Abstract
The hallmark of multiple sclerosis (MS) pathogenesis is the breakdown of peripheral tolerance in the immune system. However, its molecular mechanism is not completely understood. Since long non-coding RNAs (lncRNAs) has played important roles in regulation of immunological pathways, here, we evaluated the expression of a novel lncRNA, TOB1-AS1, and its putative associated coding genes in the mechanism of maintaining immune tolerance in peripheral blood of MS patients to assess their possible roles in MS pathogenesis. In this study, 39 MS patients and 32 healthy matched controls were recruited. Real-time PCR standard curve method was used to quantify transcript levels of TOB1-AS1, TOB1, SKP2, and TSG. In addition, the potential sex hormone receptor binding sites on target genes promoter were analyzed using JASPR software. This work demonstrates a negative correlation between TOB1-AS1 expression and EDSS of patients. Also, a robust dysregulation of co-expression of TOB1-AS1 lncRNA and the coding genes in MS patients compared to controls was observed. Such dysregulation in this pathway may be related to MS pathogenesis and response to interferon treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is caused by interactions between genetic background and environmental factors (Farsani et al. 2015). Most MS patients are diagnosed with relapsing–remitting MS (RRMS) who have period of acute relapse followed by a time of remission. The majority of RRMS patients, eventually convert to secondary progressive MS (SPMS) stage in which neurological symptoms worsen steadily (Ewing et al. 2019). MS is more common in women with a female-to-male ratio of 3:1. According to previous studies, sex hormones impact on immune function in MS development (Ysrraelit and Correale 2019). Despite many studies, the etiology of MS remains undetermined (Vila et al. 2016). It has been suggested that breakdown of self-tolerance leads to the development of self-reactive lymphocytes in MS (Dendrou et al. 2015). Self-tolerance is maintained by controlling T and B cells activation, expansion, and survival in the blood (Sakaguchi et al. 2012). Identification of genetic markers can facilitate diagnosis, prediction, and prognosis of disease and monitoring clinical course and treatment responses (Gharesouran et al. 2019).
Former studies have revealed that changes in transcriptome and gene expression lead to immune system dysregulation in MS (Gharesouran et al. 2019). Also, several lines of evidence indicated that transcription factors are the main molecules regulating the quiescence of naïve T cells (Tzachanis et al. 2004). Transducer of ERBB2,1 (TOB1) is a transcriptional repressor and is related to TGF-B family signaling that maintains T cell quiescence through suppressing expression of IL-2 (Tzachanis et al. 2001). IL-2 is important for expansion and survival of responder T cells (Naghavi Gargari et al. 2015; Sakaguchi et al. 2012). Moreover, in vitro studies indicated that TOB1 is involved in regulation of twisted gastrulation (TSG) expression, an inhibitor of CD4 + T cell activation which upregulates after T cell receptor activation and probably controls aberrant T cell activation by enhancing TGF-B signaling (Tzachanis and Boussiotis 2009). TOB1 is located on 17q21, a candidate genomic region for MS susceptibility (Haines et al. 2003). Previous studies showed the potential involvement of TOB1 in the initiation of clinical isolation syndrome (CIS) (Corvol et al. 2008) and progression of MS in secondary progressive MS patients (Zastepa et al. 2014). In addition, Schulze-Topphoff reported that by inducing experimental autoimmune encephalomyelitis (EAE), Tob1−/− mice have early onset and more aggressive type of disease in comparison with wild-type (Schulze-Topphoff et al. 2013) and Tob1 expression decreased in mRNA and protein level in EAE model (Corvol et al. 2008). Although such studies represented a probable critical role of TOB1 in MS pathogenesis, unfortunately there is little information about its associated molecules with maintaining immune quiescence (Baranzini 2014).
