method as previously described (Longa et al., 1989), in which, Score 0 for no neurological deficit; Score 1 for failure to extend the contralateral forelimb entirely; Score 2 for circling if pulled by the tail but normal posture at rest; Score 3 for circling spontaneously; and Score 4 for no spontaneous activity and a sluggish level of consciousness.
Determination of Infarct Volume
Infarct volume was evaluated using 2% 2,3,5-triphenyltetrazolium chloride (TTC) staining as previously described (Ou et al., 2017). In brief, after 24 h reperfusion, brains were isolated and cut into 2-mm thick coronal sections. These sections were then incubated with TTC solution (Sigma, 17,779, USA) for 20 min at 37 °C. Subsequently, the sections were fixed in 10% formaldehyde and observed using a digital scanner (HP Scanjet 200, USA), and infarct volume was evaluated by an image analysis software (ImageJ) according to the following formula: infarct percentage = total infarct volume/total brain volume × 100%.
Determination of Brain Water Content
After scoring neurological deficits, rats were sacrificed by cervical dislocation. The brains were weighed to obtain the wet weight. After 24 h of drying in an oven at 120 °C, the brains were weighed again to obtain the dry weight. Brain water content was calculated according to the following formula: (wet mass—dry mass)/wet mass × 100%.
Cell isolation and OGD/R Model
Rat primary cortical neurons were isolated from SD rats as described in the previous study (Zhou et al., 2016). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) at 37 °C in 5% CO2. Cells were transfected with either negative control or miR-149-5p mimic plasmids and divided into 4 groups, control, OGD/R, OGD/R + negative control (NC mimics), and OGD/R + miR-149-5p mimics groups. To simulate I/R injury, primary cortical neurons were induced by OGD/R as previously described (Morán et al., 2017). In brief, cortical neurons were exposed to glucose-free DMEM in an incubator of 5% CO2 and 95% N2 (OGD) for 2 h. Then cells were transferred into glucose-containing DMEM in an incubator of 5% CO2 and 95% O2 (reoxygenation) for 12 h. Cells were collected for subsequent experiments.
Luciferase Reporter Assay
Targetscan was used to predict putative binding sites between miR-149-5p and Notch2. The wild-type (WT) and mutant (MUT) putative binding sites of Notch2 were cloned into the pmiRGLO luciferase reporter vector (Promega, Madison, WI, USA). Then miR-149-5p mimics or miR-NC was co-transfected with Luc-Notch2-WT or Luc-Notch2-MUT into cortical neurons using Lipofectamine 2000 reagent (ThermoFisher Scientific). After transfection for 48 h, the relative luciferase activity was detected using the Dual Luciferase Assay System (Promega).
Cell Transfection
MiR-149-5p mimics and negative control (miR-NC) were obtained from GenePharma Co., Ltd. (Shanghai, China). To overexpress Notch2, the full length of Notch2 was synthesized (GenePharma) and cloned into pcDNA3.1 Expression Vector to generate pcDNA-Notch2, and the empty vector pcDNA3.1 was used as the negative control (pc-NC). 50 nM MiR-149-5p mimics/miR-NC or 5 ng overexpression vector were transfected into primary cortical neurons using Lipofectamine 2000 reagent (ThermoFisher Scientific). After transfection for 48 h, cells were exposed to OGD/R treatment. The sequences were as follows: miR-149-5p mimics: 5′-UCAGUGCAUCA CCGACCCCUGUAAGGGUCUGUGAUGCACUGAAU-3′; miR-149-5p inhibitor: 5′-UCCUUCGGAAGUGUCACGUTTACGUGACAAGUUCCGAGATTT-3′; miR-NC: 5′-AACCAGGUCUGUGAUGCACUGA-3′ and 5′-ACCAAGUUCUGUGAUG CACUGA-3′; pcDNA-Notch2: 5′-GCAGTTATTTFAFTFAATA-3′; si-Notch2: 5′-CTT CCAGGTTCGATTAATT-3′.
RNA Isolation and qRT-PCR
Total RNA of rat brains and cultured cells was extracted using Trizol reagent (ThermoFishier Scientific). A total of 2 µg RNA was reversely transcribed into cDNA using Superscript II reverse transcriptase kit (Invitrogen). All PCR reactions were performed on the 7500 realtime PCR machine (Applied Biosystems) with a SYBR Premix Ex Taq TM II (Takara). Notch2 expression level was normalized to β-actin, and miR-149-5p level was normalized to U6. The relative expression change of targets was calculated using the 2−ΔΔCt method. The primers were miR-149-5p forward 5′-CGTG CCGTTCATTTGGTATAAAC-3′ and reverse 5′-GAGCAGGGTCCGAGGT-3′; U6 forward 5′-GCTTCGGCAGCACATATACTTTAAAT-3′ and reverse 5′-AGTGCGAA CTGTGCCGAT-3′; Notch2 forward 5′-GGTCCACGGCGGACCGGT-3′ and reverse 5′-GACCCCGAGAACGTGGTGCGC-3′; β-actin forward 5′-GTGGGCCGCCCC A GGCAACA-3′ and reverse 5′-CTCCTTAATGTCACGCACGAATTC-3′.
Western Blot
Total protein samples of rat brains and cultured cells were extracted by RIPA lysis buffer (Beyotime, Shanghai, China). Approximately, equal amounts of protein samples were separated by SDS-PAGE and transferred onto PVDF membrane (Millipore, USA). The membranes were incubated with specific primary antibodies including Notch2 (1:500, Abcam), Bcl-2 (1:500, Abcam), Bax (1:500, Abcam), COX-2 (1:500, Abcam), Fas (1:500, Abcam), Hsp70 (1:500, Abcam) and β-actin (1:2000, Abcam) overnight at 4 °C. On the following day, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti‑rabbit second antibody (1:10,000, Abcam) for 2 h at room temperature. The bound proteins were visualized using the ECL chemiluminescent detection kit, and the intensity was analyzed using ImageJ software.
ELISA
The expression levels of pro-inflammatory cytokines TNF-α, IL-4, IL-6, and IL-1β in the supernatant of cultured cortical neurons were detected using the specific ELISA kits (Puxin, Shanghai, China) according to manufacturers’ instructions, respectively.
Caspase Activity Assay
Caspase 3 activity was detected using the caspase 3 detection kit (Promega) according to the manufacturer’s instructions. Briefly, cortical neurons were incubated with Caspase-Glo reagent (provided by the specific kit) for 20 min, and the absorbance values at 560 nm were measured using a microplate reader.
Apoptosis Analysis
Cell apoptosis was detected using the Annexin-V-fluorescein isothiocyanate Apoptosis Detection Kit (Beyotime) according to the manufacturer’s instructions. In brief, after treatment, cells were stained with 10 µL fluorescein isothiocyanate-Annexin-V for 1 h in the dark. The apoptotic cells (Annexin-V positive cells) were analyzed using a flow cytometer (Beckman, Boulevard Brea, CA, USA).
Statistical Analysis
All data were presented as mean ± standard deviation (mean ± SD). Statistical analysis was performed using SPSS statistical software (version 16.0, SPSS, Chicago, IL, USA). All experiments were repeated three times independently. Difference was determined by Student’s t-tests (two groups) and one-way ANOVA (multiple groups). P < 0.05 was considered as the significant threshold.
Results
MiR-149-5p was Significantly Downregulated in I/R and OGD Model
To explore the role of miR-149-5p in I/R injury, in vivo I/R rat model and in vitro OGD model were used. We found that the expression of miR-149-5p was significantly downregulated in the brain tissues of the rat model induced by MCAO/R (p < 0.01, Fig. 1A) and primary cortical neurons induced by OGD/R (p < 0.01, Fig. 1B). The intracerebroventricular injection of miR-149-5p mimics into rats significantly enhanced the expression of miR-149-5p in the brain tissues compared with that of I/R + NC mimics group (p < 0.01, Fig. 1A). Moreover, the transfection of miR-149-5p mimics into cortical neurons also markedly increased the expression of miR-149-5p compared with that of OGD/R + NC mimics group (p < 0.01, Fig. 1B). These results indicated that miR-149-5p is potentially associated with I/R injury.