Recently, it has been discovered that long non-coding RNAs (lncRNAs) can regulate the transcription of some coding genes, especially adjacent genes, and play important roles in immune cell responses. The aberrant expression of lncRNAs may lead to pathogenesis of diseases (Atianand and Fitzgerald 2014). According to RNA sequencing analysis, lncRNAs’ expression is altered in diverse neurological disorders including neurodegenerative diseases and neurologic autoimmune disorders (Gharesouran et al. 2019). Recent studies have shown evidence of lncRNAs role in the pathogenesis of MS as well as patients’ response to treatment; however, their expressions have not been thoroughly investigated in MS (Dastmalchi et al. 2018). TOB1-Anti Sense 1 (TOB1-AS1) is a novel lncRNA, located on the antisense strand of TOB1. Recently, RNA sequencing data showed that both TOB1-AS1 and TOB1 expression increase during differentiation of primary to effector T helper 1 (Spurlock III et al. 2015), and it is one of the Th1-spesific lncRNAs (Collier 2014). Moreover, the same as TOB1 (Maik-Rachline et al. 2019), this lncRNA has a role in cancer and functions as a novel tumor suppressor by sponging mir-27b in cervical cancer (Yao et al. 2018), gestational choriocarcinoma (de Mello et al. 2017), and breast cancer whose expression is positively correlated with ESR1 expression (Oskooei and Ghafouri-Fard 2019). Previous studies have revealed increased expression of mir-27b in MS patients and its role in MS pathogenesis (Dolati et al. 2018). Besides, this lncRNA is located on an expression quantitative trait locus (eQTL) after glucocorticoid receptor activation in major depressive disorder (Arloth 2014). Altogether, we could hypothesize that TOB1-AS1 may play a role in neurologic autoimmune disease pathogenesis including MS by impacting on expression of its neighbor gene, TOB1, or interacting with some proteins and RNAs in tolerogenic pathways (Mowel et al. 2018). On the other hand, in vitro studies have shown that TOB1 level is regulated by S-phase kinase-associated protein 2 (SKP2) which accelerates TOB1 ubiquitination and degradation (Hiramatsu et al. 2006). Since ubiquitin–proteasome system profile in MS patients is different from healthy controls (HCs) (Minagar et al. 2012), studying SKP2 expression in MS patients may uncover some vague and undiscovered aspects of MS pathogenesis.
In the present study, the expression of TOB1-AS1, TOB1, SKP2, and TSG genes as the putative key players in maintenance immune quiescence was analyzed in RRMS and SPMS patients versus HCs. Furthermore, since interferon-beta (INF-B), as an anti-inflammatory cytokine, regulates T cell activity and is used as a first-line therapy for MS (Dehghanian et al. 2018), the correlation among the expression of these genes and the probable impact of the INF-B treatment on the expression of target genes in patients’ peripheral blood mononuclear cells (PBMCs) were explored thoroughly.
Materials and Methods
Subjects
Thirty-nine MS patients were enrolled from the comprehensive MS clinic of Sina Hospital at Tehran University of Medical Sciences (Tehran, Iran). All patients were diagnosed according to McDonald criteria (McDonald et al. 2001). Included patients were diagnosed as RRMS or SPMS who did not take any immunomodulatory agents and glucocorticoids for at least the last 3 months in the time of sampling. In addition, RRMS patients who were under INF-B treatment were enrolled in this study. The exclusion criteria for patients were as follows: Hospitalization, pregnancy or breastfeeding, smoking (as cigarette smoking affects immune responsiveness) (Qiu et al. 2017), and having any other inflammatory or neurological disorders at the time of sampling. Moreover, 32 two age- and gender-matched volunteers were included in control group with no current chronic or acute infection and lack of personal or family neurological or autoimmune disorders. Furthermore, pregnant, lactating, and smoking individuals were excluded from the control group. The Declaration of Helsinki was regarded for researching on human participation. The study was approved by the ethics committee of Tarbiat Modares University and informed consent had been obtained from all participants prior to blood sampling.
RNA Extraction and cDNA Synthesis
PBMCs were isolated from fresh blood by Ficoll-Paque™ Lymphodex (InnoTrain, Sweden) density gradient centrifugation. Subsequently, total RNA was extracted using RiboEx solution (GenALL, Korea), following manufacturer’s instruction. The isolated RNAs were treated with DNase I (Fermentas, Lithuania) for 20 min at 37 °C. RNA concentration and integrity were examined by spectrophotometry and on 1% agarose electrophoresis gel, respectively. The reverse transcription was performed using 3 µg of total RNA, 200 units of M-MulV reverse transcriptase (Thermo scientific, USA), Oligo(dT)18, and random hexamer primers (MWG, Germany) in a total volume of 20 µl of reaction mixture, according to the manufacturer’s instruction.
Real-Time PCR
Specific primers were designed by AlleleID software and were analyzed in NCBI-primer blast to confirm their gene specificity (Table 1). The expression of target genes was evaluated using real-time PCR standard curve method by StepOne (Applied Biosystems, USA) with HOT FIREPol Eva Green (Solis BioDyne, Estonia). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was applied, as the internal control, to normalize the relative expression of the target genes. PCR was performed through the following instructions: an initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, and extension at 72 °C for 30 s. All reactions were tested at least twice and specificity of PCR products was verified by polyacrylamide gel electrophoresis and melt curve analysis. The expression of target genes mRNA was analyzed using 2−ΔCt and relative fold change of each gene was calculated by 2−ΔΔCT (Livak and Schmittgen 2001).
In Silico Analysis of Sex Hormones Binding Sites
To investigate the existence of potentially sex hormone receptor binding sites on the sequences of TOB1, TOB1-AS1, SKP2, and TSG genes, we used Jasper (http://jaspar.genereg.net/) (Sandelin et al. 2004) online software with a profile score thresholds of 80%, 90%, and 95%.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6.01 (GraphPad Software, Inc., San Diego, CA, USA) and the statistical package for the social sciences (SPSS, version 21). All categories of data were evaluated for normality distribution by Shapiro–Wilk test. Student’s t test and Mann–Whitney U test were used for parametric and non-parametric continuous variable, respectively. The expression levels of selected genes based on disease durations (RRMS and SPMS) were compared by one-way ANOVA, Tukey’s multiple comparison test. Correlations analyses were performed based on normality distribution by Pearson’s or Spearman's correlation coefficient. Controls and patients were matched for the purpose of gender by χ2 test. Eventually, the specificity and sensitivity of the expression of target genes as a biomarker were examined by the Receiver Operating Characteristic (ROC) curve. A p value ≤ 0.05 was considered significant.
Results
Clinical Characteristics of Patients
The mean age of disease onset was 30.1 ± 9 years and the mean of disease duration was 6.8 ± 6 years. Seventeen of RRMS patients and all of the SPMS patients were not treated by any immunomodulatory agents or glucocorticoids for at least 3 months in the time of sampling. Moreover, fourteen RRMS patients were being treated with CinnoVex (CinnaGen, Iran), an INF-B-based drug at doses of 30 µg once a week, in the research time. The clinical and demography characteristics of all MS patients and HCs are displayed in Table 2.
Upregulation of TOB1, TSG, and SKP2 Genes in PBMCs of MS Patients
The evaluation of target genes expression according to t-test revealed a significant increase of TOB1 (p = 0.0267, 1.6-fold), SKP2 (p = 0.0054, twofold), and TSG (p = 0.0108, 2.4-fold) in patients versus control individuals, although there was no significant difference in TOB1-AS1 expression in participants (p = 0.6725) (Fig. 1).
Analysis of TOB1 (a), TOB1-AS1 (b), SKP2 (c), and TSG (d) genes expression in MS patients (n = 39) in comparison with HCs (n = 32). The mRNA expression of TOB1 (a), SKP2 (c), and TSG (d) were upregulated significantly in MS patients (p < 0.05). However, we did not find any remarkable difference in TOB1-AS1 (b) in MS patients in comparison with HCs (p > 0.05). The gene expression values of each sample were normalized against GAPDH expression
Different Expression of TSG and SKP2 Among Subgroups of MS
One-way ANOVA test revealed a significant difference of TSG expression just in controls versus RRMS patients (p = 0.0155, Fig. 2a). Moreover, SKP2 expression levels demonstrated differences between our RRMS patients and healthy controls (p = 0.0218, Fig. 2b).
The Putative Effect of INF-B on the TOB1 Gene Expression
The assessments of the INF-B impact on the target genes expression showed clear differences in some groups. There were obvious differences between TOB1 expression in treated patients and untreated patients (p = 0.0324, Fig. 3a) and male treated patients versus male controls (p = 0.0033, Fig. 3b) according to Mann–Whitney U test.
Upregulation of SKP2, TSG, and TOB1 Genes in Male MS Patients
Using Mann–Whitney U test, we noticed a remarkable increase of SKP2 (p = 0.0247, Fig. 4a) and TSG (p = 0.0275, Fig. 4b) expression in male versus female in the controls, but interestingly, there was no significant difference in the expression of TOB1 (p = 0.0708, Fig. 4c) and TOB1-AS1 (p = 0.4816, Fig. 4d) in male controls versus female controls. In the following, statistical analysis revealed increased expression of SKP2 (p = 0.0019, Fig. 4e), TSG (p = 0.0079, Fig. 4f), and TOB1 (p = 0.0147, Fig. 4g), in male patients in comparison with male controls but not in TOB1-AS1 expression (p = 0.6597, Fig. 4h). However, there was no significant difference in their expression between female patients and female controls (data not shown).
The comparison of the expression of target genes in stratification for gender. The expression level of SKP2 (a) and TSG (b) were different between male controls and female controls. In addition, there was significant difference between male controls and male patients for the expression of SKP2 (e), TSG (f), and TOB1 (g). However, there were no differences in TOB1 expression between male and female controls (c) and in the case of TOB1-AS1 expression (d and h)
In Silico Analysis of Putative Sex Hormone Receptors Binding Sites on the Genes
The potential promoter regions of TOB1, TOB1-AS1, SKP2, and TSG were obtained from the Eukaryotic Promoter Database (EPD) (epd.vital-it.ch). Two sequences of GGA/TACANNNTGTTCT and GGTCAnnnTGACC were selected as androgen and estrogen receptor binding sites’ consensus sequences based on the Jasper algorithm (Driscoll et al. 1998; Roche et al. 1992), respectively. The numbers of potential binding sites for estrogen and androgen receptors in the promoters of selected genes are shown in Table 3. However, the number of androgen receptor hormone binding sites was lower than estrogen receptor binding site on examined genes; they were observed in each of the four target genes.