MiR-149-5p was significantly downregulated in I/R and OGD models. Shown are the expression of miR-149-5p evaluated by qRT-PCR (A) in the brain tissues of rats in the sham group, I/R group, I/R + NC mimics group and I/R + miR-149-5p mimics group, and B in primary cortical neurons in the control group, OGD/R group, OGD/R + NC mimics and OGD/R + miR-149-5p mimics group. A total of 24 rats were used, with six in each group. Data are presented as mean ± SD. **p < 0.01 vs sham group or control group; ##p < 0.01 vs I/R + NC mimics group or OGD/R + NC mimics group
Overexpression of miR-149-5p-Alleviated Cerebral I/R Injury In Vivo
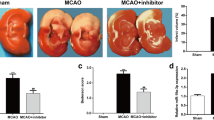
We then assessed the effect of miR-149-5p on infarct volumes, neurological scores, and brain water content. As shown in Fig. 2A and B, MCAO/R significantly increased the infarct volume of rats compared with the sham operation (p < 0.01), and the intracerebroventricular injection of miR-149-5p mimics effectively reduced infarct volume of MCAO/R-induced rats compared with that of I/R + NC mimics group (p < 0.01, Fig. 2A and B). Meanwhile, MCAO/R treatment significantly elevated neurological score and brain water content of rats compared with the sham operation (p < 0.01), and intracerebroventricular injection of miR-149-5p mimics effectively attenuated the effects of MCAO/R on the neurological score and brain water content in rats compared with NC mimics (p < 0.05, Fig. 2C and D). These results demonstrated that overexpression of miR-149-5p could effectively alleviate cerebral I/R injury in vivo.
Overexpression of miR-149-5p-alleviated cerebral I/R injury in vivo. At 2 h after ischemia, rats were intracerebroventricularly injected with miR-149-5p mimics or NC mimics followed by 24 h of reperfusion. A The representative TTC stains of six corresponding coronal brain sections, where ischemic infarctions are white. B Infarct volume evaluated by ImageJ. C Neurological scores. D Brain water content. A total of 24 rats were used, with six in each group. Data are presented as mean ± SD. **p < 0.01 vs sham group; ##p < 0.01 vs I/R + NC mimics group
Overexpression of miR-149-5p Reduced the Expression of Pro-Inflammatory Cytokines in OGD-Treated Primary Cortical Neurons
It has been reported that secretion of pro-inflammatory cytokines from cortical neurons is one of the key elements for cerebral I/R injury (Zhao et al., 2015). Hence, we investigated the effect of miR-149-5p on the expression of several pro-inflammatory cytokines. We found that OGD/R treatment significantly increased the expression of inflammatory cytokines TNF-α, IL-4, IL-6, IL-1β, and COX-2 in primary cortical neurons compared with the control (p < 0.01), and overexpression of miR-149-5p reduced OGD/R-induced elevation of these inflammatory cytokines in primary cortical neurons compared with NC mimics group (p < 0.01, Fig. 3A–E). These results suggested that miR-149-5p might function via anti-inflammation in I/R injury.
Overexpression of miR-149-5p reduced the expression of inflammatory cytokines in OGD-treated primary cortical neurons. Primary cortical neurons were transfected with miR-149-5p mimics or control mimics and then subjected to OGD/R treatment. Shown are the expressions of inflammatory cytokines TNF-α (A), IL-4 (B), IL-6 (C), and IL-1β (D) detected by respective ELISA kits and the expression of COX-2 (E) detected by Western blot. Data are presented as mean ± SD. **p < 0.01 vs control group; ##p < 0.01 vs OGD/R + NC mimics group
Overexpression of miR-149-5p Inhibited OGD/R-Induced Neuronal Apoptosis In Vitro
Previous studies have demonstrated that neuronal apoptosis was also a leading cause of I/R injury (Liang et al., 2014). Then we explored the effect of miR-149-5p on neuronal apoptosis and found that OGD/R treatment significantly enhanced apoptosis of primary cortical neurons compared with the control (p < 0.01), and overexpression of miR-149-5p effectively reduced OGD/R-induced neuronal apoptosis compared with NC mimics group (p < 0.01, Fig. 4A). Meanwhile, OGD/R treatment significantly elevated caspase 3 activity and the expression of Bax and Fas, while decreased the expression of Bcl-2 and Hsp70 in cortical neurons compared with the control (p < 0.01). Moreover, overexpression of miR-149-5p effectively reversed the effects of OGD/R treatment on the expression of these apoptosis-related indexes (p < 0.01, Fig. 4B and C). These results demonstrated that miR-149-5p exerted a protective role in cerebral I/R injury through anti-apoptotic activity.