Co-expression Analysis
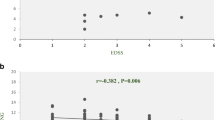
The correlations between the expression profiles of the selected genes together and with demographic features were analyzed. Spearman's analysis revealed a significant correlation between TOB1-AS1 expression profile with TOB1 in patients (p < 0.0001, r = 0.61), but not in the controls (Fig. 5a). Moreover, TOB1-AS1 expression correlated with SKP2 and TSG expression in both patients and controls (Fig. 5b, c, respectively). Besides, we found a significant correlation between TOB1 expression and TSG expression just in controls (p = 0.0297, r = 0.3847), but not in patients (Fig. 5d). In addition, we found a significant correlation just between TSG expression in patients with the age of onset (p = 0.006, r = 0.42) and the age at the time of analysis (p = 0.004, r = 0.44) (Data not shown). Also, TOB1-AS1 expression had reverse correlation with expanded disability status scale (EDSS) (p = 0.0348, r = − 0.348) (Fig. 6).
Correlation analysis between genes expression in controls and patients. The expression level of TOB1-AS1 correlated with TOB1 expression level in patients, but there was not any significant correlation between these genes in controls (a). In addition, there was significant correlation in both patients and controls between TOB1-AS1 and both SKP2 expression (b) and TSG expression (c). Moreover, there was a remarkable correlation between TOB1 and TSG expression just in controls (d)
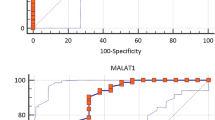
SKP2 and TSG as a Probable Biomarker to Clinical Diagnosis of MS
Finally, we analyzed the sensitivity and specificity of SKP2 and TSG as a probable biomarker to clinical diagnosis of MS by performing the ROC curve analysis and calculating the area under the curve (AUC) values. SKP2 and TSG had AUC value of 0.69 (95% CI 0.57 to 0.80, p = 0.006) and 0.67 (95% CI 0.55 to 0.78, p = 0.013), respectively. A value above 0.5 for AUC is considered to have reasonable discriminating ability to diagnose patients with or without the disease (Mandrekar 2010). The optimal cut-off values for SKP2 and TSG expression to discriminate HCs and MS patients were 9.08 (sensitivity: 61% and specificity: 75%) and 8.32 (sensitivity: 51.2% and specificity: 84.4%), respectively (data not shown).
Discussion
Several lines of evidence have proven that MS complications can be connected with defect in self-tolerance homeostasis (Gonsette 2012). However, the question, as how immune cells abnormally activate against myelin sheath, remains unanswered yet. In this work, we highlighted the interaction of an lncRNA, TOB1-AS1, and some putative associated coding genes with immune-tolerance regulatory role, TOB1, SKP2, and TSG, in MS pathogenesis.
Obtained results demonstrated the upregulation of TOB1 in PBMCs of MS patients in comparison with HCs. Such result in this study did not show a likely role for TOB1 in the pathogenesis of MS, as Basdeo et al. did not find significant difference in TOB1 expression in CD4 + T cells of CIS, RRMS, and controls (Basdeo et al. 2016). In addition, our results did not show any correlation between EDSS score of patients and TOB1 expression. However, the previous studies revealed that TOB1 expression decreased during disease progression (Corvol et al. 2008) and Zastepa et al. study showed that probably TOB1 expression decreases from CIS to SPMS (Zastepa et al. 2014). Therefore, further experiment is needed to determine the exact roles of this gene in MS disease.
Previous observations revealed that TSG expression is sharply upregulated after T cell activation and leads to inhibition of cytokine production of activated T cells (Tzachanis and Boussiotis 2009). In addition, modulating of TSG signaling has been proposed to control autoimmune disease (Tzachanis et al. 2007). However, its role in autoimmune pathogenesis including MS disease has not been discovered yet. In the current study, upregulation of TSG in MS patients might be a reaction of immune system against aberrant activation of immune cells. However, the expression of TOB1 had positive correlation with TSG just in controls. Apparently, TOB1 regulated TSG expression in a positive way, but in MS condition this regulatory system does not work completely efficiently. This result is in contrast with an in vitro study revealing TOB1 negatively regulates TSG expression (Tzachanis et al. 2007). This discrepancy can be because of environment-dependent regulation of gene expression (Martins et al. 2017) which needs to be clarified in further studies. Moreover, there were direct correlations between TSG expression in patients and age at the time of analysis as well as age of disease onset. Perhaps as the age increases, the TSG expression increases; thus it can confirm the hypothesis that severity of MS, when disease starts in higher ages, is more than that of the patients with early age at disease onsets (Cierny et al. 2017). So apparently, upregulation of TSG expression in order to prevent aberrant-activated immune responses occurs imperfectly in MS patients.