Overexpression of miR-149-5p inhibited OGD/R-induced neuronal apoptosis in vitro. Primary cortical neurons were transfected with miR-149-5p mimics or control mimics and then subjected to OGD/R treatment. Shown are (A) apoptosis of cortical neurons evaluated by flow cytometry, B caspase 3 activity detected using the caspase 3 detection kit, and C expression of Bcl-2, Hsp70, Bax, and Fas detected by Western blot. Data are presented as mean ± SD. **p < 0.01 vs control group. ##p < 0.01 vs OGD/R + NC mimics group
Notch2 was a Target of miR-149-5p
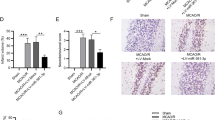
To investigate the specific mechanism of miR-149-5p in I/R injury, Targetscan was used to predict the potential targets of miR-149-5p, and the results indicated that Notch2 is a potential target of miR-149-5p (Fig. 5A). Meanwhile, overexpression of miR-149-5p significantly reduced the relative luciferase activity of Luc-Notch2-WT in cortical neurons compared with miR-NC (p < 0.01) but did not affect the luciferase activity of Luc-Notch2-MUT (Fig. 5B). Moreover, OGD/R treatment significantly increased the expression of Notch2 in cortical neurons compared with the control (p < 0.01), and overexpression of miR-149-5p effectively reduced OGD/R-induced elevation of Notch2 expression compared with the NC mimics (p < 0.01, Fig. 5C and D). In addition, cortical neurons were transfected with pcDNA-Notch2 or pc-NC, and then exposed to OGD/R treatment. The transfection efficiency was confirmed, and the results revealed that OGD/R treatment significantly increased Notch2 expression compared with the control (p < 0.01). In addition, Notch2 overexpression effectively increased the expression of Notch2 in OGD/R-treated cortical neurons compared with pc-NC (p < 0.05, Fig. 5E and F), but significantly reduced miR-149-5p the expression in OGD/R-treated cortical neurons (p < 0.01, Fig. 5G). These results demonstrated that Notch2 is a target of miR-149-5p and might partially mediate the role of miR-149-5p in I/R injury.
Notch2 was a target of miR-149-5p. A The putative binding site between miR-149-5p and Notch2 predicted by Targetscan. B Relative luciferase activity of Luc-Notch2-WT/MUT in cortical neurons detected by dual luciferase reporter system. **p < 0.01. C, D Expression of Notch2 evaluated by qRT-PCR (C) and Western blot (D) in primary cortical neurons transfected with miR-149-5p mimics or control mimics and subsequently subjected to OGD/R treatment. Data are presented as mean ± SD. **p < 0.01 vs control group. ##p < 0.01 vs OGD/R + NC mimics group. E–G Expression of Notch2 evaluated by qRT-PCR E and Western blot (F) in primary cortical neurons transfected with pcDNA-Notch2 or pc-NC and subsequently exposed to OGD/R treatment. G Expression of miR-149-5p evaluated by qRT-PCR. **p < 0.01 vs control group; #p < 0.05 vs OGD/R + pc-NC group
Overexpression of Notch2 Attenuated the Inhibitory Effect of miR-149-5p Mimics on OGD/R-Induced Neuronal Apoptosis In Vitro
To further determine whether Notch2 mediated the effect of miR-149-5p on I/R injury, cortical neurons were transfected with NC mimics, miR-149-5p mimics, or miR-149-5p mimics plus pcDNA-Notch2, and then subjected to OGD/R treatment. Flow cytometry assay revealed that OGD/R treatment significantly induced neuronal apoptosis compared with the control (p < 0.01), and overexpression of miR-149-5p effectively reduced OGD/R-induced neuronal apoptosis compared with the NC mimics (p < 0.01), while co-transfection of miR-149-5p mimics and pcDNA-Notch2 significantly attenuated the inhibitory effect of miR-149-5p mimics on apoptosis in OGD/R-treated cortical neurons (p < 0.01, Fig. 6A). As expected, OGD/R treatment significantly elevated caspase 3 activity and Bax and Fas expressions while decreased the expression of Bcl-2 and Hsp70 in cortical neurons compared with the control (p < 0.01). Moreover, overexpression of miR-149-5p effectively reversed the effects of OGD/R treatment on the expression of these apoptosis-related indexes (p < 0.01), and these effects of miR-149-5p mimics were reversed by additional Notch2 (p < 0.01, Fig. 6B and C). Together, these results demonstrated that miR-149-5p exerted a protective role in I/R injury through by the Notch2-mediated anti-apoptotic and anti-inflammatory pathway.