In the case of SKP2, however, an ex vivo investigation on type 1 diabetogenic T cells revealed that changes in expression level of SKP2 can provide a way to improve cell-based immunotherapy in autoimmune responses (Wang et al. 2012). Significantly, upregulation of SKP2 in our study provided evidence to considerate SKP2 as a trigger for autoimmune disorders including MS disease. Also, this outcome can confirm the important role of the ubiquitin–proteasome system in MS pathogenesis (Chen et al. 2019; Minagar et al. 2012).
TOB1-AS1 lncRNA can inhibit cell proliferation (Yao et al. 2018). In this study, TOB1-AS1 expression did not reveal a significant difference between patients and HCs. Hence, it can be considered that expression level of this lncRNA does not change in MS patients. However, according to our co-expression analysis, the exact answer can only be provided by assessment of its expression level in a large cohort of patients. As a matter of fact, high inter-individual expression variability of lncRNAs may lead to reduced representation of variably expressed lncRNAs in limited number sample size (Kornienko et al. 2016). In line with this fact that lncRNAs regulates the expression of neighbor genes and plays an important role in immune cell function (Atianand and Fitzgerald 2014), we found a significant correlation between TOB1-AS1 and TOB1 expressions in patients but not in controls. Since differential correlation analysis can identify specific molecular signatures that underlie diverse biological processes, for example, in disease pathogenesis or prognosis (Singh et al. 2018), this observed correlation implies the presence of a probable regulatory mechanism between TOB1-AS1 and TOB1 expressions in MS patients. In this sense, this correlation may be involved in MS pathogenesis. Interestingly, a powerful correlation was found between TOB1-AS1 and both SKP2 and TSG expression. It seems that these correlations are indirect. According to co-expression analysis, when TOB1 expression increases, probably in association with TOB1-AS1, the SKP2 expression increases to balance TOB1 level, and TOB1 regulates TSG expression as already mentioned. In addition, TOB1-AS1 expression correlated negatively with EDSS of patients, which shows the possible role of TOB1-AS1 in MS development. Accordingly, these results warrant further exploration in a well-designed functional study to demonstrate the underlying network of TOB1-AS1 function and related molecules in MS pathogenesis.
Further analysis showed different expression of SKP2 and TSG in subtypes of MS and HCs. Since MS subtypes show different expression profiles in PBMCs (Ratzer et al. 2013), our data suggest a probable role of SKP2 and TSG expression in these different profiles. Different expression of TSG and SKP2 just in RRMS can support the proposal that neurodegenerative process in progressive MS is independent from the inflammatory responses which predominate in RRMS (Correale et al. 2016). In categories based on therapy, INF-B seemingly influences TOB1 expression in positive way through increasing its expression in the INF-B-treated patients. It can be deduced that one of the mechanisms of INF-B for enhancing immune system homeostasis (de Andrés et al. 2007; Korporal et al. 2008), might be a modification of TOB1 expression.
According to significant differences in immune function between males and females and sex-specific expression pattern of MS (Ullah et al. 2019), we evaluated the possible differences of expression of target genes between males and females in both control and patient groups. SKP2 and TSG expression rose in female controls versus male controls and in male patients versus male HCs. In the follow-up evaluations, according to the evidence of sex hormone effects on immune tolerance and MS pathogenesis (Ysrraelit and Correale 2019), our in silico analysis suggested the existence of some putative estrogen and testosterone receptor binding sites on the promoter of examined genes. Therefore, we hypothesize that one of the beneficial effects of testosterone and estrogen treatment to reduce the percentage of circulating CD4 + cells (Greer and McCombe 2011) may be resulted from SKP2 and TSG expression regulation. Furthermore, the upregulation of SKP2 in males can be a reason for severity of MS in males (Whitacre et al. 1999). Of course, these predictions of estrogen and testosterone receptor binding sites need to be confirmed through more precise bioinformatics analysis and molecular experiments. Finally, the area under the ROC curve suggested that expression of SKP2 and TSG in the PBMCs may have a moderate predictive power in order to diagnose MS alongside with other biomarkers such as IL-2, TNF-a, IL-1B, and IL-4 (Preedy and Patel 2015).