Overexpression of Notch2 attenuated the inhibitory effect of miR-149-5p mimics on OGD/R-induced neuron apoptosis in vitro. Cortical neurons were transfected with NC mimics, miR-149-5p mimics, or co-transfected with miR-149-5p mimics and pcDNA-Notch2, and then submitted to OGD/R treatment. A Apoptosis of cortical neurons evaluated by flow cytometry. B Caspase 3 activity detected using the caspase 3 detection kit. C Expression of Bcl-2, Hsp70, Bax, and Fas detected by Western blot. Data are presented as mean ± SD. **p < 0.01 vs control group; ##p < 0.01 vs OGD/R + NC mimics group; &&p < 0.01 vs OGD/R + miR-149-5p mimics group
Discussion
So far, ischemic stroke represents a considerable social and healthy burden due to the complex processes involved in I/R injury, including apoptosis and inflammation (Yu et al., 2019). Although reperfusion of ischemic brain tissues is the most common therapeutic strategy to prevent ischemic stroke, it may further cause brain damages and even death (Benakis et al., 2016; Choudhury & Ding, 2016). Therefore, identifying potential diagnostic and therapeutic targets may contribute to developing effective agents or drugs for ischemic stroke. In this study, we confirmed the downregulation of miR-149-5p in MCAO-induced rats and determined that miR-149-5p was significantly downregulated in OGD-induced primary cortical neurons. Overexpression of miR-149-5p effectively protected brain damages in vivo and reduced cortical neurons apoptosis and expression of pro-inflammatory cytokines in vitro. Moreover, miR-149-5p negatively regulated Notch2 by binding to 3′-UTR and inhibiting its transcription, and Notch2 overexpression reversed the inhibitory effect of miR-149-5p overexpression on cortical neurons apoptosis in vitro. Our study revealed a new molecular mechanism involved in the neuroprotective function of miR-149-5p in cerebral I/R injury.
As a class of non-coding RNAs, miRNAs are crucial regulators in the development of cerebral I/R injury through different manners. For example, miR-128-3p, miR-148a-3p, and miR-210 reportedly inhibit oxidative stress pathway (Jiang et al., 2015; Li et al., 2019b; Zeng et al., 2019). MiR-1247-3p, miR-224-3p and miR-19a reportedly protect neurons from apoptosis (Deng et al., 2019; Ge et al., 2019; Zhang et al., 2019). However, some miRNAs, which play essential roles in other human diseases, have not been well studied in cerebral I/R injury. Here, we revealed that miR-149-5p was downregulated in MCAO-induced rats and OGD-induced primary cortical neurons. Overexpression of miR-149-5p effectively reduced MCAO/R-induced infarct volume, neurological score, and brain water content in rats. These results demonstrated that targeting miR-149-5p might be an effective therapeutic strategy for brain ischemia. One previous study indicated that resveratrol administration upregulated miR-149-5p and then protected against ischemia via p53 in rat model (Teertam et al., 2020). Our study confirmed the protective role of miR-149-5p in cerebral I/R injury and demonstrated that well understanding of the specific mechanism of miR-149-5p might contribute to developing new therapeutic strategies for ischemic stroke.
Apoptosis, also called programmed cell death, is a crucial mechanism for secondary damage in brain tissues after cerebral I/R injury (Kalinichenko & Matveeva, 2007). Increasing evidence has demonstrated that apoptosis participates in the activation of physiological cellular processes, and promotes pathological damages under environmental stimulation, resulting in disorders such as stroke (Radak et al., 2017). To explore the potential mechanism of miR-149-5p, we used Targetscan and found that Notch2 was the only candidate involved in cell apoptosis except for p53 in the previous study (Teertam et al., 2020). The direct relationship between miR-149-5p and Notch2 was determined by luciferase reporter assay. Overexpression of miR-149-5p significantly reduced OGD-induced cortical neurons apoptosis, and this effect was reversed by co-transfection of miR-149-5p and pcDNA-Notch2. The expression levels of Bcl-2 and Hsp70, two anti-apoptotic proteins, and Bax and Fas, two pro-apoptotic proteins, have been used as significant indicators for determining the occurrence of apoptosis (Liu et al., 2017; Roufayel & Kadry, 2019) because of their close correlation with the apoptosis phenotype. These results revealed that miR-149-5p/Notch2 pathway participated in cerebral I/R injury through regulating neurons apoptosis.
It has been reported that cytokines are upregulated in the brain after various stress stimulation, including cerebral ischemia (Jin et al., 2010). TNF-α, IL-4, IL-6, and IL-1β are among the most important cytokines associated with inflammation in cerebral ischemia (Han & Yenari, 2000). Inhibition of TNF-α attenuates ischemic brain injury, and administration of recombinant TNF-α antibody after stroke onset aggravates ischemic brain damage (Yang et al., 1998). Previous studies reported that mice with IL-1β deficiency had smaller infarcts than wild-type mice (Yamasaki et al., 1995). A previous study has revealed that Notch1 participated in inflammatory responses after cerebral I/R injury by promoting the release of inflammatory cytokines (Li et al., 2019a). Here, our results demonstrated that miR-149-5p overexpression effectively reduced OGD/R-induced release of inflammatory cytokines in primary cortical neurons. However, whether the effect of miR-149-5p on inflammation is mediated by Notch2 needed to be further confirmed.