Conclusion
In conclusion, our findings propose a link between a novel lncRNA and some coding genes, TOB1, TSG, and SKP2 that play significant roles in the maintaining immune tolerance. Besides, TOB1-AS1 expression may decrease during MS progression. This study demonstrates robust dysregulation of these genes in MS patients compared to controls. Such dysregulation in maintaining immune tolerance may act as putative agents to MS pathogenesis and response to interferon treatment. As our results depicted, INF-B can possibly modify TOB1 expression in treated patients. However, our study has a limitation regarding small sample size. The logic of this research might then be applied to set up further studies with the purpose of finding the functional mechanism of TOB1-AS1 and its related gene network in the pathogenesis of MS and response to therapy.
References
Arloth, J. (2014). Expression quantitative trait loci as possible biomarkers on depression: Candidate gene and genome-wide approaches.
Atianand, M. K., & Fitzgerald, K. A. (2014). Long non-coding RNAs and control of gene expression in the immune system. Trends in Molecular Medicine,20(11), 623–631.
Baranzini, S. E. (2014). Role of antiproliferative gene Tob1 in the immune system. Clinical and Experimental Neuroimmunology,5(2), 132–136.
Basdeo, S. A., Kelly, S., O’Connell, K., Tubridy, N., McGuigan, C., & Fletcher, J. M. (2016). Increased expression of Tbet in CD4 + T cells from clinically isolated syndrome patients at high risk of conversion to clinically definite MS. SpringerPlus,5(1), 779.
Chen, D., Liu, X., Xia, T., Tekcham, D. S., Wang, W., Chen, H., … Liu, X. (2019). A multidimensional characterization of E3 ubiquitin ligase and substrate interaction network. IScience, 16, 177–191.
Cierny, D., Lehotsky, J., Hanysova, S., Michalik, J., Kantorova, E., Sivak, S., … Jesenska, L. (2017). The age at onset in Multiple Sclerosis is associated with patient’s prognosis. Bratislavske Lekarske Listy, 118(6), 374–377.
Collier, S. P. (2014). TMEVPG1, A Long Noncoding RNA within the Immune System. Vanderbilt University.
Correale, J., Gaitán, M. I., Ysrraelit, M. C., & Fiol, M. P. (2016). Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain,140(3), 527–546.
Corvol, J.-C., Pelletier, D., Henry, R. G., Caillier, S. J., Wang, J., Pappas, D., … Oksenberg, J. R. (2008). Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proceedings of the National Academy of Sciences United States of America, 105(33), 11839–11844.
Dastmalchi, R., Ghafouri-Fard, S., Omrani, M. D., Mazdeh, M., Sayad, A., & Taheri, M. (2018). Dysregulation of long non-coding RNA profile in peripheral blood of multiple sclerosis patients. Multiple Sclerosis and Related Disorders,25, 219–226. https://doi.org/10.1016/j.msard.2018.07.044.
de Andrés, C., Aristimuño, C., de las Heras, V., Martínez-Ginés, M. L., Bartolomé, M., Arroyo, R., … Sánchez-Ramón, S. (2007). Interferon beta-1a therapy enhances CD4 + regulatory T-cell function: An ex vivo and in vitro longitudinal study in relapsing−remitting multiple sclerosis. Journal of Neuroimmunology, 182(1), 204–211.
de Mello, J. B. H., Cirilo, P. D. R., Michelin, O. C., Domingues, M. A. C., Rudge, M. V. C., Rogatto, S. R., et al. (2017). Genomic profile in gestational and non-gestational choriocarcinomas. Placenta,50, 8–15.
Dehghanian, F., Kay, M., & Hojati, Z. (2018). Interferon gamma versus beta-interferon in pathogenesis of multiple sclerosis: Battle of two interferons. In A. Minagar (Ed.), Neuroinflammation (pp. 429–448). Amsterdam: Elsevier.
Dendrou, C. A., Fugger, L., & Friese, M. A. (2015). Immunopathology of multiple sclerosis. Nature Reviews Immunology,15(9), 545–558.
Dolati, S., Marofi, F., Babaloo, Z., Aghebati-Maleki, L., Roshangar, L., Ahmadi, M., … Yousefi, M. (2018). Dysregulated network of mirnas involved in the pathogenesis of multiple sclerosis. Biomedicine & Pharmacotherapy, 104, 280–290.
Driscoll, M. D., Sathya, G., Muyan, M., Klinge, C. M., Hilf, R., & Bambara, R. A. (1998). Sequence requirements for estrogen receptor binding to estrogen response elements. Journal of Biological Chemistry,273(45), 29321–29330.
Ewing, E., Kular, L., Fernandes, S. J., Karathanasis, N., Lagani, V., Ruhrmann, S., … Gomez-Cabrero, D. (2019). Combining evidence from four immune cell types identifies DNA methylation patterns that implicate functionally distinct pathways during Multiple Sclerosis progression. EBioMedicine, 43, 411–423.
Farsani, Z. S., Behmanesh, M., & Sahraian, M. A. (2015). Interleukin-10 but not transforming growth factor-β1 gene expression is up-regulated by vitamin D treatment in multiple sclerosis patients. Journal of the Neurological Sciences,350(1), 18–23.
Gharesouran, J., Taheri, M., Sayad, A., Ghafouri-Fard, S., Mazdeh, M., & Omrani, M. D. (2019). A novel regulatory function of long non-coding RNAs at different levels of gene expression in multiple sclerosis. Journal of Molecular Neuroscience,1, 1–7.
Gonsette, R. E. (2012). Self-tolerance in multiple sclerosis. Acta Neurologica Belgica,112(2), 133–140.
Greer, J. M., & McCombe, P. A. (2011). Role of gender in multiple sclerosis: Clinical effects and potential molecular mechanisms. Journal of Neuroimmunology,234(1), 7–18.
Haines, J., Stewart, G., Compston, A., Ebers, G., Peltonen, L., D’Alfonso, S., … Eraksoy, M. (2003). A meta-analysis of whole genome linkage screens in multiple sclerosis. Journal of Neuroimmunology, 143(1–2), 39–46.
Hiramatsu, Y., Kitagawa, K., Suzuki, T., Uchida, C., Hattori, T., Kikuchi, H., … Yamamoto, T. (2006). Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Research, 66(17), 8477–8483.
Kornienko, A. E., Dotter, C. P., Guenzl, P. M., Gisslinger, H., Gisslinger, B., Cleary, C., … Barlow, D. P. (2016). Long non-coding RNAs display higher natural expression variation than protein-coding genes in healthy humans. Genome Biology, 17(1), 14.
Korporal, M., Haas, J., Balint, B., Fritzsching, B., Schwarz, A., Moeller, S., … Wildemann, B. (2008). Interferon beta–induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells. Archives of Neurology, 65(11), 1434–1439.
Maik-Rachline, G., Hacohen-Lev-Ran, A., & Seger, R. (2019). Nuclear ERK: Mechanism of translocation, substrates, and role in cancer. International Journal of Molecular Sciences,20(5), 1194.
Mandrekar, J. N. (2010). Receiver operating characteristic curve in diagnostic test assessment. Journal of Thoracic Oncology,5(9), 1315–1316.
Martins, A. J., Narayanan, M., Prüstel, T., Fixsen, B., Park, K., Gottschalk, R. A., … Wendelsdorf, K. V. (2017). Environment tunes propagation of cell-to-cell variation in the human macrophage gene network. Cell Systems, 4(4), 379–392.
McDonald, W. I., Compston, A., Edan, G., Goodkin, D., Hartung, H., Lublin, F. D., … Reingold, S. C. (2001). Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology, 50(1), 121–127.
Minagar, A., Ma, W., Zhang, X., Wang, X., Zhang, K., Alexander, J. S., … Albitar, M. (2012). Plasma ubiquitin–proteasome system profile in patients with multiple sclerosis: Correlation with clinical features, neuroimaging, and treatment with interferon-beta-1b. Neurological Research, 34(6), 611–618.
Mowel, W. K., Kotzin, J. J., McCright, S. J., Neal, V. D., & Henao-Mejia, J. (2018). Control of immune cell homeostasis and function by lncRNAs. Trends in Immunology,39(1), 55–69.
Naghavi Gargari, B., Behmanesh, M., & Sahraian, M. A. (2015). Effect of vitamin D treatment on interleukin-2 and interleukin-4 genes expression in multiple sclerosis. Physiology and Pharmacology,19(1), 14–21.
Oskooei, V. K., & Ghafouri-Fard, S. (2019). Are long non-coding RNAs involved in the interaction circuit between estrogen receptor and vitamin D receptor? Meta Gene,19, 1–9.
Preedy, V. R., & Patel, V. B. (2015). Biomarkers in cancer. Berlin: Springer.
Qiu, F., Liang, C.-L., Liu, H., Zeng, Y.-Q., Hou, S., Huang, S., … Dai, Z. (2017). Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget, 8(1), 268.
Ratzer, R., Søndergaard, H. B., Christensen, J. R., Börnsen, L., Borup, R., Sørensen, P. S., et al. (2013). Gene expression analysis of relapsing–remitting, primary progressive and secondary progressive multiple sclerosis. Multiple Sclerosis Journal,19(14), 1841–1848.
Roche, P. J., Hoare, S. A., & Parker, M. G. (1992). A consensus DNA-binding site for the androgen receptor. Molecular Endocrinology,6(12), 2229–2235.
Sakaguchi, S., Powrie, F., & Ransohoff, R. M. (2012). Re-establishing immunological self-tolerance in autoimmune disease. Nature Medicine. https://doi.org/10.1038/nm.2622.