Conclusion
In summary, our study demonstrated that miR-149-5p played a protective role in cerebral I/R injury through downregulating Notch2 and subsequent inhibition of inflammation and apoptosis, which extended our understanding of miRNAs in ischemic stroke.
Data Availability
The dataset generated during this study is available from the corresponding author upon reasonable request.
References
Barthels, D., & Das, H. (2020). Current advances in ischemic stroke research and therapies. Biochimica et Biophysica Acta. Molecular Basis of Disease, 1866, 165260. https://doi.org/10.1016/j.bbadis.2018.09.012
Benakis, C., et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nature Medicine, 22, 516–523. https://doi.org/10.1038/nm.4068
Chai, Z., Gong, J., Zheng, P., & Zheng, J. (2020). Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion injury by targeting IGFBP3 in vivo and in vitro. Biological Research, 53, 17. https://doi.org/10.1186/s40659-020-00280-9
Chen, L., Heikkinen, L., Wang, C., Yang, Y., Sun, H., & Wong, G. (2019). Trends in the development of miRNA bioinformatics tools. Briefings in Bioinformatics, 20, 1836–1852. https://doi.org/10.1093/bib/bby054
Chen, X., Zhang, S., Shi, P., Su, Y., Zhang, D., & Li, N. (2020). MiR-485-5p promotes neuron survival through mediating Rac1/Notch2 signaling pathway after cerebral ischemia/reperfusion. Current Neurovascular Research, 17, 259–266. https://doi.org/10.2174/1567202617666200415154822
Chen, Z., Yang, J., Zhong, J., Luo, Y., Du, W., Hu, C., Xia, H., Li, Y., Zhang, J., Li, M., Yang, Y., Huang, H., Peng, Z., Tan, X., & Wang, H. (2020). MicroRNA-193b-3p alleviates focal cerebral ischemia and reperfusion-induced injury in rats by inhibiting 5-lipoxygenase expression. Experimental Neurology, 327, 113223. https://doi.org/10.1016/j.expneurol.2020.113223
Choudhury, G. R., & Ding, S. (2016). Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiology of Disease, 85, 234–244. https://doi.org/10.1016/j.nbd.2015.05.003
Deng, Y., Ma, G., Dong, Q., Sun, X., Liu, L., Miao, Z., & Gao, F. (2019). Overexpression of miR-224–3p alleviates apoptosis from cerebral ischemia reperfusion injury by targeting FIP200. Journal of Cellular Biochemistry, 120, 17151–17158. https://doi.org/10.1002/jcb.28975
D’Souza, B., Meloty-Kapella, L., & Weinmaster, G. (2010). Canonical and non-canonical Notch ligands. Current Topics in Developmental Biology, 92, 73–129. https://doi.org/10.1016/s0070-2153(10)92003-6
D’Souza, B., Miyamoto, A., & Weinmaster, G. (2008). The many facets of Notch ligands. Oncogene, 27, 5148–5167. https://doi.org/10.1038/onc.2008.229
Ge, X. L., Wang, J. L., Liu, X., Zhang, J., Liu, C., & Guo, L. (2019). Inhibition of miR-19a protects neurons against ischemic stroke through modulating glucose metabolism and neuronal apoptosis. Cellular & Molecular Biology Letters, 24, 37. https://doi.org/10.1186/s11658-019-0160-2
Ghafouri-Fard, S., Shoorei, H., & Taheri, M. (2020). Non-coding RNAs participate in the ischemia-reperfusion injury. Biomedicine & Pharmacotherapy, 129, 110419. https://doi.org/10.1016/j.biopha.2020.110419
Granger, D. N., & Kvietys, P. R. (2015). Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biology, 6, 524–551. https://doi.org/10.1016/j.redox.2015.08.020
Han, H. S., & Yenari, M. A. (2003). Cellular targets of brain inflammation in stroke. Current opinion in investigational drugs (London, England: 2000), 4, 522–529.