Sandelin, A., Alkema, W., Engström, P., Wasserman, W. W., & Lenhard, B. (2004). JASPAR: An open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Research,32(suppl_1), D91–D94.
Schulze-Topphoff, U., Casazza, S., Varrin-Doyer, M., Pekarek, K., Sobel, R. A., Hauser, S. L., … Baranzini, S. E. (2013). Tob1 plays a critical role in the activation of encephalitogenic T cells in CNS autoimmunity. Journal of Experimental Medicine, 210(7), 1301–1309.
Singh, A. J., Ramsey, S. A., Filtz, T. M., & Kioussi, C. (2018). Differential gene regulatory networks in development and disease. Cellular and Molecular Life Sciences,75(6), 1013–1025.
Spurlock, C. F., III, Tossberg, J. T., Guo, Y., Collier, S. P., Crooke, P. S., III, & Aune, T. M. (2015). Expression and functions of long noncoding RNAs during human T helper cell differentiation. Nature Communications,6, 6932.
Tzachanis, D., & Boussiotis, V. A. (2009). Tob, a member of the APRO family, regulates immunological quiescence and tumor suppression. Cell Cycle,8(7), 1019–1025.
Tzachanis, D., Freeman, G. J., Hirano, N., Van Puijenbroek, A. A. F. L., Delfs, M. W., Berezovskaya, A., … Boussiotis, V. A. (2001). Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nature Immunology, 2(12), 1174–1182.
Tzachanis, D., Lafuente, E. M., Li, L., & Boussiotis, V. A. (2004). Intrinsic and extrinsic regulation of T lymphocyte quiescence. Leukemia & Lymphoma,45(10), 1959–1967.
Tzachanis, D., Li, L., Lafuente, E. M., Berezovskaya, A., Freeman, G. J., & Boussiotis, V. A. (2007). Twisted gastrulation (Tsg) is regulated by Tob and enhances TGF-β signaling in activated T lymphocytes. Blood,109(7), 2944–2952.
Ullah, M. F., Ahmad, A., Bhat, S. H., Abu-Duhier, F. M., Barreto, G. E., & Ashraf, G. M. (2019). Impact of sex differences and gender specificity on behavioral characteristics and pathophysiology of neurodegenerative disorders. Neuroscience & Biobehavioral Reviews,102, 95–105.
Vila, A., Casabella, A. M., Baig, M., Bidot, C. J., & Jones, B. C. (2016). Multiple sclerosis: Enigmatic factors and new controversies. Clinical Case Reports and Reviews. https://doi.org/10.15761/CCRR.1000288.
Wang, D., Qin, H., Du, W., Shen, Y.-W., Lee, W.-H., Riggs, A. D., et al. (2012). Inhibition of S-phase kinase-associated protein 2 (Skp2) reprograms and converts diabetogenic T cells to Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences USA,109(24), 9493–9498.
Whitacre, C. C., Reingold, S. C., O’looney, P. A., Blankenhorn, E., Brinley, F., Collier, E., … Gilmore, W. (1999). A gender gap in autoimmunity: Task force on gender, multiple sclerosis and autoimmunity. Science, 283(5406), 1277–1278.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif.),25(4), 402–408. https://doi.org/10.1006/meth.2001.1262.
Yao, J., Li, Z., Yang, Z., Xue, H., Chang, H., Zhang, X., … Guo, K. (2018). Long noncoding RNA TOB1-AS1, an epigenetically silenced gene, functioned as a novel tumor suppressor by sponging miR-27b in cervical cancer. American Journal of Cancer Research, 8(8), 1483.
Ysrraelit, M. C., & Correale, J. (2019). Impact of sex hormones on immune function and multiple sclerosis development. Immunology,156(1), 9–22.
Zastepa, E., Fitz-Gerald, L., Hallett, M., Antel, J., Bar-Or, A., Baranzini, S., … Haegert, D. G. (2014). Naive CD4 T-cell activation identifies MS patients having rapid transition to progressive MS. Neurology, 82(8), 681–690.
Acknowledgements
The authors gratefully acknowledge the contribution of the patients and controls for their participation in this study.
Funding
This work is supported by the Iran National Science Foundation and the Department of research Affairs of Tarbiat Modares University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethics committee of Tarbiat Modares University and with the 1964 Helsinki Declaration.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dehghanzad, R., Pahlevan Kakhki, M., Alikhah, A. et al. The Putative Association of TOB1-AS1 Long Non-coding RNA with Immune Tolerance: A Study on Multiple Sclerosis Patients. Neuromol Med 22, 100–110 (2020). https://doi.org/10.1007/s12017-019-08567-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-019-08567-1