Jiang, Y., Li, L., Tan, X., Liu, B., Zhang, Y., & Li, C. (2015). miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. Journal of Neurochemistry, 134, 173–181. https://doi.org/10.1111/jnc.13097
Jin, R., Yang, G., & Li, G. (2010). Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. Journal of Leukocyte Biology, 87, 779–789. https://doi.org/10.1189/jlb.1109766
Kalinichenko, S. G., & Matveeva, N. (2007). Morphological characteristic of apoptosis and its significance in neurogenesis. Morfologiia (Saint Petersburg, Russia), 131, 16–28.
Kuts, R., Melamed, I., Shiyntum, H. N., Frank, D., Grinshpun, J., Zlotnik, A., Brotfain, E., Dubilet, M., Natanel, D., & Boyko, M. (2019). A middle cerebral artery occlusion technique for inducing post-stroke depression in rats. Journal of Visualized Experiments: JoVE. https://doi.org/10.3791/58875
Li, H., Ma, J., Fang, Q., Li, H., Shen, H., Li, X., Xue, Q., Zhu, J., & Chen, G. (2019). Botch protects neurons from ischemic insult by antagonizing Notch-mediated neuroinflammation. Experimental Neurology, 321, 113028. https://doi.org/10.1016/j.expneurol.2019.113028
Li, R., Li, X., Wu, H., Yang, Z., Fei, L., & Zhu, J. (2019). Theaflavin attenuates cerebral ischemia/reperfusion injury by abolishing miRNA-128-3p-mediated Nrf2 inhibition and reducing oxidative stress. Molecular Medicine Reports, 20, 4893–4904. https://doi.org/10.3892/mmr.2019.10755
Liang, K., Ye, Y., Wang, Y., Zhang, J., & Li, C. (2014). Formononetin mediates neuroprotection against cerebral ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2 ratio and upregulation PI3K/Akt signaling pathway. Journal of the Neurological Sciences, 344, 100–104. https://doi.org/10.1016/j.jns.2014.06.033
Liu, Q. S., Deng, R., Li, S., Li, X., Li, K., Kebaituli, G., Li, X., & Liu, R. (2017). Ellagic acid protects against neuron damage in ischemic stroke through regulating the ratio of Bcl-2/Bax expression. Applied Physiology, Nutrition, and Metabolism, 42, 855–860. https://doi.org/10.1139/apnm-2016-0651
Liu, W., Miao, Y., Zhang, L., Xu, X., & Luan, Q. (2020). MiR-211 protects cerebral ischemia/reperfusion injury by inhibiting cell apoptosis. Bioengineered, 11, 189–200. https://doi.org/10.1080/21655979.2020.1729322
Longa, E. Z., Weinstein, P. R., Carlson, S., & Cummins, R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20, 84–91. https://doi.org/10.1161/01.str.20.1.84
Lu, T. X., & Rothenberg, M. E. (2018). MicroRNA. The Journal of Allergy and Clinical Immunology, 141, 1202–1207. https://doi.org/10.1016/j.jaci.2017.08.034
Morán, J., Perez-Basterrechea, M., Garrido, P., Díaz, E., Alonso, A., Otero, J., Colado, E., & González, C. (2017). Effects of estrogen and phytoestrogen treatment on an in vitro model of recurrent stroke on HT22 neuronal cell line. Cellular and Molecular Neurobiology, 37, 405–416. https://doi.org/10.1007/s10571-016-0372-1
Ou, J., Kou, L., Liang, L., & Tang, C. (2017). MiR-375 attenuates injury of cerebral ischemia/reperfusion via targetting Ctgf. Bioscience reports. https://doi.org/10.1042/bsr20171242
Pan, D., & Rubin, G. M. (1997). Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell, 90, 271–280. https://doi.org/10.1016/s0092-8674(00)80335-9
Phipps, M. S., & Cronin, C. A. (2020). Management of Acute Ischemic Stroke. BMJ (Clinical Research Ed.), 368, l6983. https://doi.org/10.1136/bmj.l6983
Powers, W. J. (2020). Acute Ischemic Stroke. New England Journal of Medicine, 383, 252–260. https://doi.org/10.1056/NEJMcp1917030
Prabhakaran, S., Ruff, I., & Bernstein, R. A. (2015). Acute stroke intervention: A systematic review. JAMA, 313, 1451–1462. https://doi.org/10.1001/jama.2015.3058
Radak, D., Katsiki, N., Resanovic, I., Jovanovic, A., Sudar-Milovanovic, E., Zafirovic, S., Mousad, S. A., & Isenovic, E. R. (2017). Apoptosis and acute brain ischemia in ischemic stroke. Current Vascular Pharmacology, 15, 115–122. https://doi.org/10.2174/1570161115666161104095522
Roufayel, R., & Kadry, S. (2019). Molecular chaperone HSP70 and key regulators of apoptosis—a review. Current Molecular Medicine, 19, 315–325. https://doi.org/10.2174/1566524019666190326114720
Siebel, C., & Lendahl, U. (2017). Notch signaling in development, tissue homeostasis, and disease. Physiological Reviews, 97, 1235–1294. https://doi.org/10.1152/physrev.00005.2017
Teertam, S. K., Jha, S., & Prakash Babu, P. (2020). Up-regulation of Sirt1/miR-149–5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. Journal of Clinical Neuroscience, 72, 402–411. https://doi.org/10.1016/j.jocn.2019.11.043
Wang, J., Cao, B., Han, D., Sun, M., & Feng, J. (2017). Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging and Disease, 8, 71–84. https://doi.org/10.14336/ad.2016.0530
Xu, R. D., Feng, F., Yu, X. S., Liu, Z. D., & Lao, L. F. (2018). miR-149–5p inhibits cell growth by regulating TWEAK/Fn14/PI3K/AKT pathway and predicts favorable survival in human osteosarcoma. International Journal of Immunopathology and Pharmacology, 32, 2058738418786656. https://doi.org/10.1177/2058738418786656
Yamasaki, Y., Matsuura, N., Shozuhara, H., Onodera, H., Itoyama, Y., & Kogure, K. (1995). Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke, 26, 676–680. https://doi.org/10.1161/01.str.26.4.676 discussion 681.
Yang, G. Y., Gong, C., Qin, Z., Ye, W., Mao, Y., & Bertz, A. L. (1998). Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport, 9, 2131–2134. https://doi.org/10.1097/00001756-199806220-00041
Ye, X., & Chen, X. (2019). miR-149-5p inhibits cell proliferation and invasion through targeting GIT1 in medullary thyroid carcinoma. Oncology Letters, 17, 372–378. https://doi.org/10.3892/ol.2018.9628
Yu, S., Yu, M., He, X., Wen, L., Bu, Z., & Feng, J. (2019). KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging cell, 18, e12940. https://doi.org/10.1111/acel.12940
Zeng, J., Zhu, L., Liu, J., Zhu, T., & Xie, Z. (2019). Metformin protects against oxidative stress injury induced by ischemia/reperfusion via regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxidative Medicine and Cellular Longevity, 2019, 8768327. https://doi.org/10.1155/2019/8768327
Zhang, R., Zhou, W., Yu, Z., Yang, L., Liu, G., Yu, H., Zhou, Q., Min, Z., Zhang, C., Wu, Q., Hu, X. M., & Yuan, Q. (2019). miR-1247-3p mediates apoptosis of cerebral neurons by targeting caspase-2 in stroke. Brain research, 1714, 18–26. https://doi.org/10.1016/j.brainres.2019.02.020
Zhang, X., Wang, S., Wang, H., Cao, J., Huang, X., Chen, Z., Xu, P., Sun, G., Xu, J., Lv, J., & Xu, Z. (2019). Circular RNA circNRIP1 acts as a microRNA-149–5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Molecular Cancer, 18, 20. https://doi.org/10.1186/s12943-018-0935-5
Zhao, X., Wang, H., Sun, G., Zhang, J., Edwards, N. J., & Aronowski, J. (2015). Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. Journal of Neuroscience, 35, 11281–11291. https://doi.org/10.1523/jneurosci.1685-15.2015
Zhou, H. J., Wang, L. Q., Xu, Q. S., Fan, Z. X., Zhu, Y., Jiang, H., Zheng, X. J., Ma, Y. H., & Zhan, R. Y. (2016). Downregulation of miR-199b promotes the acute spinal cord injury through IKKβ-NF-κB signaling pathway activating microglial cells. Experimental Cell Research, 349, 60–67. https://doi.org/10.1016/j.yexcr.2016.09.020
Funding
This work was supported by the Science and Technology Project of Health Commission of Sichuan Province (Grant No. 18PJ430).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
All animal procedures were approved by the Animal Care and Use Committee of Chongqing Medical University.
Informed Consent
All authors have agreed to the contents of this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoya Wang and Qingbao Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, X., Xu, Q. & Wang, S. Overexpression of miR-149-5p Attenuates Cerebral Ischemia/Reperfusion (I/R) Injury by Targeting Notch2. Neuromol Med 24, 279–289 (2022). https://doi.org/10.1007/s12017-021-08685-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-021-08685-9